

|
Chapter 8
Clinical Disorders and the Sensory System
From R. C. Schafer, DC, PhD, FICC's best-selling book:
“Basic Principles of Chiropractic Neuroscience”
The following materials are provided as a service to our profession. There is no charge for individuals to copy and file these materials. However, they cannot be sold or used in any group or commercial venture without written permission from ACAPress.
All of Dr. Schafer's books are now available on CDs, with all proceeds being donated
to chiropractic research. Please review the complete list of available books.
The Analysis of Pain in the Clinical Setting Common Causes of Pain and Paresthesia Pain of Mechanical Origin Pain Induced by Change of Position Pain Induced by Chemical Irritation Pain of Psychic Origin Assessing the Origin of Pain Posterior Lemniscal vs Anterolateral Spinothalamic System The Qualities of Pain Pain Threshold Measuring Sensitivity Effects of Spinal Manipulation in Pain Control Myofascial Trigger Points Common Locations Predisposing Factors Precipitating Factors Etiologic Hypotheses Correlation with Acupoints Visceral Pain Splanchnic Pain Parietal Reflexes Visceral Pain Mimicking Musculoskeletal Disorders Musculoskeletal Disorders Mimicking Visceral Disease Paresthesia Common Types Meralgia Paresthetica Notalgia Paresthetica Diagnostic Considerations The Clinical Differentiation of Pain Evaluating the Sensory System Evaluating Cranial Nerve Sensory Fibers BibliographyChapter 8 Clinical Disorders and the Sensory System
This chapter describes those sensory mechanisms, joint signals, and abnormal sensations (eg, pain, thermal abnormalities) that have particular significance within clinical diagnosis. The basis and differentiation of pain are described, as are the related subjects of trigger points and paresthesia. The chapter concludes with a description of the neurologic basis for the evaluation of the sensory system and the sensory fibers of the cranial nerves.
THE ANALYSIS OF PAIN IN THE CLINICAL SETTINGAlthough all pain does not have organic causes, there is no such thing as "imagined" pain. Pain that can be purely isolated as a structural, functional, or an emotional effect is rare. More likely, all three are superimposed upon and interlaced with each other in various degrees of status. This is also true for neural, vascular, lymphatic, and hormonal mechanisms.
Common Causes of Pain and Paresthesia
The common causes of pain and paresthesia are:(1) obvious direct trauma or injury;
(2) reflex origins in musculoskeletal lesions, which deep pressure often exaggerates, such as trigger areas;
(3) peripheral nerve injury (eg, causalgia), which results in an intense burning superficial pain;
(4) the presence of nerve inflammations and degeneration of the peripheral or CNS, which frequently cause other changes indicative of such lesions;
(5) reflexes from visceral reflexes;
(6) vascular disease; and
(7) sensory root pressure.Sensations Transmitted by the Sensations Transmitted by the Posterior Lemniscal System Anterolateral Spinothalamic System Touch signals that require a Touch and pressure signals capable highdegree of intensity of only crude localization and graduations or stimulus discrimination. localization. Signals of movement against Pain and thermal (hot and cold) the skinand data concerning signals fine degrees of pressure Sexual sensations. intensity. Positional data and phasic Tickle and itch signals. signals (eg,vibration).The Qualities of Pain
The perception of pain may vary from mild discomfort to excruciating, intolerable misery. While the effects of pain can be measured in the office, it is clinically impossible to measure pain accurately by graduated degrees because it is subjective. We must generally rely on the communicative ability of the patient to assess the quantity and quality of pain being experienced.
Pain may be referred to in terms of:(1) time of occurrence as in posttherapy pain;
(2) duration or length of time experienced as in acute or chronic pain;
(3) intensity such as in mild pain or severe pain;
(4) location as in superficial, deep, or central pain;
(5) mode of transmission as in referred or projected pain;
(6) ease of transmission as in inhibited or facilitated pain;
(7) manner experienced as in sharp, burning, throbbing, or dull pain;
(8) general causative agent as in self-inflicted pain, spontaneous, organic;
(9) direct source as in gallbladder or sacroiliac pain; or
(10) autonomic relationship such as related to sympathetic or parasympathetic origin.From a purely clinical viewpoint, it seems that differences in the quality of pain are due to:
(1) different patterns of stimulation of nociceptors,
(2) different locations in the body, and/or
(3) simultaneous stimulation of other types of receptors along with pain receptors.The Intensity of Pain
In studying pain-sensitive tissues, Kellgren/Samuel found that the musculoskeletal structures most sensitive to noxious stimulation were periosteum and joint capsules. Subchondral bone, tendons, and ligaments are moderately pain sensitive, while muscle and cortical bone were slightly less sensitive. The least sensitive tissues were articular cartilage and fibrocartilage. Bone and joint pathology, although appearing well advanced on x-ray films, may be "silent" until the periosteum or joint capsule becomes involved. It is for this reason that IVDs which have become severely degenerated rarely cause discomfort until more sensitive adjacent tissues become involved. A complete muscle rupture is not as painful as a moderate strain/sprain in which the tendinous and ligamentous attachments to periosteum are involved.
There are three commonly held fallacies about pain:(1) persons who are critically ill or gravely injured always experience intense pain;
(2) the greater the pain, the greater the amount of tissue damage; and
(3) severe chronic pain is symptomatic of incurable illness.Many people who are critically ill (eg, suffering from terminal cancer) or gravely injured do not inevitably experience intense pain. Some do, others do not. The intensity of pain is not directly proportional to the severity or extent of tissue damage; it depends whether pain pathways are intact and activated. In addition, the personality of the neurotic patient may accentuate the pain and the Spartan-like personality may diminish its presence.
The general behavior of a patient in pain can provide a measure of its intensity and the patient's ability to cope with it. If the patient is able to concentrate on something else, or if he completely forgets the pain when asked something unrelated, then the pain is probably not severe. If the patient is able to continue normal activities (eg, working, sleeping), then the pain may be a nuisance but it would not be considered severe even if the patient may relate it in terms reflecting unbearable agony. Through actions, words, gestures, facial expressions, and voice tones and inflections, the patient slowly, and often indirectly, gives us a picture of how much suffering is occurring.
According to Janse, the acuity of interpreted pain depends greatly on the degree and clarity of consciousness possessed by a particular individual; ie, the greater the degree of mentality, the greater degree of estimating and interpretive capacity of the individual. The more keenly the mind is developed, the more pain sensitive it becomes. People in the sciences and skilled professions appear to show a greater degree of pain symptoms than those in the physical trades where less intellectual development is required.
Pain Threshold
The threshold of pain is defined as the smallest intensity of a stimulus at which the subject perceives pain. Some authorities define it as the lowest intensity of stimulus that will excite the sensation of pain even when the stimulus is applied continuously. Beecher described it as "the first barely perceptible pain to appear in an instructed subject (usually revealed by a verbal statement) under given conditions of noxious stimulation." A literature search will reveal widely diverse definitions of pain threshold.
The threshold of pain is approximately the same for everyone, but how each individual grades pain and responds to it can differ greatly from one person to another.
Most people will perceive pain when skin temperature reaches 45ºC. It is at this temperature that tissue damage begins to occur because of thermal injury. Almost all subjects perceive pain before the temperature reaches 47ºC. Thus, it is almost never true that some people are unusually sensitive or insensitive to pain. However, this is not to say that different people do not react differently to the same intensity of pain.
Conditioning impulses entering the sensory areas of the CNS from various parts of the cerebrospinal and peripheral nervous systems can determine whether incoming sensory impulses will be transmitted extensively or weakly to other areas of the brain. See Table 8.3.

It has been found that the perception of pain may be strongly modulated according to an individual's experiences and his direction of attention and intention. Since different people have experiences, expectations, and motivations that cannot be equal, their perceptions are not likely to be the same except in areas where general experiences overlap. Even then, two people will rarely report a simultaneous experience in the same terms or with the same emphasis. Therefore, the same event, as determined by sensory data reaching consciousness, may be experienced as distinctly different events in different people. Some other factors that determine pain responsiveness are an individual's mood, personality, race, sex, age, and health status.
Distribution and Timing
Besides intensity and threshold, two other qualities of pain are its distribution and periodicity. It appears that distribution is governed primarily by the myotome, dermatome, or sclerotome area in which the pain may be perceived. Concerning the periodicity of pain, various physiologic or psychologic factors may influence whether the pain is worse or better at certain times of the day or night, or following certain activities.
Measuring Sensitivity
A patient's gross sensitivity to a painful stimulus should be measured clinically to(1) determine the integrity of pain pathways,
(2) evaluate the level of patient sensibility, and/or
(3) determine the patient's pain threshold.
Subjective Methods
The pain threshold and tolerance of a patient are frequently determined in general practice by(1) pricking the skin with a pin or
(2) pressing an area of tender tissue.The patient is asked to say "now" when a painful sensation is first felt and say "stop" when the sensation becomes unbearable. This gives the subjective points of
(1) threshold and
(2) end-point.The span between these points is a measure of tolerance. See Table 8.4.

Pain tolerance can be defined as the greatest intensity of painful stimulation that a subject is able to tolerate. It is common practice, however, to refer to pain tolerance as the quantity of painful stimuli that a subject can bear voluntarily between the low and upper pain thresholds. As with pain threshold, an exact definition of pain end-point and tolerance is controversial. There does not appear to be a definite correlation between the tolerance of pain (reaction interval) and its threshold. During clinical studies, three arbitrary phases are often recorded:
When the intensity of a noxious stimulus is slowly increased, there comes a point where pain is experienced by the subject and reported (initial verbal complaint). This can be considered the individual's low pain threshold.
If the intensity continues to increase, it reaches the degree where it "hurts a lot." This can be considered the individual's severe pain threshold.
If the intensity is still increased, it reaches a point characterized by a rapid pulse increase and automatic withdrawal. This can be considered the individual's upper pain threshold.
Objective Methods
Severe or prolonged pain produces several noticeable signs of which the examiner should be aware. With severe pain, there is an increase in pulse rate, respiratory rate, perspiration, and vasomotor tone. There is also an initial rise in blood pressure and possibly nausea, vomiting, and pallor.
Common Patterns of Pain
Determination of the lesion site is often aided by noting the distribution of pain along the course of the involved nerve and the areas of cutaneous sensory disturbance, if any are expressed. The pain may be localized at the site of involvement, below the site of involvement, or be widespread because of referred or projected somatic impulses. It also may be a manifestation of psychic conversion.
Dermatomes, Myotomes, and Sclerotomes
The differentiation of somatic pain reference often rests upon the examiner's knowledge and consideration of dermatomes, myotomes, sclerotomes, and their courses. Grieve points out that the regional distribution and innervation of musculoskeletal and associated tissues evolve from embryonic ectoderm, endoderm, and mesoderm, and these primitive germ layers retain the parenthood of their source connections with mesodermal somites and the ectodermal segments of the spinal cord and the ectodermal nuclei of the cranial nerves. In general, dermatomes overlie myotomes and myotomes overlie sclerotomes. However, areas of the buttock, face, heart, diaphragm, thenar eminence, and upper thorax have a cutaneous innervation that differs from that of the deeper tissues.
Dermatomes. Dermatomes are areas of sensation on the skin (skin segments) that are primarily supplied by the dorsal root of a single spinal segment. Dermatomal pain follows the anatomical course of the involved nerve fibers, and pain limited in distribution to one or more dermatomes is called radicular pain. It is typically sharp, sometimes shooting, and well localized. Specific determination of the segmental level of involvement is usually impossible because, in most cases, each dermatome overlaps about 50% or more of adjacent skin segments. For example, the superior aspect of the T5 dermatome is superimposed on the inferior aspect of the T4 dermatome and the inferior aspect of the T5 dermatome is superimposed on the superior aspect of the T6 dermatome.
Myotomes. Myotomes are muscles or groups of small muscles innervated by a single spinal segment. Some authors refer to them as a muscle mass whose total population of motor units is supplied by one nerve root. Because most muscles are supplied by more than one nerve root, most muscles help to form more than one myotome.
Sclerotomes. Sclerotomes are areas of bone, cartilage, and other deep tissues innervated from a single spinal segment. A sclerotome, whose parent is a single mesodermal somite, forms the embryonic tissue that ultimately develops into the skeleton. In contrast to dermatomal pain, sclerotomal pain is typically deep, aching, and poorly localized. An example of its significance is seen with the patient whose pain does not correspond to the site of a known lesion. The pain will be described as traveling down one side of a limb and then abruptly spiral down the other side in a manner that does not correspond to any nerve or muscle. The patient may be describing a sclerotome pattern arising from C6 or L4, for example.
Referred Pain
Pain is said to be referred when it originates at one site in the body and is perceived in another such as when it initiates from a deep visceral lesion and is felt to be located in the surface of the body.
Common syndromes that are known to refer pain to the spine are listed in Table 8.5.

Distress sensed at the point of injury is called homotopic pain. In contrast, heterotopic pain and tenderness are distressing sensations that are referred; ie, perceived to arise from an area other than its origin.
Radiating pain may or may not imply intrinsic nerve involvement; ie, it may arise from any structure containing nociceptors. When pain is referred, it is usually expressed in a structure that developed from the same dermatome (ie, the same embryonic segment) as the irritated structure. Shepard points out that pain caused by an irritation of the heart is commonly referred to the chest, axilla, and/or inside the left arm; the diaphragm to the neck and shoulder; the stomach to between the scapulae; the appendix to the umbilical area; and the ureter to the groin or testes.
One mechanism widely held responsible for referred pain is convergence. This theory assumes that the localization of pain depends on what area or areas of the brain are stimulated. For example, heart pain referred to the arm is explained by the fact that pain fibers from the heart and pain fibers from the arm have an identical final common pathway.
Projected Pain
While most authors treat referred pain and projected pain as synonyms, Lynch/Kessler define projected pain as that which is composed of both dermatomal and sclerotomal expression. They believe it shows a direct irritation of nerve pathways projecting afferent input from a particular site (eg, a nerve root). Thus, an IVD lesion or IVF osteophyte may excite fibers in a nerve root servicing sensory or motor functions and thus produce symptoms and signs confined to the relevant dermatome, myotome, and sclerotome. Peripheral entrapment syndromes, however, primarily exhibit as paresthesias long before pain manifests.
The classic courses of referred/projected pain are shown in Table 8.6.
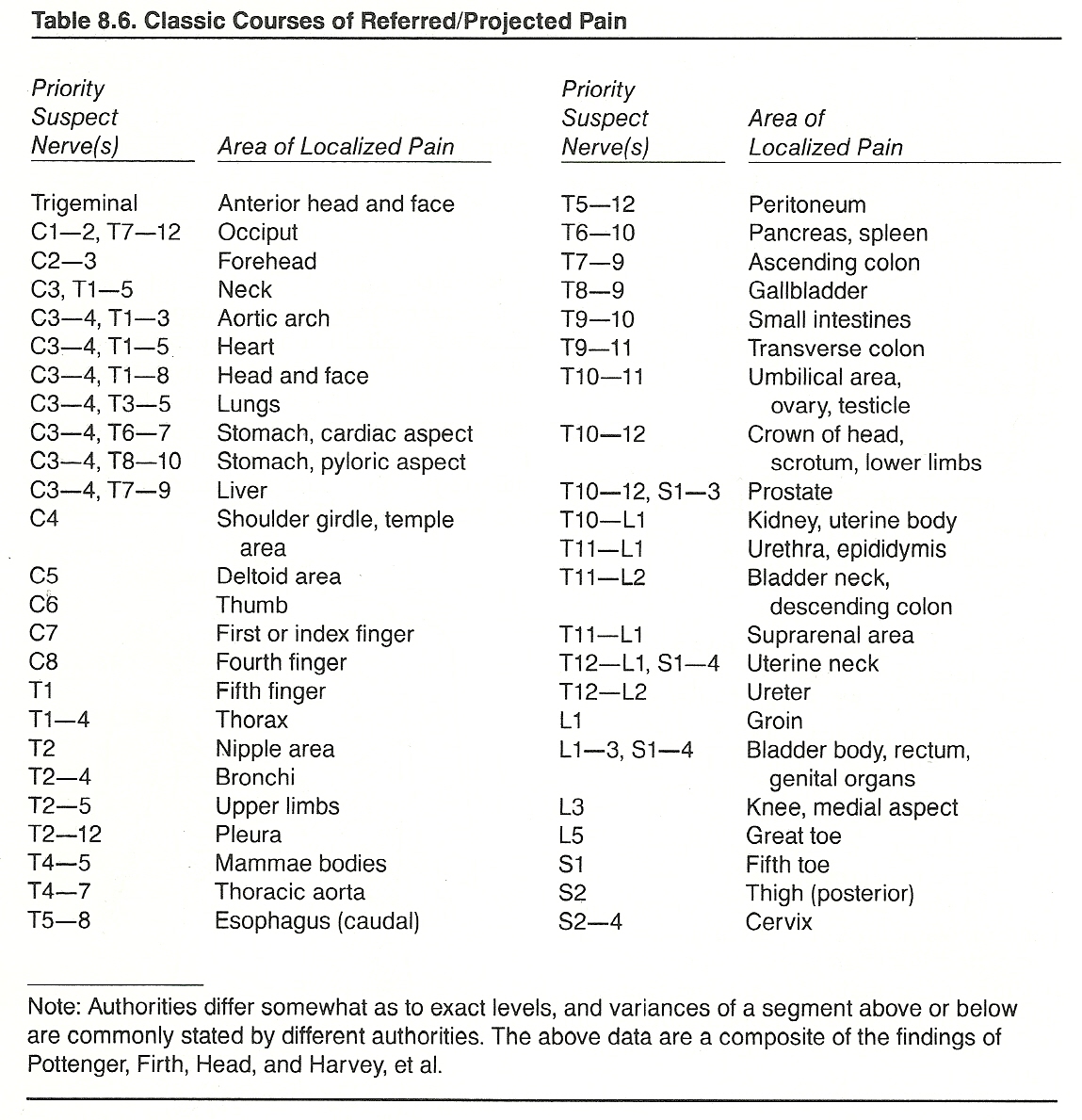
Prolonged Pain
When the normal pattern of adequate activity and rest becomes unbalanced in either direction, malfunction and deterioration are likely to result. Inadequate physical activity can lead to a large number of systemic changes that serve as precursors to chronic pain of somatic origin.
The common points of differentiation of pain by duration and intensity are shown in Table 8.7.
Table 8.7. Differentiation of Pain by Duration and Intensity
Type of Pain Suggested Etiology
Continuous, dull, Expanding inflammation,
spontaneous muscle strain
Continuous, sharp, Neuritis
with paresthesia
Exacerbative or Vasospasm (sympathicotonic)
paroxysmal
Intermittent, sharp, Neuritis
with paresthesia
Intractable pain Metastatic invasion,
obstructed duct
Remittent pain Colic, neuralgia
Pain responses initiated through reduced physical activity may become strengthened in time by learning (operant conditioning) processes, according to Fordyce. This process, the learned pain syndrome, is similar to any behavior that is modified by positive and negative reinforcements or mimicry (eg, drug tranquillity, financial gain, increased attention, reduced responsibilities, relief, sympathy). It is characterized by dependency, disability exaggeration, disuse atrophy, dramatization of complaints, and drug overuse (the "Five Ds").
Persistent pain unaffected by activity, rest, or position is likely to have a visceral, obstruction, or neoplastic origin. There are some exceptions to this, of course, such as most headaches, toothaches, earaches, etc. A psychologic origin may be a consideration, but psychogenic pain is unrelieved by analgesic-producing therapies unless the patient is highly receptive to suggestion.
Recurrent Pain
Acute pain or a dull ache recurring at nonspecific intervals is a common characteristic of chronic visceral disease. It is expressed in those dermatomes supplied by neurons whose cell bodies lie adjacent in the cord to the cell bodies of the afferent neurons from a viscus that is or has been the site of distress. This pain may occur when there is no other evidence of pathology, and it is often precipitated at a time of psychic stress or hormonal change (eg, grief, worry, fatigue, menstruation, weather changes, etc).
The cause of recurring pain, states Pottenger, is often a hypersensitive viscerosomatic reflex (ie, a functional cord conditioning lowering the threshold response) established by a previous visceral pathology and initiated by a stimulus that would be insufficient to produce overt malfunction under normal conditions. If this hypothesis is true, then a somatosomatic reflex from an old musculoskeletal injury could produce a similar effect.
Nocturnal Pain
Nocturnal pain that is relieved during the day may have a positional explanation or be the effect of gout or a bone disease. Anxiety states that are worse at night often increase physical pain of other origins during the evening hours. Febrile diseases usually have their crises during the early morning hours.
Effects of Spinal Manipulation in Pain Control
The positive benefits of chiropractic adjustments for the relief of back pain have been written of extensively in chiropractic and osteopathic literature for over 90 years. However, except for German journals, it has only been recently that such reports have begun to appear in allopathic literature. Livingston, Paris, Burns, and Tobis/Hoebler, for example, found that a large percentage of injuries or disorders of the spine can be helped by manipulative treatment. Two other examples are described below:
A study by Terrett/Vernon assessed the response of paraspinal cutaneous pain tolerance measurements to spinal manipulation. They found that a statistically significant elevation of pain tolerance (140%) occurred after manipulation as compared to the control group.
A syndrome of back pain is described by Glover that consists of skin hyperesthesia associated with tender areas, a dull ache, and limitation of spinal movement by pain. The tender areas followed the distribution of sclerotomes rather than dermatomes. After one successful manipulation, the syndrome usually disappeared within a few minutes. The hyperesthesia was thought to be the skin component of pain arising in deep mesodermal structures such as nipping of the synovial membrane between the apophyseal facets or the sacroiliac joint, or to tension of a joint ligament. In either case, manipulation restored the normal function of the joints and relieved the patient's pain.
Trigger points are functional transient entities; ie, like a pea-shaped spasm, there is no overt histologic change. During palpatory examination, a trigger point (myodysneuria) is noted as a small, deep, hypersensitive area within a myofascial structure. From this focus, high-intensity impulses bombard the CNS and give rise to a deep-aching, sharply demarcated area of referred pain as contrasted with the ischemic compression nerve pain of prickling, tingling, and numbing that follows the segmental distribution of an entrapped peripheral nerve.
One or more trigger points may be localized in one muscle or group, or it also may involve remote muscles or groups. Primary trigger points in the gluteus medius, for example, are commonly related to secondary trigger points in the neck and shoulder girdle. Thus, while trigger points in the neck and upper thoracic muscles may be found to be responsible for or contributing to tension headaches, a group of "mother" trigger points should be sought on the dorsal aspect of the ilial crests.
(1) well-defined pathways (eg, motor reflexes, sensory changes),
The autonomic concomitants are similar to those seen with Meridian acupoints. Travell believes that these are frequently expressed as decreased skin resistance, increased pilomotor reaction in the reference area, vasodilation (possibly with dermatographia), and skin temperature changes (coolness). In the typical myofascial syndrome, laboratory analyses and roentgenography fail to show significant bone, joint, or metabolic changes.
Pain originating from viscera features a diffuse dull ache that is entirely unrelated to posture. There are, generally, no articular signs of muscle guarding (splinting), blocked motion, or sharp pain during movements of the part, unless somatic soft tissues are also involved (eg, peritonitis). The major causes of true visceral pain are chemical irritation, distention, ischemia, and/or visceral spasm.
Musculoskeletal Disorders Mimicking Visceral Disease
The term paresthesia refers to a perverted sensation or uncomfortable sensation not amounting to pain but it may be concomitant with pain. The most common paresthesias are sensations of numbness, tingling, formication or itching, weight or bearing down, coldness, faintness, fullness, girdle sensation, precordial constriction, and weakness or debility.
(1) at the L1–L3 IVFs from subluxation encroachment or arthritic hyperplasia or
The typical patient inflicted reports a sense of numbness or burning in the anterolateral aspect of the thigh, which is rarely painful until the late stages. Care must be taken to differentiate this syndrome from trochanteric or iliopectineal (iliopsoas) bursitis because both refer pain to the anterolateral thigh. It also should be kept in mind that pronation or supination of the ankle, genu varum, genu valgum, genu recurvatum, flexion contracture, etc, can cause or contribute to anterosuperior thigh pain.
Testing sensory perception helps to determine the condition of the peripheral receptors, their afferent fibers, the sensory columns of the spinal cord, and the higher sensory areas of the nervous system. If not conducted on a regional basis, some examiners leave this portion of the neurologic examination to the last because it is the most tedious and challenging part of the investigation.
(1) qualitative, to determine the elements of sensation that are affected;
(1) adjustments to the spine can significantly affect temperature (probably through changes in blood flow) in tissues distant from the spine;
Interpretation of current localized skin temperature methodology remains controversial. The American Chiropractic Association has not adopted a specific policy on this procedure.
Localized Tenderness. Tenderness is a frequently encountered sensory symptom that can be defined as pain upon pressure. Rebound pain or rebound tenderness refers to the sensation or intensification of discomfort when pressure is released; eg, rebound pain at McBurney's point is a classic symptom of acute appendicitis. It should be remembered whenever muscle tenderness is found that the sensory innervation of muscle follows the motor innervation and not that of the cutaneous zones.
Method of holding the palpating finger.
It is necessary to perform deep palpation by considerable pressure to elicit tenderness along the nerve pathway and thus determine its course. To avoid finger fatigue during continuous deep pressure, the index finger is placed on the top surface of the middle finger for support and the thumb is placed against the under surface of the middle finger to support the bottom of the palpating middle finger.
Method of following a nerve.
Once a sensitive point along a nerve pathway is found by digital exploration, palpation is continued along the anticipated course of the nerve. If tenderness is lost, pressure is applied above or below the expected course by palpating in a half-moon direction. The unactive hand usually holds a skin pencil that can be used to mark the course of tenderness.
Unpalpable nerve pathways.
If a tender nerve passes under a bone or thick muscle where tenderness cannot be elicited, the examiner must try to anticipate its route and pick it up past the obstruction where the nerve will again be elicited by tenderness. This is common when a nerve passes under the scapula or clavicle and the pathway must be reaffirmed.
Method of tracing nerves from the spine.
Standard paraspinal palpation is performed, but then the palpation continues along the nerve pathway step-by-step until the pathologic zone is reached. Sometimes nerve pressure upon a tender nerve tract may relieve the tenderness or numb it to the degree that it cannot be traced further. For that reason, it may be difficult to retrace a nerve from its spinal origin to the periphery.
Method of tracing nerves to the spine.
First, the tender point along the nerve pathway near the inflammatory area should be elicited, but deep palpation is avoided over the actual tender zone of the affected area. Palpation should be made a short distance toward the spinal origin of the nerve supply. If pressure on the nerve numbs its sensitivity, only the distal portion of the nerve will be affected. Deep pressure along the nerve pathway will frequently relieve pain or at least afford temporary relief. This is often a therapeutic aid but a diagnostic obstacle.
Dual routes. It is often found that the nerve will branch and the tender pathway can be traced to two points of the spinal column. This is most likely because multiple organs are involved or because a nerve supply from different segments of the spine is given off to the same pathologic zone that has become involved.
Locating tender points along the nerve pathway. It is important to realize that as the nerve is traced from its spinal origin to the region of some pathologic zone, or vice versa, certain points along the nerve pathway may prove more tender than others. These points, likely trigger points or acupoints, should be marked. Pressure along the nerve may excite a sharp and decisive pain in the pathologic area. This site also should be marked and noted.
Tracing of the peripheral nerve rami.
Nerves and their branches that innervate internal viscera cannot be directly traced because they are too deep to be palpated. How then in such a condition can the examiner elicit information that will help to determine correctly the location of the etiologic spinal lesion? The explanation is that if a deep nerve is sensitive or tender, usually its branches are equally sensitive or tender. If we cannot trace a nerve to the end site of a trunk or cavity of the organ, it may be possible to trace a peripheral branch of the same nerve back to its spinal origin. Needless to say, an excellent working knowledge of neurology is essential for this particular work. If the peripheral nerve branches can be traced to the proper point or locality of the spinal lesion, then an excellent tool of analysis is at hand.
Visual Acuity and Color Perception. The ability to recognize objects and to read the standard Snellen eye charts can be tested. Jaeger or similar test cards can be used at the bedside. If the examiner is not concerned with errors of refraction, the patient can be tested with the glasses on. Color perception also should be determined, and this can be tested by colored yarn or test cards.
Some of the gross signs are as follows: Bitemporal hemianopsia indicates a lesion at the optic chiasma.
Homonymous contralateral hemianopsia points toward a lesion at the optic tract or optic radiation.
Homonymous contralateral quadrantopsia indicates a lesion in the upper or lower area of optic radiation.
Complete blindness of one eye indicates a lesion of the optic nerve. Isolated blind spots (scotomata) surrounded by areas of normal vision may be the result of optic neuritis, optic atrophy, cataracts, detached retina, or lesions of the optic chiasm.
The Fundi. The fundi are examined with an ophthalmoscope, preferably through clinically dilated pupils if legally allowed in the state of practice. Papilledema appears with increased intracranial pressure. Doubtful signs should be confirmed by a specialist. Papilledema first appears as a loss of sharpness of the disc margins, which progresses until evidence of the margin disappears. The normal optic disc is glistening white with sharply defined edges. There is a normal minimal indistinctiveness of the disc margins along the nasal aspect of the disc. Optic atrophy appears as a combination of pallor of the lateral aspect and a lack of normal vascularity in the lateral area. The retinal vessels should be inspected for signs of arteriosclerosis, diabetes, retina detachment, pigmentation, hemorrhage, and exudation.
After ruling out local ocular causes of disturbed vision, patterns of visual loss offer one of the best means of locating intracranial lesions when such lesions affect the visual pathway. A sharp disc outline and pulsations of the disc veins are of particular neurologic significance because these findings indicate normal intracranial pressure.
The Corneal Reflex. The cornea and conjunctiva are tested for light touch perception with a thin wisp of cotton or a fine hair. The patient should be looking upward, and the cornea should be approached from the side. Each eye should be tested separately, and both eyes should blink quickly with the stimulation. The corneal reflex involves three corneal nerves: III (to open eyelid), V (for sensitivity), and VII (to close eyelid). This reflex is frequently diminished unilaterally from either a central or peripheral lesion. Positive signs will be seen in tic douloureux, herpes zoster, alcoholism, neuritis, and cerebellopontine angle tumors. Irritating lesions result from encephalitis, tabes, and tetanus, and clonic spasm is occasionally seen in petit mal attacks.
Rinne's Test. To test for deafness, a tuning fork is vibrated and placed next to each ear opening (for air conduction) and then against the mastoid bone (for bone conduction) to see which tone is heard longer. Normally, the tone will be heard longer through the otic canal via air conduction.
Caloric test. Hot and cold waters are alternately douched into the patient's ear, after it has been determined that the eardrum is not perforated. The patient should be seated with the head tilted backward about 60º to bring the horizontal semicircular canal into a vertical plane. Normally, hot water (not exceeding 120ºF) produces rotary nystagmus, first away and then toward the irrigated ear. Cold water produces an opposite effect nystagmus first toward and then away from the ear irrigated. The final response normally will appear within 30–40 seconds. Lack of response shows decreased or absent vestibular nerve function; ie, no nystagmus results if the labyrinth is diseased. The test should be discontinued if there is no reaction within 3 minutes.
In describing manipulative treatment based upon a study of postural reflexes, Stejskal states that labyrinthine postural reflexes have relatively little clinical importance. Rather, he emphasizes the importance of the stretch reflex of the axial musculature, of the visual afferentation that is closely connected with the deep neck reflexes, and of the influence of the respiratory phase.
American Hospital Association: Stress.
MYOFASCIAL TRIGGER POINTS
A little-recognized cause of spasm and myofascial pain is often an irritable trigger point in or near a muscle. When a trigger point becomes irritated, the pain may manifest at the site or be referred to a remote area. Many cases of torticollis, shoulder pain, tennis elbow, substernal aches, lumbago, sciatica, hip pain, knee pain, and ankle pain can be traced to trigger-point mechanisms. Such conditions are frequently misdiagnosed as myalgia, myofascitis, nonarticular rheumatism, and sometimes as muscle strain or joint sprain. This is especially true when symptoms persist long after precipitating events.
Sola reports that myofascial pain may be the most common pain problem faced by most physicians. It may present as a primary complaint or as a crippling adjunct to any number of other problems (eg, unequal leg lengths, disuse, immobilization, chronic strains, poor posture, gait disturbances, connective-tissue diseases, arthritides).
Kraus states that visceral or somatic trigger-point irritation can produce a degree of spasm of the paravertebral muscles ipsilaterally in 2 3 segments on the same side as the entering afferent. If the irritation is severe, this effect can spread up, down, and contralateral (eg, as in renal colic). In this regard, Stoddard reminds us that the sharp "textbook" demarcation made between the somatic and autonomic nervous systems is erroneous.
Common Locations
Although one or more trigger points may occur in any muscle, they usually form in clusters. Certain muscles and muscle groups (eg, the antigravity muscles) appear to be more liable than others.
The major trigger points producing posterior thoracic pain (low, middle, upper areas) are related to the most common muscles involved in Table 8.8.
Table 8.8. Major Trigger Points Causing Posterior Thoracic Pain
Area of Primary Suspect
Perceived Pain Muscles
Low thoracic area Iliocostalis thoracis Rectus abdominis
Longissimus thoracis Serratus posterior
Multifidi inferior
Middle thoracic Iliocostalis thoracis Scaleni
area Infraspinatus Serratus posterior
Latissimus dorsi superior
Levator scapulae Serratus anterior
Multifidi Trapezius
Rhomboids
Upper thoracic Levator scapulae Scaleni
area Multifidi Supraspinatus
Rhomboids Trapezius
Predisposing Conditions
Trigger point syndromes often appear related to a lack of appropriate (but not excessive) exercise; thus, they are less common (but not absent) in athletes and laborers than they are in sedentary workers.
The major conditions that appear to predispose myofascial trigger mechanisms near the spine are chronic strain as the result of prolonged repetitive movement, subluxation, fatigue, tension, infection, neural lesions, nutritional deficiencies, syndromes of the menopause or male climacteric, and hypometabolic states exhibiting creatinuria.
Precipitating Factors
Clinicians for many years have found highly localized, exquisitely sensitive areas within or near a painful region. When pressure is placed on the sensitive spot (trigger point), local pain, referred pain, or both may be initiated. Besides deep pressure, an application of heat, cold, electrical stimulation, needling, or some other stimulus may evoke a painful trigger-point reaction. The force of such a reaction appears to be moderated by several factors (eg, conditioning, genetic predisposition, hormonal balance, scar tissue from previous injury, and prolonged emotional stress).
It is generally considered that trigger points are "weak" points within myofascial tissue which are particularly sensitive to stress-induced change. That is, they may remain quiescent until a certain stress (mechanical or metabolic) triggers a syndrome that involves some positive feedback cycles such as sensory and motor reflexes, sympathicotonia, vascular responses, and, possibly, extracellular fluid changes. These can eventually lead to hypertonia, fatigue, and endogenous pain or the intensification of traumatic pain.
According to Travell, the most common precipitating factor is thought to be motion that produces stretching of the tissue containing the focal spot. This ignites a self-perpetuating cycle of pain spasm pain that may persist even after the precipitating cause has been removed (eg, subluxation). Other precipitating factors include acute trauma, an inflammatory process, unusual or excessive exercise, chills, IVD herniation producing nerve root pressure, immobilization, and severe anxiety.
The cycles of physiologic responses arising from trigger points involve
(2) anticipated autonomic feedback reflexes, and
(3) hypothesized microscopic tissue changes. Motor and sensory reactions usually manifest as local and general muscle fatigue, hypertonia, weakness, possibly a fine tremor, hyperirritability, pain, and hypoesthesia.
Etiologic Hypotheses
Kraus states that the frequent result of chronic muscle tension and spasm is trigger-point development. The effect can be referred pain on pressure over the trigger area, hyperalgesia, joint stiffness, movement limitation, weakness, and autonomic dysfunction occurring in the area of reference (target area). Once one muscle has been affected by trigger points, its whole system of related antagonists and synergists is affected. This muscular dysfunction leads to episodes of tension, spasm, radiating pain, and more trigger points unless proper therapy is applied.
The exact etiology of trigger-point development is unknown. Several hypotheses are described below, and it should be noted that many facets of each overlap to some degree.
The Neurophysiologic Theories
Although the exact physiologic mechanisms of trigger point pain are unknown, Sola offers a neurophysiologic explanation. He feels that, because of physiologic defense mechanisms such as splinting and bracing of muscles, vasomotor changes, increased sympathetic discharge, and hormonal and other humoral changes in plasma and extracellular fluids, the spastic muscle or its fascia (which is probably more sensitive than surrounding tissue due to previous injury or a genetic weakness) fatigues and signals its distress to the CNS. Several responses may result. For example, various muscles associated with the trigger point may become more tense and begin to fatigue because of motor reflexes or because sympathetic responses may lead to vasomotor changes within and around the trigger point.
Zimmermann reports that local ischemia following vasoconstriction or increased vascular permeability following vasodilation may lead to changes in the extracellular environment of the cells involved, release of algesic agents (eg, bradykinins, prostaglandins), osmotic changes, and pH changes -- all which may increase the sensitivity or activity of nociceptors in the area. The sympathetic hyperactivity also may cause smooth muscle contraction in the vicinity of nociceptors, thus increasing their activity. This increased nociceptor input may then contribute to the cycle by increasing motor and sympathetic activity, which, in turn, leads to increased pain. This pain may be shadowed by growing fatigue that adds an overall mood of distress to the patient's status and feeds back to the cycle.
As tense muscles in the affected area begin to fatigue in an environment of local sympathicotonia and biochemical change, Sola believes that latent trigger points within the involved muscles also may begin to fire --thus adding to the positive feedback cycle and spreading the pain to these muscles or muscle groups. Finally, the stress of pain and fatigue, coupled with both increased muscle tension and sympathicotonia throughout the body (conceivably with ipsilateral emphasis through the sympathetic chain), may lead to focal exacerbations or trigger points in other muscles that are far remote from the initial area of pain.
Common sites and reference zones are shown in Table 8.9.
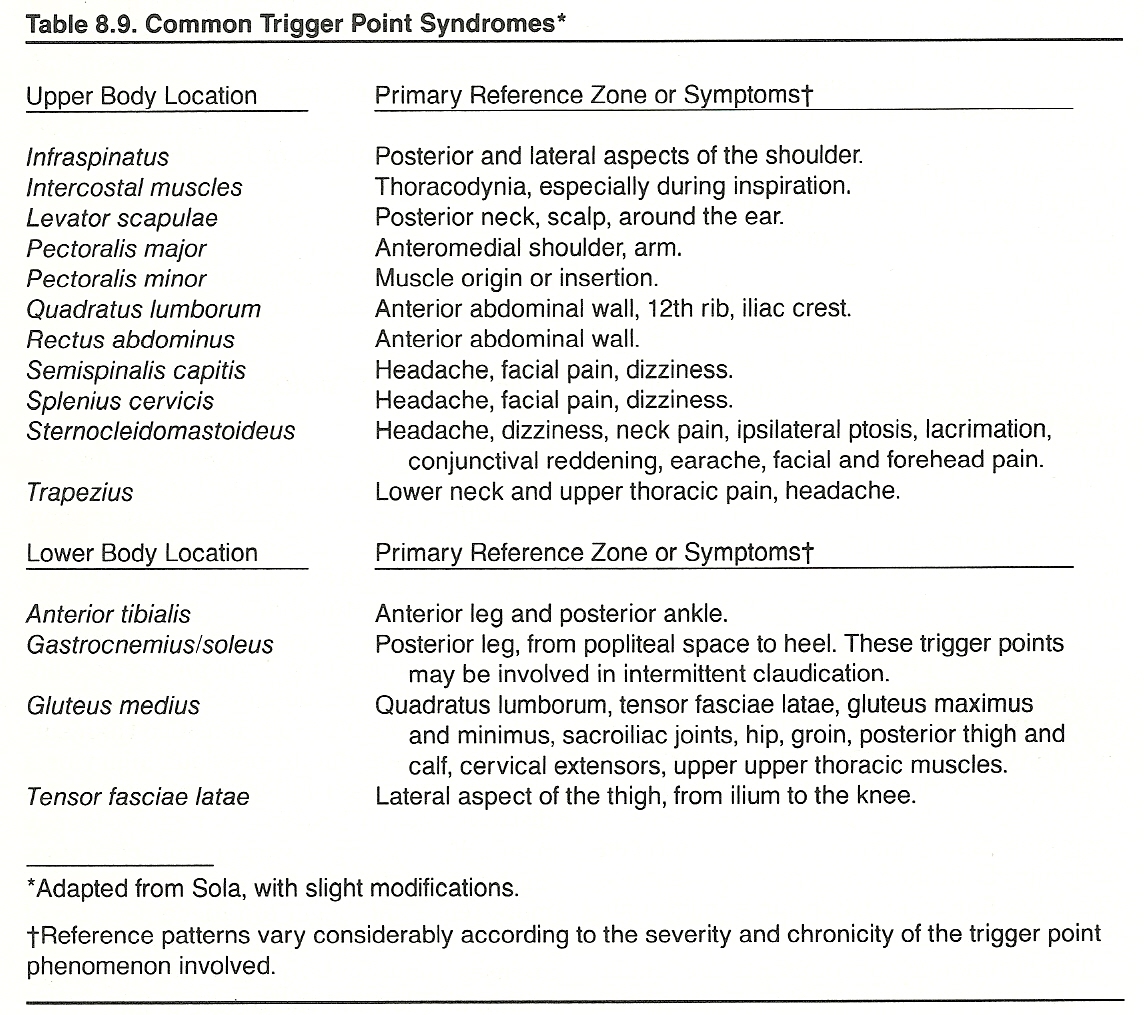
Sandman states that the focus of pain appears to be from exercise of an ischemic muscle and/or chemoreceptor and mechanoreceptor stimulation from pressure by accumulated metabolic debris or irritation by released acetylcholine, blood serum, bradykinin, histamine, inflammatory exudates, substance P, and 5-hydroxytryptamine. He feels that the amount of degeneration or pathologic alteration created may relate directly to the duration these conditions are allowed to exist within a muscle.
The Neurochemical Theory
Simons offers a neurochemical explanation of trigger point development that also deserves consideration. He feels that a traumatically induced tear in the sarcoplasmic reticulum initially causes the release of calcium that acts in conjunction with adenosine triphosphate (ATP) to stimulate continuously local contractile activity. This uncontrolled contraction shortens and tenses fibers within the involved muscle bundle(s). Such increased physiologic activity can initiate a subsequent increase in sustained, uncontrolled, localized metabolic activity by the muscles that is capable of producing substances that cause a hypersensitivity of involved sensory nerve fibers and, possibly, stimulate localized reflex vasoconstriction to help control what otherwise might be a rapidly increasing metabolic activity. The result is local tenderness, referred pain, and decreased blood flow within the involved muscle area.
Once the local energy and nutrient supply become restricted in this manner, ATP stores become depleted. When this occurs, the local physiologic contracture of muscle fibers is converted to an energy-deficient contraction. Thus, the sarcoplasmic reticulum of the muscle must be repaired. If sufficient energy is not available, the calcium pump (which is the most energy-sensitive step in the contractile mechanism) will respond with continued muscle contraction, creating an even greater energy depletion.
It has been found clinically that normal function may be restored by stretching the locked actin and myosin filaments far enough apart to eliminate contraction (eg, deep friction massage). Following stretching, enough ATP will then accumulate to restore a normal sarcoplasmic reticulum, according to Simons, which would allow the inhibited circulation to remove the accumulation of metabolic by-products slowly.
Correlation with Acupoints
Trigger points associated with myofascial and visceral pains often lie within the areas of referred pain, but many are found far from them. These properties of trigger points, their widespread distribution, and the pain relief produced by stimulating them resemble those of acupuncture. A study by Melzack (coauthor of the "gate theory") and associates determined a correlation between trigger points and acupuncture points for pain on the basis of two criteria: spatial distribution and associated pain pattern. A high degree (71%) of correspondence was found. This correlation suggests that trigger points and acupuncture points for pain, though discovered independently and labeled differently, represent the same phenomenon.
VISCERAL PAIN
Viscerally produced pain is often difficult to distinguish from deep somatic pain except for colic. A distended viscus can produce intermittent regular pain, sometimes of an extreme degree, which is characteristic and quite unlike the intermittent pain produced by somatic structures. Nociceptive impulses from viscera travel mainly through C-fiber (unmyelinated) afferents.
Splanchnic Pain
Although research has not been directed to visceral nociceptors to the extent it has to somatic nociceptors, specific visceral nociceptors have been shown to exist by Cervero and others. In general, visceral organs have sensory receptors primarily for pain, especially terminals sensitive to tension and pressure forces such as seen in distention or prolonged isometric muscle contraction of hollow organs. For example, a ruptured, ulcerated, burned, or severed viscus is not painful in itself, but one distended by gas or a space-occupying mass is (eg, tumor, aneurysm, swollen gland or capsule).
Receptors for light touch, compression, heat, and cold are either not found within visceral walls or are very sparsely distributed. For this reason, highly localized types of damage to an internal organ rarely give rise to pain. However, any stimulus that causes diffuse stimulation of nociceptors throughout a viscus can cause pain that may be extremely severe. An occlusion of the blood or lymph supply also can do this. Thus, any stimulus that excites pain-sensitive receptors in diffuse areas of a viscus can elicit visceral pain.
Parietal Reflexes
Visceral pain also may have an underlying parietal cause or contribution. Some pain sensations are transmitted from the viscera to nerve fibers that innervate the parietal peritoneum, pleura, and pericardium. These are primarily spinal cord arcs (viscerosomatic reflexes). The parietal surfaces of visceral cavities are supplied mainly by sensory fibers that penetrate centrally from more superficial branches. Thus, parietal pain and tenderness are due to this extension innervation. It is frequently sharp and pricking in quality but may be perceived as a burning sensation or a dull ache.
Visceral Pain Mimicking Musculoskeletal Disorders
Most visceral sensations travel the same pathway as somatic sensations in the spinothalamic tracts and the thalamic radiation. The major exceptions to this are the few (but potent) sensory fibers that travel to the brain along with the vagus nerve.
Pathologic conditions in many organs of the body have been noted to exhibit painful symptoms that are directly related to specific cutaneous zones. These skin areas have been noted to be so characteristic that they have become useful to the physician during diagnosis. Although pain has been known to be referred from one organ to another (viscerovisceral reflex), pain is most often referred from a visceral organ and interpreted as pain in the skin (viscerosomatic). In fact, many splanchnic ailments exhibit no other sign except that of pain referred to the periphery.
It is hypothesized that when visceral pain fibers are intensely irritated, especially by overdistension or an impaired blood supply, the intense stimulation produces such a bombardment of impulses to the cord that some spill over to affect synapses that connect with cutaneous afferents from the skin and subcutaneous tissues. This can produce referred hyperesthesia and deep tenderness. The patient then has the feeling that the sensations are originating in peripheral tissues. In this context, it should be noted that a paravertebral inflammatory reaction need not be from infection but of irritation from malfunction in a part of the gastrointestinal tract that reflexly produces vasospasm in the para-articular tissues and hence pain.
Many back pains and certain types of headaches appear to be caused by muscular spasm, with the spasm originating reflexly from much weaker pain impulses originating elsewhere in the body. Other examples are the severe cluster-like temporal headaches associated with gastric ulcers or the migraine-type headaches associated with many gynecologic syndromes. Another example is the pain associated with a ureter or prostate, which can result in reflex spasm of the lumbar muscles. Thus, irritation produced by malfunction of an abdominal/pelvic organ or valve may produce many signs and symptoms that can confound the diagnostician.
Some comparative characteristics between splanchnic and somatic pain are shown in Table 8.10.
Table 8.10. Splanchnic vs Somatic Pain
Splanchnic Disorders Somatic Disorders
Origin Diseased organ Neuromuscular tissue
Location and Deep, poorly localized, often Same.
radiation radiates or is referred to
the surface
Distribution Segmental Dermatome, myotome,
sclerotome.
Intensity Correlates with the Same.
intensity of stimulation
Quality Gripping, cramping, aching, Vague, aching, dull,
squeezing, crushing, heavy, boring, or
stabbing, burning. pounding character or
that is intensified by
movement, compression,
or pulsation.
Alleviating Intensified by motor activity Relieved by rest and
exacerbating or compression of an involved inactivity.
factors viscus that correlates with
secretory or motor rhythms of
the involved viscus
Associated Patient presents a trial-and- Patient avoids movement,
factors error behavior, often based pressure, and presents
on past experience, in an awkward motions because
attempt to relieve the pain. of the protective spasm
associated.
In investigating the history of any visceral problem, the sympathetic and parasympathetic innervation to the viscera and the structures related to the viscera must be considered. Next, the somatic and visceral reflexes that affect other tissues must be appraised. Visceral dysfunction frequently refers pain to the spinal segments supplying the involved viscera and also alters the function of other viscera sharing the same nerve supply. In addition, the probability should be considered of somatic dysfunction occurring in segmentally involved areas causing visceral dysfunction or in other somatic structures sharing the same nerve supply.
Just as visceral disease can exhibit solely as signs of a somatic nature, musculoskeletal disorders can mimic visceral disease. Several examples are described below.
Headaches
Headaches, the most frequent symptom in America today, are usually attributed to tension, migraine, abnormal sinus, tumor, vascular disorders, or hysteria. Often neglected causes are overall postural strain and trauma to the cervical spine, and headaches caused by viscerosomatic reflexes from the gallbladder, stomach, and duodenum are much more common than suspected. Nausea or vomiting with headaches is usually considered to have a neurologic basis of a vascular nature; however, a vagal disturbance due to upper cervical malfunction may be the cause.
Vertigo
Vertigo makes basilar insufficiency or Meniere's syndrome suspect, but vertebral artery ischemia due to upper cervical dysfunction or an atlanto-occipital subluxation may be the reason. In blackout spells of an unexplained nature, epilepsy and arterial occlusive disease are suspect, but somatic dysfunction involving the cervical ganglia may be the cause.
Chest Pain
Chest pain usually points to acute or chronic coronary insufficiency, dissecting aneurysm, or it may be of esophageal or pleural origin. It also may be referred from the gallbladder, stomach, duodenum, or pancreas. However, chest pain also may be the result of somatic rib cage dysfunction, costochondral or costovertebral strain, or pseudoangina.
Chest Pains Associated with Cough
In chest pains associated with a cough, acute and chronic pulmonary infections, pneumonia, lung abscess, and chronic bronchitis are usually suspected, along with pleuritis and lung or pleural tumors. Bronchial tumor, pulmonary embolism, broncholith, bronchiectasis, postnasal discharge, or the inhalation of irritants also should be considered. Reflex considerations would include costovertebral dysfunction, costochondral dysfunction or separation, or costal fracture. Reflexes from clavicular strains affecting recurrent laryngeal nerves, cervical subluxation, or cerumen impaction should not be overlooked.
Respiratory Symptoms
Ventilatory impairment is usually suspected in dyspnea or of metabolic origin, but it may be the result of rib cage dysfunction, spondylitis, or paravertebral muscle spasm and pain. Air hunger at rest is a cardinal sign of anxiety, and it is often seen in chronic obstructive pulmonary disease. However, it is also a reflex sign of rib cage dysfunction.
Upper Extremity Symptoms
Several diseases may refer pain to the shoulder or arm. This is seen often in disorders other than those of the gallbladder such as coronary artery disease, empyema, pneumothorax, pericarditis, mediastinal lesions, peptic ulcer, diaphragmatic hernia, perisplenitis, and subphrenic abscess. Pain radiating to the arm may be referred from coronary disease. It may also be a reflex from brachial neuropathy caused by dysfunction or degenerative disease of the cervical spine. The referred scapular pain of gallbladder disease and other visceral disorders have been mentioned. Frequently overlooked is the gallbladder reflex causing cardiac arrhythmia. Pain, numbness, or tingling in the hands is seen in many neuropathies. However, the cause may be musculoskeletal in origin such as degenerative disease or somatic dysfunction of the cervical spine, brachial plexus entrapment due to clavicular and upper rib dysfunction, carpal tunnel syndrome, or cervical cord compression.
PARESTHESIA
Paresthesia is commonly witnessed as the familiar prickling and tingling felt when one's arm or leg has "gone to sleep." Sensations as of crawling insects are not uncommon; the "hot feet" of many elderly persons with arteriosclerosis and the "burning hands" of people who work in hot water are other familiar examples. Local paresthesia is common in lesions of the cerebral cortex and constitutes the preliminary aura with which many attacks of epilepsy are ushered in. Well-developed tabes dorsalis shows many curious or distressing varieties of paresthesia, as do many other varieties of peripheral neuritis.
Common Types
Numbness
Numbness and tingling may occur in the feet during the initial stages of locomotor ataxia, apoplexy, tumor of the brain, spinal meningitis, neuritis, myelitis, and neurasthenia to name a few associated disorders. It indicates the loss of sensory function.
Itching
Formication is a sensation of insects crawling upon the skin. It occurs in diabetes, jaundice, and skin diseases. General formication is more common in cases of hysteria, neuresthesia, allergies, and drug intoxication.
Precordial Constriction
Precordial constriction is a feeling of tightness in the chest that is near the point of suffocation. It is encountered in those diseases accompanied by intense dyspnea such as bronchial asthma, emphysema, angina pectoris, meteorism, and heart trouble.
Girdle Sensation
A girdle sensation is an important and common paresthesia found in disease of the nervous system. It is a subjective sensation of a tight band drawn around the waist and often associated with locomotor ataxia, myelitis, and tumor of the spinal cord.
Bearing Down
The sensation of weight is most commonly found in the pelvis and is more frequently found in women than in men. It is symptomatic of prolapse of the uterus, pelvic tumors, or falling of the abdominal viscera.
Fullness
The sensation of fullness is usually perceived when the abdomen is distended by gas or fluid as in gastritis and ascites, or it may be present when there is pressure by an enlarged or prolapsed organ.
Coldness
The sensation of coldness is present at the beginning of a fever or during the chill stage but also occurs in a few cases in which the body temperature is normal. These latter instances are usually diseases of the nervous system in which the sensation is only mentally initiated. Such sensations of coldness are found in neurasthenia, hysteria, and chorea.
Faintness
Faintness is a feeling of extreme bodily weakness with a cloudiness of the intellect and occurs from cerebral anemia. Early chiropractors often attributed this to a subluxation in the region of T2 in which the heart is affected to such an extent that the brain is not receiving sufficient oxygen to maintain consciousness. It was also felt it may be caused by an atlas subluxation in which the vasomotor nerves of the vessels of the brain are impinged, thus causing a spasm of these muscles and rendering a part or whole of the cerebrum anemic to some extent. It occurs in diseases of the heart and hydrothorax, pleurisy with effusion, or sometimes as a result of great emotion, fatigue, or excessive heat.
Vertigo
This is a subjective sensation of a loss of equilibrium. When it appears that the patient himself is falling, rising, or whirling, it is called subjective vertigo. When the objects around the patient appear to be in a state of motion, it is called objective vertigo. Both subjective and objective vertigo may be classed as horizontal if present when the patient is lying down. Horizontal vertigo always disappears when the patient assumes an erect position. Vertigo itself is a symptom and not a disease. It is often found in disorders of the heart, liver, stomach, disease of the semicircular canals of the internal ear, and in acute alcohol intoxication.
Weakness
Weakness or debility attends the onset of all febrile diseases and appears toward the close of wasting diseases. It may be especially marked in diseases such as diabetes mellitus, cancer, anemia, influenza, tuberculosis, and neurasthenia.
Meralgia Paresthetica
This syndrome, often referred to as lateral femoral cutaneous neuropathy, features a patch of anesthesia, paresthesia, or hyperesthesia, with or without pain, on the anterior surface of one or both thighs in the distribution of the lateral femoral cutaneous nerve. Investigator Sites defines the condition simply as a sensory disturbance affecting the lateral cutaneous nerve.
The lateral femoral cutaneous nerve arises from the posterior divisions of L1–L3, appears at the lateral border of the psoas, passes obliquely across the iliacus to the ASIS, and then travels under the inguinal ligament to enter the anterolateral aspect of the thigh. The focus of irritation is often found
(2) in the pelvis from repetitive trauma as seen in athletic injuries and late pregnancy. Nevertheless, pathology or impingement anywhere along the course of the nerve may be responsible, whether it be intraspinal, intersegmental, paraspinal, retroperitoneal, pelvic, or, rarely, abdominal.
Notalgia Paresthetica
Pleet/Massey describe this disorder of unknown etiology as a sensory neuritis that affects the posterior rami of several thoracic spinal nerves (T2–T6), producing itching, burning, dysesthesia, and hyperesthesia. They point out that the posterior rami of these spinal roots traverse at a 90º course through the multifidus spinae muscles, making them unique among the posterior rami.
DIAGNOSTIC CONSIDERATIONS
General somatic sensations are divided into two major classes: exteroception and proprioception. Exteroception includes the sensations of pain, temperature, and light touch from the surfaces of the body. Proprioception includes the sensations of muscle, tendon, ligament, and joint activities, along with vibratory awareness, deep pain, and deep pressure perceptions. In evaluating exteroceptive qualities, tactile, pain, and temperature tests should be conducted. In judging proprioceptive qualities, position, motion, vibration, deep pain, stereognosis, and two-point discrimination tests should be conducted.
Daube/Sandok recommend that the examination of sensation should consist of three portions:
(2) quantitative, to determine the degree of involvement when sensation is impaired; and
(3) anatomical, to map out the areas of sensory impairment.
The Clinical Differentiation of Pain
One of the first steps in the differential analysis of pain is to determine whether the patient's pain is being expressed superficially or deeply within the body part and then to determine whether the pain is of somatic, splanchnic, or psychic origin. If this can be determined, the physician is then in an advantageous position to locate the specific tissues involved.
Significance of Localization
The localization of pain usually depends on whether the source of the pain is superficial or deep. Kellgren has shown that, in general, the closer the involved tissue is to the surface of the body, the better the site of pain corresponds to the site of noxious stimulation. His research team also found that pain of deep musculoskeletal origin was usually delocalized; ie, the site at which the pain is perceived rarely corresponds exactly to the site of its origin.
Pain can be localized only because most of the stimuli that excite nociceptors also excite touch, pressure, or stretch receptors at the same time. Thus, localized pain arises only because more than one type of receptor is activated. If it were not for this important fact, pain would be practically impossible, if not completely impossible, to localize.
Superficial vs Deep Pain
Pain perceived to be on or near the skin (superficial) is usually sharp, localized, of short duration with a sudden onset. In contrast, deep pain is dull, diffuse, of long duration with a gradual onset.
Deep physical pain can arise from either a splanchnic or a somatic disorder. It can rarely be distinguished easily from pain that is derived from the viscera if the sources are linked with the same segment. Deep pain may give rise to variations in sudomotor, visceromotor, and myotomic activity; but, it is not associated with a loss of muscle strength, a loss of normal reflexes, or a loss of sensory perception (eg, light touch, pressure, temperature, position, two-point discrimination).
Pain of deep somatic origin often radiates into a characteristic pattern that tends to follow dermatome or sclerotome pathways. The extent of this radiation, which almost always radiates distally rather than proximally, depends on the intensity of the noxious stimulation.
When a tissue of a particular sclerotome is irritated, the pain will be perceived to arise from any or all tissues innervated by the same segmental nerve even when they are healthy. Inman/Saunders attribute this to a lack of precision in the central neural connections rather than being related to impulse overflow down a nerve. They feel that the problem is central, not peripheral. This effect has been seen in many chiropractic offices when-severe knee pain, which failed to be corrected by repeated surgery, was relieved when adjustments corrected a lumbar lesion.
Similar experiences with chronic elbow pain are commonplace after a lower cervical (C5 C7) subluxation has been corrected. When pain expresses over a sclerotome, state Inman/Saunders, there need not be anything wrong with the area in which the pain seems to arise.
Painful Muscles
Pain originating from muscle tissue has peculiar characteristics. It can arise from an injury to muscle tissue elicited by making the muscle contract against resistance without allowing it to shorten; ie, preventing movement of adjacent joints. This test, although it may be of help in differentiating myalgia from the pain of other etiologies, is not absolute because it is not always possible, even with great care, to avoid some indirect pressure or tension on adjacent structures. An additional feature is that the pain which arises from a chronic contraction of the involved muscle is not increased by contracting the muscle further.
Any type of excessive motor fiber stimulation results in pathologic, involuntary, and painful muscle spasm. Severe spasm places considerable tension on highly sensitive periosteum via tendinous attachments. The spasm itself may be the result of toxic irritation of the anterior horn cells; encroachment irritation of the nerve root; irritation, stretching, or pressure upon a nerve trunk or plexus; irritation or pressure upon peripheral nerve branches; muscle spasm secondary to trauma of an adjacent structure; primary muscle spasm from direct irritation or trauma; or psychogenic muscle spasm.
Tendon Pain
Pain in damaged tendons usually arises when the attached muscle is contracted. The tendon fibers that are torn will usually not cause pain when the muscle is relaxed; but, with the least muscle shortening, pain ensues. The pain of true tendinitis is often superficial, resulting from a tenosynovitis. It is evoked by passively moving the tendon to and fro within its sheath.
Lymph Stasis
Although skeletal muscle tissue lacks an intrinsic lymph supply, a muscle's connective-tissue sheath and tendons are richly endowed with lymphatic vessels. During the normal physiologic exchange of fluids through capillary walls, the quantity of fluid leaving the capillary is usually greater than that entering the venule.
The related lymphatic network takes up the excess and eventually delivers it to the venous system. It is this process that allows a continuous exchange of tissue fluids and maintains a constant pressure of interstitial fluid. The flow of lymph is increased during activity as is capillary circulation, but this flow can be restricted by excessive pressure exerted by a constantly hypertonic or phasic contracted muscle.
De Sterno explains that inhibited lymph drainage contributes to muscular pain during prolonged activity by (1) causing a buildup of interstitial fluids that increase hydrostatic pressure and (2) encouraging the accumulation of metabolic waste products that would normally be drained by the lymphatics and venules.
Painful Joints
Pain may arise from any joint tissue containing nociceptors such as ligaments, tendon insertions, periosteum, fibrocartilages (slightly), capsules, and vascular walls. Authorities disagree whether the synovial lining contains nociceptors.
Ligament Pain
Pain is elicited from irritable ligaments by stretching and deep pressure. Joint ligaments can be stretched by passive movements of the related joint to the limit of the range of motion. When accessible to palpation, an irritated ligament will be tender; and if it can be squeezed, pain will be evoked. Ligamentous pain is the type that develops when a joint is under extremely prolonged stretch (tension). A hypomobile joint should be the first suspicion in such cases. Generally, chronic ligamentous pain comes on slowly after assuming some posture in which the involved joint(s) is held at a limit of motion. The ache arises from the irritation of the intraligamentous and periosteal receptors.
Painful Adhesions
Adhesions do not in themselves contain nociceptors. During movement, however, pain arises when they stretch or occlude adhering, connecting, or congruent pain-sensitive tissues (eg, periosteum, vascular walls, joint or visceral capsules). The cause may be from direct compression or tensile forces or be the product of ensuing stasis, ischemia, or distention. The pain is immediate in onset and not delayed as when the ligaments are relaxed. Another diagnostic clue is that there is a pronounced structural hypomobility when adhesions are present.
The most common situation encountered is the painful adhesions that develop after surgery or major trauma. However, adhesions may develop as the result of adhesive capsulitis, rheumatoid arthritis, and septic arthritis.
The pain originating in capsules tightened by adhesions occurs at once when the capsule is stretched. If the adhesions are stretched further, a sharp pain may ensue, leaving the surrounding muscles flaccid. The intensity of such a pain varies with the site and size of the adhesions. For the most part, pain arising from adhesions is only momentary because motion is quickly halted when the sharp pain is felt.
Cartilage Pain
As with adhesions, pain arises from most cartilaginous tissues only when they are displaced or swollen and stretch or pressure is applied upon adjacent pain-sensitive receptors. The periphery of intervertebral fibrocartilage and the menisci of the knee and jaw contain a few nociceptors, but the degree that they are involved in a patient's report of pain is difficult to determine. A cartilaginous loose body (eg, in the knee) will certainly produce pain if it is caught between two apposing articular surfaces (joint block). If adjacent tissues are inflamed, then both compression and tensile forces will cause pain.
Evaluating the Sensory System
Testing should be conducted in a warm room when the patient is relaxed. Examination begins with the face and scalp, then proceeds anteriorly and posteriorly to the soles of the feet. Reactions in both the right and left sides of the body and the upper and lower extremities should be compared.
According to Cabot, the most important types of sensations evaluated during the examination are anesthesia, hyperesthesia, paresthesias, pain, and disorders of the special senses. These disturbances may all be seen in different stages or types of lesions of the spinal cord or peripheral nerves. They are less common in brain lesions. Brain stem lesions can usually be determined through the pupillary reaction to light, corneal reflex, gag reflex, jaw reflex, and ciliospinal reflex.
Mechanoreceptive Sensations
The mechanoreceptive somatic senses are stimulated by mechanical displacement of some tissue of the body. These senses include touch, pressure, vibration, and tickle senses (all which are called the tactile senses) and the position sense, which determines the relative positions and rates of movement of different segments of the body.
The Tactile Senses
The perception of light touch is called esthesia; increased sensation, hyperesthesia. The term graphesthesia refers to the sense by which figures or numbers written on the skin are recognized by the patient without being seen.
The patient should close the eyes and be instructed to answer "Yes" when the touch is felt. The examiner frequently outlines with a skin pencil any area that shows a tactile alteration. Lighter and heavier touches may be attempted for comparison, and a graphic record is prepared when appropriate. Some examiners prefer to use a wisp of cotton for the very light touch and their finger or a blunt instrument is used to evaluate moderate touch. Light stroking stimulates only the receptors of the skin, thus normal responses are lost in pyramidal tract disease.
Decreased sensation is called hypesthesia; loss of sensation, anesthesia. Sensory loss may be the result of the same factors that produce pain; however, upper motor neuron lesions also may exist. In such cases, abnormal reflexes and other signs are associated such as loss of superficial reflexes, exaggerated deep tendon reflexes, and paralysis (spastic or rigid).
Superficial Pain Perception
During examination, the patient's eyes should be closed. The patient is touched lightly with the point of a pin and directed to say "Yes" when the pressure is detected as being "sharp." The procedure then proceeds as in the tactile test. Reactions are then compared using the point and head of the pin or a bent pin, noting the patient's ability to appreciate the difference quickly. If known beforehand, the unaffected side should be tested first taking care to use the same degree of stimulation over unaffected and affected areas.
Pain is technically called algesia; a decrease in pain sensation, hypalgesia (hypoalgesia); an absence of pain sensation, analgesia; increased pain sensation, hyperalgesia as seen in irritative lesions.
Hypalgesia and Analgesia
Diminished or lack of superficial pain sensation commonly occurs in peripheral neuritis, disorders of the spinothalamic tract, and hysteria. The distribution of anesthesia depends, like the distribution of paralysis, on the lesion. Hemianesthesia is seen most often in hysteria and organic brain lesions. Cord lesions such as transverse myelitis or compression of the cord usually produce anesthesia in the area supplied by the spinal nerves below the lesion. Peripheral nerve lesions may produce anesthesia of the skin supplied by the nerve in question. Areas of hysterical anesthesia (with hyperesthesia and paresthesia) usually do not correspond to the distribution of any set of nerves or centers and are distinguished by this fact.
Hyperesthesia
An increase in pain sensation is most often recognized as hypersensitivity to light tough, abnormal tenderness, or, in the special senses such as hypersensitivity to light or noise. It is most common in peripheral nerve lesions and in hysteria. Increased superficial pain sensation is seen in cases of posterior root irritations caused by local inflammatory processes. A ticklish person is one whose superficial reflexes (skin and muscles) are normally very lively.
Deep Pain Perception
Tenderness on pressure along the various nerves should be tested. Tendons and muscles should be squeezed firmly. The patient should be able to detect a painful sensation. Certain areas are more important than others such as squeezing the Achilles tendon, where upon absence of pain is called Abode’s sign. In tabes dorsalis, the patient will not feel pain upon normally painful pressure.
Thermal Perception
Decreased temperature sensation is called thermhypesthesia; increased sensation, thermhyperesthesia.
In evaluating temperature perception (thermesthesia), metal tubes (eg, 5 cc steritube containers) are commonly used: one containing hot water and the other containing cold water. The patient's eyes should be closed, and the patient should be asked to name the temperature as it is applied to the skin. The patient should be able to perceive which is hot and which is cold, and the examiner should note the quickness of response, mistakes, etc. The procedure then proceeds in a manner similar to that used for the tactile and pain tests.
Areas of impaired temperature sensation usually follow a pattern similar to the areas of impaired pain sensations. The receptors in the skin for the sense of cold and heat are thought to consist of both naked and encapsulated endings, and the neurons serving the sensory path for temperature perception follow the same course as those of the pain pathway. The two systems are so closely associated in the CNS that they can scarcely be distinguished anatomically, and an injury to one usually affects the other to a similar degree. Thus, for most practical clinical purposes, testing either pain or temperature sensibility accomplishes the same result.
Local Temperature Changes
During health, bilateral skin temperature readings are about equal unless an ipsilateral inflammatory reaction exists. For example, a painful joint with overlying hyperemia or a local sympathetic block may alter this pattern. Upon this fact, many instruments have been developed to measure skin temperature differentials. The conclusions drawn from the interpretation of these recordings, however, have been controversial for many years.
In recent years, the study of skin temperature differentials has advanced considerably, with the introduction of liquid crystal thermography, thermal cameras, infrared detector instruments, and improved thermograms. Rask concluded that thermography of the spine was more accurate in evaluating and differentiating IVD syndromes than myelography, and Hendler and associates found current techniques valuable in differentiating somatic from psychogenic causes of pain.
There is little doubt that there is a correlation between sympathetic activity and skin temperature. Normell/Wallin demonstrated concomitant changes in evoked skin sympathetic activity in a peripheral nerve and in skin temperature in the corresponding skin area that strongly supports the concept that the cutaneous thermoregulatory response is a consequence of changes in efferent sympathetic nerve activity.
Effects of Chiropractic Adjustments on Peripheral Temperature
In studying the effects of spinal adjustments on distal skin temperature, McCullough, Haldeman, Pierce/Stillwagon, and Kelso and associates have noted before and after changes following chiropractic adjustments. In a recent study, Harris/Wagnon concluded that
(2) changes in fingertip temperature varied, depending upon which region of the spine was adjusted;
(3) adjustments within the classic sympathetic outflow caused activation of the sympathetic nervous system; and
(4) adjustments outside this region caused apparent sympathetic inhibition. The changes in temperature recorded in this study were quite small, but their presence strengthens the contention that manual adjustments of the spine can cause measurable changes in the physiology of distal tissues.
Vibration Perception
A decreased perception of vibratory sensation is recorded as pallhypesthesia; loss of sensation, pallanesthesia. Pallesthesia refers to the normal perception of vibration from the fork when placed against any subcutaneous bony prominence.
During typical evaluation, a vibrating tuning fork is placed over bony prominences (eg, the lower ends of the radius and ulna, the ASIS, the patellae, and the external and internal malleoli), then the patient is asked to state when he feels a vibration or humming sensation. When the vibratory sense is lost, the patient cannot differentiate between a vibrating fork and a silent one.
Testing begins at the head and proceeds to the feet, and compared with the examiner's hand or other bony prominences. The nonvibrating fork is alternately placed over a point to determine if the patient is guessing. If the patient fails to feel the vibration, it suggests an impairment of the posterior columns of the spinal cord; ie, those tracts that convey the vibratory impulses. Normal vibratory perception is commonly impaired in tabes dorsalis, subacute sclerosis of the cord following pernicious anemia, and in generalized arteriosclerosis. It is first detected with a C256 fork and later by a C128.
Position and Motion Senses
Joint sensation is called arthresthesia; decreased sensation, arthrhypesthesia; loss of sensation, arthranesthesia; increased sensation, arthrhyperesthesia.
With the eyes closed and extremities relaxed, the patient is asked to tell what position a particular joint has been placed or what joint is being moved. Each part should be tested several times. The patient should be able to perceive joint motion with the eyes closed. The examiner should be cautious that the patient completely relaxes the joints being manipulated. The best joints to test appear to be those of the great toe and thumb.
Stereognotic Perception
Stereognosis refers to the ability to sense the form, nature, and solidity of objects through the sense of touch. Familiar objects as keys, coins, marbles, pencils, etc, are commonly used. Different objects are placed in either hand of the patient when the patient's eyes are closed. The patient is asked to handle them and to name them. Loss of the ability to recognize familiar objects occurs in contralateral brain lesions in the parietal area.
Two-Point Discrimination
There is loss of the normal ability to recognize two points simultaneously applied to the skin as distinct from a single point when there is impairment of the proprioceptive pathway. In testing, it is common to use the two ends of an opened paper clip or the points of a compass.
Tenderness
The perception of tenderness is a highly subjective symptom influenced by many individual structural, functional, and psychologic factors that often make it an unreliable sign.
Paravertebral Tenderness
Determining the specific location of paravertebral tenderness is often helpful in its differentiation. For example, paravertebral tenderness is frequently found at the apices of spinal curves and rotations and not infrequently where one curve merges with another. Tenderness about spinous or transverse processes is usually of low intensity and suggests articular strain. Tenderness noted at the points of nerve exit from the spine and continuing in the pathway of the peripheral division of the nerves is a valuable aid in spinal analysis; however, the lack of tenderness is not a clear indication of lack of spinal dysfunction.
Nerve Tracing
Nerve tracing is the art of following by palpation a tender nerve from its spinal origin to some inflammatory or pathologic lesion or zone or the act of tracing a tender nerve from an inflammatory zone to its spinal exit. A nerve may be traced if it is tender and situated at a point where it may be reached while palpating. To be traced, it is necessary that the nerve be hypersensitive, regardless of cause.
Tenderness is a frequently encountered sensory symptom that may be defined as pain on pressure. Pain produced by external pressure results from trigger points, traumatic lesions of sensitive subdermal tissue, or the development of a toxic accumulation or deep-seated inflammatory irritation. There may be a condition that would cause tenderness when pressure is applied, but no abnormal sensation is felt if there is no pressure. It is upon this fact that nerve tracing is based. Nerve tracing is a method of following the course of tenderness over nerves that are irritated or impinged that will usually assist in locating the cause of pain, tenderness, and headache.
Nerve tracing as a diagnostic procedure is a technique once widely practiced by pioneer chiropractors. Although it has fallen into disuse in recent years (like outlining the borders of organs through percussion), the art has considerable merit. Firth, Janse, and Harper mentioned the importance of nerve tracing in their writings on spinal analysis. More recently, Kirby, using the early works of Gregory as reference, offers a concise explanation of the methodology from which the following has been adapted.
Points to be considered in nerve tracing:
Not all nerve pathways follow the course shown in anatomy textbooks. Some deviate and wander somewhat wildly along their classic course. In these cases, by palpating in a fantailed manner, tenderness may be found and nerve tracing may then proceed as normal. Gregory felt that when a nerve is tender at all, it is tender throughout its entire length. Consequently, any tender nerve will be tender near its spinal origin.
Gregory illustrated these wandering nerves by describing a patient with pain over the right hypochondriac region when a full breath was drawn. He expected to locate the tender zone and trace it from this region back to the spine following the intercostal spaces and nerves. For the first 1| inches, this was true; then suddenly the tender pathway took an upward direction through the axillary region to the front of the shoulder, under the clavicle, and back to the origin at C6 on the same side as the tender zone. If this seems to be a peculiar tracing, then understanding the ramifications of the long posterior thoracic nerve will provide an explanation.
Clinical Reflexes
Testing the superficial, deep, and visceral reflexes evaluates the integrity of the peripheral receptors and the respective motor response. See Table 8.11. Such reflexes evaluate both the sensory afferent and motor efferent portions of the arc.
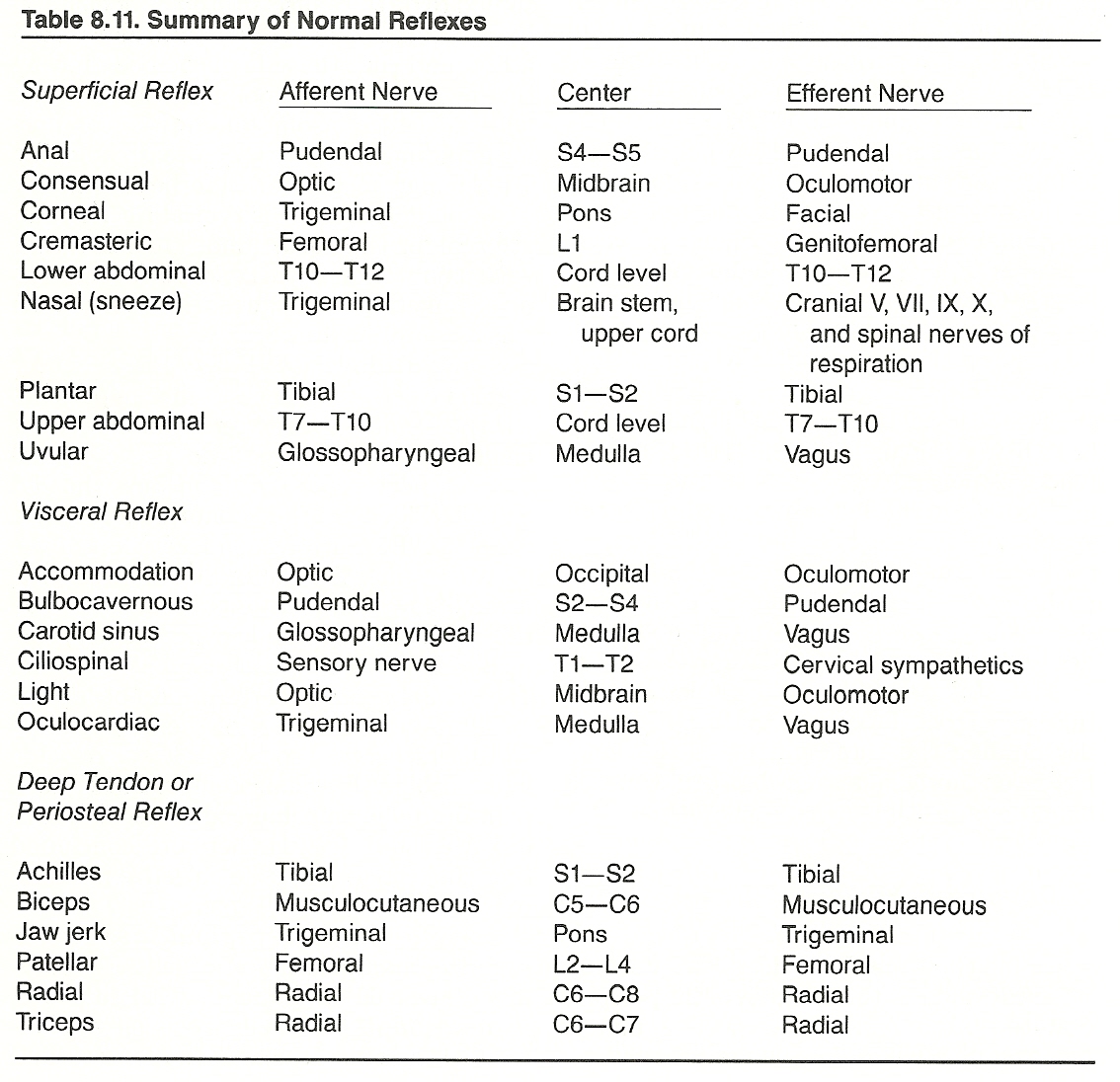
Skin Resistance
Electrodermograph recordings of skin resistance are sometimes used as an aid during the diagnosis of musculoskeletal and internal disorders, but their interpretation is controversial. Due to cutaneous overlap, segmental localization is imprecise, therefore, neither segment nor organ specific.
General Bilateral Differences
The common asymmetry of the conduction of the skin between the two complete sides of the body has not yet been explained, state Bauch/Hartig, but CNS influences are assumed to be the cause. In studies of bilateral basal skin resistance (BSR), Varni and associates found that the side of the body having the lower BSR corresponded with the side with the lesser vasomotor constriction during experimental stress (eg, breath holding). Their findings suggested that the asymmetry of autonomic activity is typical rather than atypical for the normal individual and that various autonomic variables form patterns of asymmetry. Not all authorities agree with this assumption, however.
Correlation with Pain
Riley/Richter studied electrical skin resistance in patients with neck and upper extremity pain without clinical or radiographic evidence of spinal cord or nerve root irritation but who did exhibit evidence of an abnormality with a cervical IVD or involving a facet joint of the cervical spine. They found that, in general, areas of low skin resistance (assumed to be the result of sympathetic hyperactivity) corresponded well to areas of subjective pain. The skin resistance pattern did not lead to localization of the level of the abnormality within the cervical spine, but it was of value in confirming the clinical impression that a pain-producing mechanical or degenerative lesion existed. It was therefore concluded that the test added some objective measure to a subjective report of pain.
Correlation with Manipulative Therapy
After manipulative treatment of the segment involved in patients with lumbago, Ivanov found that electrosensitivity remained the same as that of the controls and conductivity was near normal values. Further research is necessary to substantiate Ivanov's findings, however, as many clinicians report conflicting findings.
Evaluating Cranial Nerve Sensory Fibers
The cranial nerves have a variety of functions that often become involved in combined lesions because of their proximity to each other. Examination of the cranial nerves is a specialized task that is often referred to the neurologist, but several tests can be performed by the general practitioner that may offer many distinct clues to a cranial nerve lesion. Refer to Table 4.8.
The Olfactory Nerve (Cranial I)
The olfactory nerve is a pure sensory nerve that provides the sense of smell and flavors except for salty, sweet, bitter, and acid.
The patient's sense of smell can be tested with various unlabeled solutions or oils such as cinnamon, peppermint, clove, menthol, vanilla, wintergreen, etc. One of the patient's nares is closed, and the patient is asked to smell a solution held a short distance from the nose. The tube is usually rapidly passed inward from a distance of about a yard. The patient is then requested to tell the examiner what it is. Then the opposite side is tested with a different solution. Some patients smell a substance correctly but are unable to name it.
The least amount of odor necessary for recognition is called the minimal identifiable odor (MIO). When this is determined, House and associates recommend that a steady stream of test odor should be passed through the nostril until stimulus fatigue occurs, at which time the patient is no longer aware of the odor. Each nostril is then tested at 30-second intervals with the MIO to determine the duration of fatigue. It should be kept in mind that any nasal disorder (eg, a head cold) will influence the results of such tests.
Ischemia of the nasal mucosa has been shown by Kuntz to provide the milieu for the filterable viruses associated with common upper respiratory infections, which, in turn, are often superimposed by bacterial infection. Homewood points out that an infection at the thin roof of the nasal cavity may easