

Varied Presentations of Cervical Spondylotic
Myelopathy Presenting to a Chiropractic Clinic:
A Report of 3 CasesThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Can Chiropr Assoc 2022 (Aug); 66 (2): 146–156 ~ FULL TEXT
OPEN ACCESS Chandler Bolles, DC, Patrick Battaglia, DC, DACBR, and Catherine Moore, DO
Edward Via College of Osteopathic Medicine,
Spartanburg, SC.
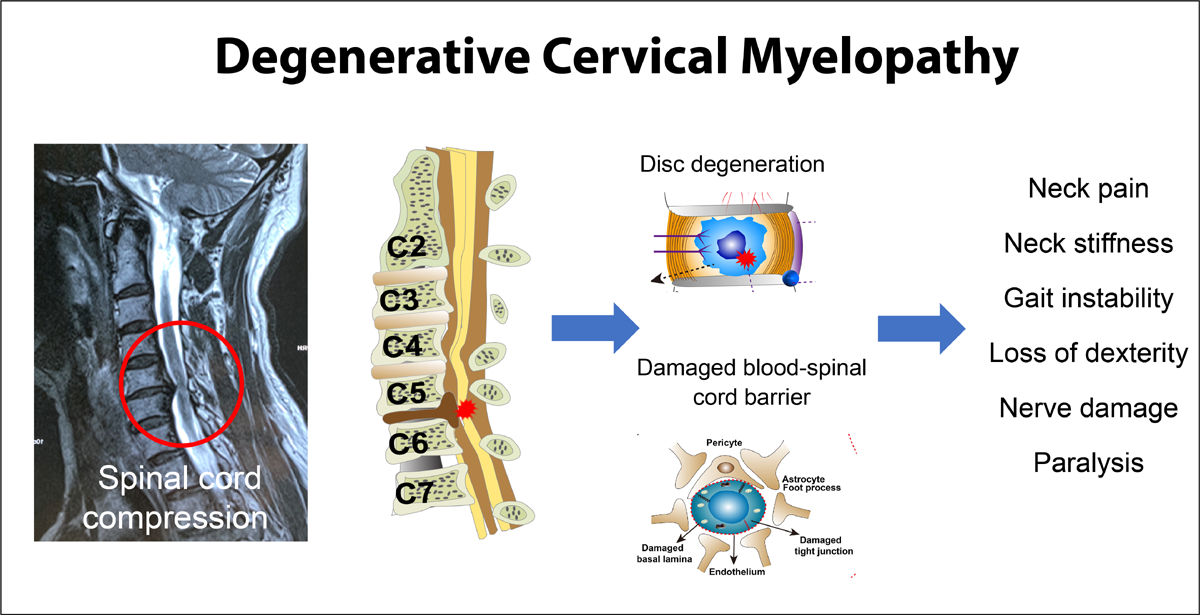
FROM: J. Clin. Med. 2021Cervical spondylotic myelopathy (CSM) is the leading cause of acquired spinal cord dysfunction worldwide and may be expected to increase in prevalence due to an aging global population. Clinical features of CSM are highly variable, and chiropractors frequently manage patients with common signs and symptoms of CSM such as neck pain, extremity weakness, and gait imbalances. Early recognition of signs consistent with myelopathy may mitigate future disability and improve quality of life. Key predictors of patient outcome are the age of initial presentation, baseline CSM severity (as measured by mJOA score), and the presence of gait disturbances. This report describes three cases of CSM presenting to a chiropractic clinic. Each case illustrates a unique manifestation of CSM, including myelopathy, myeloradiculopathy, and distal neuropathic pain (funicular referral). In addition, a review of CSM terminology, epidemiology, pathobiology, clinical features, imaging, and management is provided.
Keywords: cervical myelopathy; cervical spondylotic myelopathy; chiropractic; chiropratique; conservative management; degenerative cervical myelopathy; dysfonctionnement de la moelle épinière; myélopathie cervicale; myélopathie cervicale dégénérative; myélopathie spondylotique cervicale; spinal cord dysfunction; traitement conservateur.
From the FULL TEXT Article:
Introduction
Cervical spondylotic myelopathy (CSM) is the leading cause of spinal cord dysfunction and spastic paresis in adults aged 55 and older. [1–5] Patients with CSM often present with neck pain and stiffness, loss of manual dexterity, weakness and/or paresthesia in both the upper and lower extremities, gait imbalances, and urge incontinence. [1, 6]
Considering the prevalence of CSM is estimated at 605 per million1 and the likelihood of concurrent neck pain and neurologic signs and symptoms, it is critical that chiropractors have a thorough understanding of this entity.
We present three contrasting cases of CSM presenting to chiropractic physicians working within a federally qualified health center (FQHC) as part of an academic affiliation with Logan university. We also provide discussion on the epidemiology, pathophysiology, clinical features, and treatment options.
Case presentations
Patients provided written informed consent between August and September 2019 as this work was originally constructed for submission to the 27th Association of Chiropractic Colleges Research Agenda Conference. All patients were referred to chiropractic integrated into a Federally Qualified Health Center in St. Louis, MO.
Case 1
49-year-old male referred from primary care for initial evaluation and management of chronic, atraumatic neck and right arm pain in May 2018. He noted the neck pain started insidiously approximately four years prior in an episodic manner, worsening two years prior with new onset right upper extremity pain. The pain was subjectively noted by the patient to be in the posterior neck, right lateral shoulder, and anterior arm. He also reported worsening right hand weakness and bilateral hand numbness and pain that started insidiously eight months prior to initial presentation and has been concerned lately because he “cannot make a muscle,” meaning he could not contract his biceps brachii. No patient reported outcome measures were obtained at initial consultation.
The neck and right upper extremity pain was constant, with dull, sharp, and burning characteristics, exacerbated by any neck or upper extremity movements. He was managing this pain with prescribed gabapentin (600 mg three times daily) and cyclobenzaprine (10 mg at night before bed) with minimal relief. In addition, he reported trying his own unsupervised upper extremity strengthening exercises for this complaint without benefit.
Functionally, he was driving less because of pain related to right upper extremity movements. He also noted paroxysms of severe pain when the “air-conditioning hits my arm”, suggesting a neuropathic origin. He was working as a painter and reported impaired work performance related to inability to hold his tools and inability to “balance on a ladder.”
Review of systems revealed blurred vision (without diplopia or blindness), “balance issues” interpreted as unsteadiness with gait, and episodic urinary incontinence. Past surgical history included left sided carpal tunnel release three years prior, with residual numbness in the left hand (all five digits). The remaining past medical and surgical history was unremarkable.
At examination, his gait was grossly normal, including heel and toe walk. He could not tandem walk because of unsteadiness. Spinal inspection revealed upper thoracic kyphosis with forward head posture. He had full cervical range of motion, and full shoulder range of motion but increased pain at end ranges of all motions for both his cervical spine and shoulders. Inspection of the limbs was notable for right biceps atrophy with fasciculations, exacerbated with manual muscle testing. A small scar was noted on the left palm consistent with history of carpal tunnel release.
His neurologic examination was notable for 3/4 patellar and Achilles deep tendon reflexes bilaterally without clonus. Biceps, brachioradialis, and triceps deep tendon reflexes were 2/4 bilaterally. Upper extremity vibration sensation to a 128 Hz tuning fork was normal. There was approximately a five second delay to vibration cessation in the lower extremity. Allodynia to light touch was noted along the lateral right deltoid and lateral and anterior right arm. Manual muscle testing revealed 4/5 strength of the right biceps brachii and right hand. The remainder of his upper and lower body strength within the C5-T1 and L2-S1 myotomes was normal. He had a plantar flexor response and there was no Hoffman sign.
Maximal foraminal compression to the right reproduced the right upper extremity pain.
Radiographs dated April 2014 were available for review demonstrating straightening of the cervical spine and C5/6 discogenic spondylosis. Since the initial encounter raised concern for myelo-radiculopathy, a contemporaneous MRI was obtained in June 2018 demonstrating congenitally short pedicles with long segment central canal stenosis that was severe at C4/5 and C5/6. There was also severe bilateral neuroforaminal stenosis at these same levels. T2 hyperintensity was present in the spinal cord at the C4/5 level without cord expansion consistent with myelomalacia.
The MRI findings supported the clinical diagnosis of cervical spondylotic myelopathy with concomitant right C5 radiculopathy, and the patient was referred to neurosurgery where he underwent anterior decompression consisting of disc replacement at the C4/5 and C5/6 level. There was no posterior decompression performed.
Case 2
38-year-old female was referred from primary care in December 2017 for initial evaluation and management of acute neck pain post recent motor-vehicle accident and chronic upper and lower extremity paresthesia. The paresthesias started in 2008 after a motor-vehicle accident where she reported head trauma. She reported being told that she had “spinal cord impingement” at that time but was only offered physical therapy for her complaints which she completed without change in extremity complaints. Specifics about her physical therapy regime in 2008 were not available at the time of her chiropractic consultation in 2017. No imaging was available for review from 2008. She had neck pain at that time that mostly abated after physical therapy. However, she did have periods of episodic neck “discomfort” from 2008 until present, worsened in December 2018 with a reported low speed motor-vehicle accident. It is unknown if she sought care for this episodic pain complaint.
Regarding the neck pain, it was subjectively noted to be in the posterior, midline and described as mild. The patient was more concerned with her worsening sensorimotor complaints which consisted of weakness in both hands, right greater than left, decreased general sensation in both hands, pain in the right hand traveling to the right elbow, a “shock-like” sensation traveling from her neck to her toes on occasion, especially with head and neck movement, and decreased sensation in the toes, right greater than left. These lower extremity sensory changes resulted in feelings of “unsteadiness” when walking.
She worked as a bartender at a country club and was becoming more fearful she would drop “the expensive bottles of liquor.” Additionally, she was avoiding going down her basement stairs at home for fear of falling. No patient reported outcome measures were applied at initial consultation. Her past medical and surgical history and her review of systems were otherwise unremarkable.
At examination, inspection and palpation of the cervical spine were unremarkable. Range of motion testing was deferred considering the reported neurologic dysfunction. Her gait was unsteady, and she could not tandem walk. Her deep tendon reflexes were 3/4 upper and lower extremity, and Hoffman sign was present bilaterally. Three beats of clonus were noted in the right ankle, and one beat of clonus was present in the left ankle.
Gross motor testing as part of a neurologic screen revealed 4/5 intrinsic hand and finger flexor strength bilaterally, 4/5 right knee flexion and extension strength, and 4/5 right dorsiflexor strength. The remaining muscle groups of the upper and lower extremity were normal. No muscle atrophy or fasciculations were observed.
Radiographs were available for review dated July 2017 demonstrating straightening of the cervical spine with mild kyphosis at C5/6. Associated discogenic spondylosis and uncovertebral arthritis were present at the C5/6 level.
A working diagnosis of cervical myelopathy (degenerative or demyelinating) was made. An MRI was obtained five days after initial consultation demonstrating congenitally short pedicles at C4/5 and C5/6 with severe central canal stenosis. At C5/6 there was also a broad-based disc-osteophyte complex with spinal cord compression. T2 hyperintensity (i.e., brightness in the spinal cord likely representing myelomalacia) was present in the spinal cord at the C5/6 level. She consulted with neurosurgery in March 2018, and was lost to follow-up until September 2018, when she again consulted with neurosurgery and subsequently underwent anterior cervical diskectomy with fusion for cervical myelopathy in October 2018.
Case 3
49-year-old male was referred from primary care in January 2019 for evaluation and management of left sided back and lower extremity pain, described as “sciatica”. His history was notable for an abrupt onset of neck pain with left sided upper and lower extremity numbness after a lifting accident at work as a mechanic in November 2013. At that time, an MRI of the brain and spinal cord was obtained demonstrating central canal stenosis at the C3/4 level secondary to a disc-bone complex, with a superimposed left paracentral disc extrusion. There was related spinal cord compression and T2 hyperintensities consistent with myelomalacia. The remainder of the brain and spine MRI was normal. The patient’s numbness persisted and was accompanied with episodic neck pain through 2016. Despite being offered neurosurgery in 2013, due to insurance issues and psychosocial stressors, he did not see neurosurgery until end of year 2016. A contemporaneous cervical spine MRI was obtained in January 2017 demonstrating resorption of the cervical disc extrusion but persistence of spinal stenosis secondary to degenerative changes and unchanged myelomalacia at the C3/4 level. The patient consulted with neurosurgery in May 2018 and was told he was not a surgical candidate at that time. Approximately in 2017, the patient’s symptoms progressed to include not only numbness, but also worsening pain in the back and left lower extremity. His neck pain had resolved. His pain management from 2017 to 2019 consisted of 5 mg hydrocodone – 325 mg acetaminophen (Norco) three times daily with minimal benefit. In 2019, he was switched to buprenorphine and 300 mg gabapentin three times daily also without benefit.
At his chiropractic consultation in January 2019, his pain complaints were subjectively localized to the left-sided lower back, left gluteal, and entire left lower extremity, and rated as severe (i.e., 10/10 on a numeric rating scale). No patient reported outcome measures were obtained at initial consultation. His review of systems was notable for feeling “unsteady” with occasional falls. Otherwise, his review of systems was unremarkable. At examination, his gait was slow and unsteady but without any signs of spasticity. Inspection of the neck, back, and limbs was unremarkable without lesion or deformity. He had allodynia along the left hemithorax, left sided low back, and entire left lower extremity to light touch. All lumbar ranges of motion were limited due to pain (degrees not measured), but he had full passive hip range of motion. Upper and lower deep tendon reflexes were 2/4 throughout except 3/4 patellar reflexes. Bilateral Hoffman sign was noted. There was no ankle clonus, and he had a flexor plantar response. His straight leg raise was negative up to 90 degrees. The working diagnosis was CSM with funicular referral. There was low clinical concern for lumbosacral radiculopathy.
Conservative care emphasizing myofascial therapies as a means of desensitization was initiated for pain management, with subjective short-term benefit. A concurrent Physical Medicine and Rehabilitation (PM&R) referral was placed. At that appointment, it was agreed that the patient’s back and lower extremity symptoms were likely secondary to incomplete spinal cord injury (i.e., cervical stenosis with superimposed disc herniation) at C3/4 with subsequent neuropathic pain (funicular referral). The patient was started on Cymbalta 30 mg twice daily for their pain and was recommended to follow-up with Pain Management for consideration of cervical epidural injection and/or spinal cord stimulator trial. The Cymbalta was not helpful for managing his pain, and he declined both interventions. Also, a lumbar MRI was obtained at the time of Physical Medicine and Rehabilitation to confirm there was not concurrent lumbar spine disease, and it showed mild multilevel degenerative changes without central canal or foraminal stenosis.
The patient was lost to follow up until 2021, when he re-presented with similar but worsening complaints. Most notably, his gait and balance had worsened. He continued to decline further Physical Medicine and Rehabilitation, Pain Management, or Neurosurgical referrals and was lost to follow-up after three therapy sessions.
Discussion
We present three unique cases of cervical spondylotic myelopathy (CSM) that highlight the variable nature of the disease. A thorough understanding of this entity by chiropractors is important to optimize spine care.
History and terminology
Cervical spondylotic myelopathy (CSM) was first described by Bailey and Casamajor in 1911 and further characterized by Stookey in 1928 after describing seven patients believed to have extradural ventral chondromas in the cervical spine. [7, 8] In 1952, Brain, Northfield, and Wilkinson [9] described compression of the spinal cord secondary to cervical spondylosis and the associated neurologic signs in 38 patients. In 1972, a landmark paper by Nurick [10] investigated the degree of disability in 160 patients with CSM and corroborated the role of ischemia in long standing spinal cord compression.
CSM is characterized by spinal cord dysfunction secondary to acquired stenosis of the cervical spinal canal from vertebral degeneration (disc desiccation, osteophytic lesions, apophyseal joint hypertrophy). Degenerative cervical myelopathy (DCM) is an umbrella term, encompassing both CSM and acquired stenosis from ossification of the posterior longitudinal ligament (OPLL) or hypertrophy/ ossification of the ligamentum flavum (OLF). [1] Congenital cervical spine stenosis (CSS), such as from short pedicles, is also a risk factor for developing CSM. [1, 6] CSS has been defined as sagittal spinal canal diameter less than 13 mm, or a Torg-Pavlov measurement less than 0.82 (canal diameter/vertebral body diameter). [11]
Epidemiology
The estimated incidence of CSM in North America is 41 per million per year with an estimated prevalence of 605 per million. [1] These numbers may underestimate the true burden of disease due to classifying CSM, OPLL, OLF, and non-traumatic spinal cord injury as separate clinical entities. [1, 5] A recent systematic review and meta-analysis assessed the prevalence of spinal cord compression in asymptomatic and symptomatic cohorts using magnetic resonance imaging (MRI) and proposed the point prevalence to be higher at 2.3%. [12]
While CSM is the leading cause of spinal cord dysfunction in individuals over the age of 55, it is interesting that all three cases presented here had symptomatic presentation before the age of 50, perhaps a result of degenerative changes superimposed on a congenitally narrowed spinal canal (cases 1 and 2), and an acute disc herniation superimposed on a region of degenerative stenosis (case 3). [5] The patients described in cases 1 and 2 had confirmed congenital spinal stenosis at the affected levels. In case 3, the patient had degenerative stenosis at the C3/4 level with a superimposed disc extrusion. In this case, it is reasonable to conclude the disc herniation resulted in the described spinal cord injury (SCI) because it occurred on a background of spinal stenosis. Serial imaging of the cervical spine confirmed resorption of the disc herniation but persistence of the spinal stenosis and spinal cord changes. Other factors related to patient care seeking behaviors, such as severity of symptoms, could also explain the younger cohort of CSM patients in this series. Regardless, as spine treating clinicians, it is important that chiropractors not only appreciate the high prevalence of CSM above the age of 55, but also appreciate that patients may have congenital or developmental stenosis at younger ages predisposing to this condition.
Pathobiology
Structural degenerative changes that result in canal stenosis include osteophytic spurs and buckling of the ligamentum flavum secondary to micro-instability. [11] The long-term mechanical forces applied to the spinal cord results in impaired blood flow to the cord, which has long been heralded as a key pathophysiologic component of CSM. Chronic compression induced by degenerative changes in the cervical spine result in ischemia in both the extra- (i.e., vertebral) and intra-spinal (i.e., anterior spinal artery) blood vessels. Further, long-standing compression on extra-spinal vessels induces wall-thickening and hyalinization, further reducing regional perfusion. Additionally, chronic spinal cord compression causes stretching and flattening of penetrating vessels, reducing perfusion to axonal pathways, particularly the lateral corticospinal tract. [1] This feature may explain the distribution of motor deficits seen in CSM patients.
Emerging evidence points to the activation of an immune response in long-standing intraparenchymal ischemia. [11] This may be a key patient-specific factor explaining the highly variable nature of disease manifestation. Activation of microglia and the accumulation of macrophages at the site of compression are the main known components of this neuroinflammatory reaction. Triggering of the CX3CR1–CX3CL1 axis has been demonstrated to be a key component in the hypoxia – neuroinflammation cascade. [3]
The blood-spinal cord barrier (BSCB), an analogue to the blood brain barrier, has been implicated in acute spinal cord injury but is believed to play a central role in CSM. [1] Chronic spinal cord compression is believed to disrupt endothelial cells, permitting the entry of pro-inflammatory cells into the spinal cord paranechyma. [1]
Ischemia and neuroinflammation are presumed to activate apoptotic pathways resulting in progressive neuronal and oligodendroglial death. The apoptotic pathway is mediated by signaling through Fas, Tumor Necrosis Factor (TNF), and mitogen-activated protein (MAP) kinase. [1] In fact, Karadimas et al. [13] demonstrated neuronal and oligodendrocyte cells undergoing active apoptosis in a 5-mm area centered around the area of maximal compression in CSM patients.
Clinical features

Table 1 The diagnosis of CSM is based on clinical features with imaging confirmation. [1, 12] Common signs and symptoms are presented in Table 1. Gait dysfunction is common among people over 60 years of age. For example, Mahlknecht et al. [14] found a total of 24.0% of 60 to 97-year-old patients demonstrated neurological gait disorders, 17.4% non-neurological gait problems, and 9.2% who demonstrated a combination of both. In CSM, gait dysfunction and balance disturbances from proximal lower extremity weakness are common early manifestations of CSM but can incorrectly be attributed to old age and delay diagnosis by up to six years. [6] Similarly, an average delay in diagnosis of 2.2 years was found by Behrbalk et al. in patients presenting with symptoms compatible with CSM in a community based setting. [5, 15] In a prospective, controlled trial in one single surgical practice, myelopathic signs such as an inverted brachial reflex, Babinski reflex, Hoffman sign, and sustained clonus were more common in CSM surgical candidates compared to controls. [16] A positive Hoffman sign is suggestive of upper motor neuron pathology localized to cervical spinal cord. However, Annaswamy et al. [17] found that a positive Hoffman sign was present in 22% of patients without cervical spinal cord compromise. Therefore, a Hoffman sign must always be considered in the context of the entire clinical picture and may not indicate the presence of a cervical spinal cord lesion. The absence of sensory symptoms (such as upper and lower extremity paresthesia and neck pain) in cases of suspected CSM should prompt investigation of other motor neuron diseases such as ALS, neuromuscular junction diseases such as myasthenia gravis, or myopathies such as inclusion body myositis. [1]
Funicular referral is an uncommon feature of spinal cord compression but awareness of its existence may prevent delayed diagnosis or mismanagement in CSM. [18–20] High-lighted in case 3, this phenomenon refers to dysfunction distant or remote from an expected anatomical locus of pathology. [18] Larner [18] suggested that mechanical compression of the ascending spinothalamic tract in the cervical spine might cause this false localizing sign remote from the level of compression. Ochiai and colleagues [19] posited that a different mechanism, such as ischemia in the watershed zone of the anterior spinal artery, was a more likely explanation in CSM patients with mid-thoracic girdle sensation, a type of funicular referral. Our patient in case 3 presented with myelopathic symptoms after a previous injury, as well as neuropathic pain in the left thorax and left lower extremity. MRI revealed myelomalacia of the cervical spine and lumbar neurodynamic testing was negative, prompting a putative diagnosis of CSM with funicular referral. Chan et al. [20] described a similar presentation in two patients with CSM complaining of sciatica-like leg pain. In these two patients, early interventions were targeted at the lumbar spine. After limited improvement, both patients experienced symptom resolution after a cervical epidural steroid injection (CESI) and a selective nerve root block, respectively.
As a form of SCI, CSM can cause bladder voiding dysfunction, such as urge incontinence. [1] A lesion in the neuroaxis above S1 can lead to discoordination of micturition, resulting in reflex or spastic bladder. Voluntary inhibition of the micturition reflex may be lost in cases of SCI and can result in detrusor muscle overactivity or detrusor-sphincter dyssynergia. This discoordination can lead to high voiding pressure, residual urinary volume, and incontinence, leading to upper tract deterioration and renal failure. [21]
The natural history of CSM is variable, alternating between stepwise decline and rapid neurological deterioration. [1, 6] Clark and Robinson6 were the first to investigate the natural history of CSM in 1956 and categorized three separate patterns: 75% of patients deteriorated in a stepwise fashion, 20% had slow, steady progression of disease, and 5% developed rapid onset of symptoms and signs and subsequently remained stable for years. A 2017 systematic review demonstrated that 20–62% of patients with CSM experienced neurologic deterioration during 3–6 year follow-up. [22] This review highlighted that patients with circumferential spinal cord compression were at a greater risk of neurological deterioration than individuals with partial compression. In a multivariate analysis evaluating risk factors for patients converting to surgery after conservative care, Oshima et al. [13] found that total cervical ROM (50°), segmental kyphosis in the maximum compression segment, or the presence of a local spondylolisthesis were independently associated with an increased risk of requiring surgery.
Diagnostic imaging
Radiography is valuable as a first-line imaging modality to assess cervical alignment and provide an estimation of spinal degeneration. [1] Computed Tomography (CT) is also a useful modality when operative treatment, such as spinal fusion, is being considered. In addition to providing superior detail of bony anatomy, CT is an invaluable resource when MRI is contraindicated, such as when a patient has a non-MRI-conditional pacemaker. [1] However, MRI is the imaging of choice for evaluation of the spinal cord and recent literature suggests that MRI is safe in patients with non-MRI-conditional cardiac devices. [23] Therefore, the benefits of obtaining the MRI to confirm SCI likely outweighs any perceived risks and this should be discussed with patients. Sectional imaging with CT and MRI is used to characterize both the nature of compression (spondylosis, OPLL, OLF) and the severity of compression in the cervical spine. MRI can also detect signal intensity changes within the spinal cord parenchyma. [1, 24, 25] Uchida and colleagues [24] found that the signal intensity ratio on T1-weighted images but not T2-weighted images correlated with postoperative neurologic improvement in a cohort of patients undergoing surgery for CSM. In a retrospective study, Avadhani et al. also found that low signal intensity changes on T1-weighted images were associated with poor surgical outcomes. [25] Low intensity signal changes on T1-weighted images may represent myelomalacia, necrosis, and cystic cavitation, all of which are considered irreversible. [1, 25] Nouri et al. [4] also demonstrated that the presence of T1-weighted image signal hypointensity indicated more permanent injury and portended decreased functional recovery in patients with CSM.
The use of novel MRI techniques to diagnose CSM is an area of continued investigation as the prevalence of asymptomatic spinal cord compression in healthy populations is estimated at 24.2%. [12] Although the majority of individuals over the age of fifty demonstrate radiographic evidence of cervical degeneration, only about 25% go on to develop symptoms of neurological impairment from mechanical compression. [2] Unlike structural MRI, which relies primarily on qualitative assessment, diffusion tensor imaging (DTI) can be used to evaluate CSM patients quantitatively. [26, 27] DTI is an emerging technology which assesses the microstructural changes in the spinal cord not otherwise detected by conventional MRI. DTI uses the diffusion directionality of water molecules to study the microstructure of biological tissues. In the white matter tracts of the spinal cord, the preferential directionality of diffusion is known as fractional anisotropy (FA). [26] The white matter tracts of the spinal cord are arranged in a tightly packed orientation, leading to a high level of FA in unaffected individuals. [28]
Emerging evidence suggests that FA may have diagnostic potential as a pre- and post-operative outcome measure in CSM. [28] Findings from Lee et al. [29] support the notion that FA at the level of maximal compression in the cervical spine may have diagnostic potential in assessing the severity of myelopathy in CSM patients. Maki et al. [27] evaluated 26 surgical candidates and found that FA was a good predictive factor in determining post-operative success. Rao et al. [26] investigated the utility of FA as a biomarker for severity of CSM and a prognostic biomarker for post-operative improvement. In their study, lower FA at the level of maximal compression correlated with worse preoperative clinical severity. Results also demonstrated an inverse relationship between lower preoperative FA at the level of maximal compression and postoperative improvement. [26]
Outcomes measures
The modified Japanese Orthopedic Association (mJOA) score and the Nurick grading system are both CSM-specific indices used to grade the severity of dysfunction in patients. [1, 10] The mJOA assesses functional abilities on an 18-point scale which includes upper limb motor function, lower limb motor function, upper limb sensation and sphincter function. [1] The mJOA was adopted from the original Japanese Orthopedic Association (JOA) but was adapted to western populations. Fehlings et al. defined CSM severity as mild if mJOA scores were 15 or higher, moderate if mJOA scores ranged from 12 to 14 or severe if mJOA scores were less than 12. [30, 31] In a prospective study with 277 surgical candidates with CSM, the mJOA demonstrated higher validity with the Nurick score than previous research looking at the JOA. [31] The Nurick grading system is a 6-point ordinal scale that is primarily based on employment status and gait function. [10] However, the Nurick Scale demonstrates low sensitivity and poor responsiveness. [1, 31]
Management
The current standard of care for degenerative cervical myelopathy is surgical decompression. [2, 32] However, management strategies for patients with CSM are guided by different factors. Two salient factors include the rate of disease progression over time and the risk of acute spinal cord injury (SCI). One particular SCI is central cord syndrome; the resultant neurologic consequences following a low-energy hyperextension injury superimposed onto a pre-existing myelopathy. [1]
In some reports, non-operative management of patients with CSM have yielded similar outcomes as operative management in patients with mJOA scores ≥ 13 (as gauged by post-operative mJOA and NDI scores). [22] However, the incidence of hospitalization for spinal cord injury was 13.9 per 1,000 person-years in a non-operative group compared to 9.4 per 1,000 in the operative group, which was significant (adjusted HR = 1.57; 95% CI = 1.11–2.22; P = .011). [22] Another prospective randomized trial comparing conservative to surgical management showed that outcomes based on mJOA score, recovery rate, timed 10-meter walk, and functional daily activities were comparable after two-year follow up. [33] The results of one prospective case series demonstrated that surgery for CSM is associated with significant functional recovery, but appears to plateau after six months. [34] Boakye et al. [34] obtained data on 58,115 patients with CSM and found that complications following spine fusion were significantly more frequent among individuals with a least three pre-existing medical comorbidities.
Many reports exist describing the conservative management of CSM, but few provide detailed methods of application. In one report, conservative management included long durations of cervical traction and outcomes were categorized as: improvement, no improvement, and exacerbation. [35] In 62% of the nonsurgical patients, symptoms worsened. However, there was a strong correlation between symptom duration and treatment outcome, with longer durations portending a worse outcome. [35] Other reports also suggest gait training and cervical immobilization for patients with mild myelopathy. [13]
Chiropractors can play an important role in the early recognition and triage of patients with CSM. Early manifestations of CSM may include neck pain, for which many persons seek care from a chiropractor. Early surgical treatment of CSM, before symptoms become chronic and before the onset of irreversible spinal cord damage, is essential for optimal patient outcomes. [5, 6, 35] Therefore, early identification of signs and symptoms consistent with CSM by chiropractors may initiate appropriate testing (e.g., MRI) and timely surgical consultation, which would yield the best possible outcome.
Guidelines exist describing absolute contraindications to cervical spine manipulation but limited high-quality data guides decision making in the presence of CSM. [36] Some data suggest that cervical spine manipulation may worsen neurologic symptoms of CSM. [37] As spinal manipulation is a common treatment implemented by chiropractors, appreciating relative and absolute contraindications to manipulation ensures patient safety. Case reports describe adverse events following cervical manipulation superimposed onto a pre-existing CSM. One case series involving 27 patients highlighted that cervical spinal cord encroachment as demonstrated through advanced imaging was not an absolute contraindication to cervical manipulation. [36] However, none of the included patients had evidence of severe or acute myelopathy. A five-year retrospective series involving 22 patients found that cervical myelopathy worsened in 11 patients who underwent cervical manipulation. [38] Another case series involving three patients found that cervical spine manipulation was purported to be a causative factor in the development of CSM. [39] In spite of this, detection bias in the aforementioned studies prevents making absolute conclusions about what role, if any, manipulation played in the progression of disease independent of natural history.
Fehlings et al. [2] demonstrated that surgical decompression for the treatment of CSM was associated with improvements in functional, disability-related, and quality-of-life outcomes at one year of follow-up. The degree of improvement was correlated with preoperative mJOA scores (patients with mild disease preoperatively experienced the least amount of improvement whereas individuals with more severe disease experienced greater improvements). Another study revealed that between baseline and two years postoperatively, mJOA scores improved by 2.40 points and by 1.34 on the Nurick grading scale. [40] However, surgical intervention for CSM is not without associated risks. One in five patients in the multi-center AOSpine study experienced at least one complication following decompression surgery (most commonly dysphagia, dural tears, or infection). [40]
Limited evidence guides the decision to perform decompressive surgery in an asymptomatic patient who has evidence of cervical canal stenosis. When consulting this demographic, factors such as patient age, co-morbidities, level of activity, the rate of disease progression, and extent of radiographic findings must be considered. [41] One prospective study found that 8% of individuals with asymptomatic cord compression will go on to develop CSM after one year. Further, this study also showed that 22% in total would go on to develop CSM over the observation period (median follow-up 44 months). [15] Therefore, one in five people with asymptomatic cervical spinal cord compression may develop CSM within four-years. This information should prompt clinicians to counsel their patients on the higher risk of developing myelopathy in the presence of non-myelopathic cord compression. [42]
Conclusion
We present three unique cases of CSM and highlight the disease’s epidemiology, pathobiology, clinical features, and management strategies. CSM is the leading cause of spastic paresis in adults aged 55 and older. [5] Due to CSM’s variable disease course, it’s overlap with other conditions, and senescence, it is critical that chiropractors have a high index of suspicion for this entity so they can initiate prompt referral for co-treatment with neurosurgery. [1, 5] Owing to an ageing global population, identifying optimal treatment strategies for this disabling condition has become a public health priority, and chiropractors can play an important role with early diagnosis and rehabilitation referral. [1, 32]
Footnotes
The authors have no disclaimers, competing interests, or sources of support or funding to report in the preparation of this manuscript. The involved patients provided consent for case publication.
References:
Badhiwala JH, Ahuja CS, Akbar MA, et al. Degenerative cervical myelopathy — update and future directions. Nat Rev Neurol. 2020;16:108–124.
Fehlings MG, Wilson JR, Kopjar B, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95(18):1651–1658.
Karadimas SK, Gatzounis G, Fehlings MG. Pathobiology of cervical spondylotic myelopathy. Eur Spine J. 2015;24(Suppl 2):132–138.
Nouri A, Martin AR, Kato S, Reihani-Kermani H, Riehm LE, Fehlings MG. The relationship between MRI signal intensity changes, clinical presentation, and surgical outcome in degenerative cervical myelopathy: analysis of a global cohort. Spine. 2017;42(24):1851–1858.
Behrbalk E, Salame K, Regev GJ, Keynan O, Boszczyk B, Lidar Z. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg Focus. 2013;35(1):E1.
Bakhsheshian J, Mehta VA, Liu JC. Current diagnosis and management of cervical spondylotic myelopathy. Global Spine J. 2017;7(6):572–586.
Bailey P, Casamajor L. Osteoarthritis of the spine as a cause of compression of the spinal cord and its roots. J Nerv Ment Dis. 1911;38:588–609.
Stookey B. Compression of the spinal cord due to ventral extradural cervical chondromas diagnosis and surgical treatment. Arch Neur Psych. 1928;20(2):275–291.
Brain WR, Northfield D, Wilkinson M. The neurological manifestations of cervical spondylosis. Brain. 1952;75:187–225.
Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87–100.
Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. 2015;40(12):E675–693.
Smith SS, Stewart ME, Davies BM, Kotter MRN. The prevalence of asymptomatic and symptomatic spinal cord compression on magnetic resonance imaging: a systematic review and meta-analysis. Global Spine J. 2021;11(4):597–607.
Karadimas SK, Erwin WM, Ely CG, Dettori JR, Fehlings MG. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine. 2013;38(22 Suppl 1):S21–36.
Mahlknecht P, Kiechl S, Bloem BR, Willeit J, Scherfler C, Gasperi A, Rungger G, Poewe W, Seppi K. Prevalence and burden of gait disorders in elderly men and women aged 60–97 years: a population-based study. PLoS One. 2013;8(7):e69627.
Davies BM, Mowforth OD, Smith EK, Kotter MR. Degenerative cervical myelopathy. BMJ. 2018;360:k186.
Rhee JM, Heflin JA, Hamasaki T, Freedman B. Prevalence of physical signs in cervical myelopathy: a prospective, controlled study. Spine. 2009;34(9):890–895.
Annaswamy TM, Sakai T, Goetz LL, Pacheco FM, Ozarkar T. Reliability and repeatability of the Hoffmann sign. PMR. 2012;4(7):498–503.
Larner AJ. False localising signs. J Neurol Neurosurg Psychiatry. 2003;74(4):415–418.
Ochiai H, Yamakawa Y, Minato S, Nakahara K, Nakano S, Wakisaka S. Clinical features of the localized girdle sensation of mid-trunk (false localizing sign) appeared in cervical compressive myelopathy patients. J Neurol. 2002;249(5):549–553.
Chan CK, Lee HY, Choi WC, Cho JY, Lee SH. Cervical cord compression presenting with sciatica-like leg pain. Eur Spine J. 2011;20:S217–221.
Taweel WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85–99.
Rhee J, Tetreault LA, Chapman JR, Wilson JR, Smith JS, Martin AR, Dettori JR, Fehlings MG. Nonoperative versus operative management for the treatment degenerative cervical myelopathy: an updated systematic review. Global Spine J. 2017;7(3 Suppl):35S–41S.
Gupta SK, Ya’qoub L, Wimmer AP, Fisher S, Saeed IM. Safety and clinical impact of MRI in patients with non-MRI-conditional cardiac devices. Radiol Cardiothorac Imaging. 2020;2(5):e200086.
Uchida K, Nakajima H, Takeura N, Yayama T, Guerrero AR, Yoshida A, Sakamoto T, Honjoh K, Baba H. Prognostic value of changes in spinal cord signal intensity on magnetic resonance imaging in patients with cervical compressive myelopathy. Spine J. 2014;14(8):1601–1610.
Avadhani A, Rajasekaran S, Shetty AP. Comparison of prognostic value of different MRI classifications of signal intensity change in cervical spondylotic myelopathy. Spine J. 2010;10(6):475–485.
Rao A, Soliman H, Kaushal M, Motovylyak O, Vedantam A, Budde MD, Schmit B, Wang M, Kurpad SN. Diffusion tensor imaging in a large longitudinal series of patients with cervical spondylotic myelopathy correlated with long-term functional outcome. Neurosurg. 2018;83(4):753–760.
Maki S, Koda M, Kitamura M, Inada T, et al. Diffusion tensor imaging can predict surgical outcomes of patients with cervical compression myelopathy. Eur Spine J. 2017;26(9):2459–2466.
Shabani S, Kaushal M, Budde MD, Wang MC, Kurpad SN. Diffusion tensor imaging in cervical spondylotic myelopathy: a review. J Neurosurg Spine. 2020:1–8. doi: 10.3171/2019.12.SPINE191158.
Overley SC, Kim JS, Gogel BA, Merrill RK, Hecht AC. Tandem spinal stenosis: a systematic review. JBJS Rev. 2017;5(9):e2.
Kato S, Oshima Y, Oka H, et al. Comparison of the Japanese Orthopaedic Association (JOA) score and modified JOA (mJOA) score for the assessment of cervical myelopathy: a multicenter observational study. PLoS One. 2015;10(4):e0123022.
Kopjar B, Tetreault L, Kalsi-Ryan S, Fehlings MG. Psychometric properties of the modified Japanese Orthopaedic Association scale in patients with cervical spondylotic myelopathy. Spine. 2015;40(1):E23–28.
Fehlings MG, Badhiwala JH, Ahn H, et al. Safety and efficacy of riluzole in patients undergoing decompressive surgery for degenerative cervical myelopathy (CSM-Protect): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Neurol. 2021;20(2):98–106.
Kadanka Z, Bednarík J, Vohánka S, et al. Conservative treatment versus surgery in spondylotic cervical myelopathy: a prospective randomised study. Eur Spine J. 2000;9(6):538–544.
Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348–355.
Yoshimatsu H, Nagata K, Goto H, Sonoda K, Ando N, Imoto H, Mashima T, Takamiya Y. Conservative treatment for cervical spondylotic myelopathy. prediction of treatment effects by multivariate analysis. Spine J. 2001;1(4):269–273.
Murphy DR, Hurwitz EL, Gregory AA. Manipulation in the presence of cervical spinal cord compression: a case series. J Manipulative Physiol Ther. 2006;29(3):236–244.
McCormick JR, Sama AJ, Schiller NC, Butler AJ, Donnally CJ., 3rd Cervical spondylotic myelopathy: a guide to diagnosis and management. J Am Board Fam Med. 2020;33(2):303–313.
Malone DG, Baldwin NG, Tomecek FJ, Boxell CM, Gaede SE, Covington CG, Kugler KK. Complications of cervical spine manipulation therapy: 5-year retrospective study in a single-group practice. Neurosurg Focus. 2002;13(6):ecp1.
Kewalramani LS, Kewalramani DL, Krebs M, Saleem A. Myelopathy following cervical spine manipulation. Am J Phys Med. 1982;61(4):165–175.
Fehlings MG, Ibrahim A, Tetreault L, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine. 2015;40(17):1322–1328.
Sheikh Taha AM, Shue J, Lebl D, Girardi F. Considerations for prophylactic surgery in asymptomatic severe cervical stenosis: review article. HSS J. 2015;11(1):31–35.
Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 2017;7(3 Suppl):70S–83S.

Return to CASE STUDIES
Since 8-09-2023


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |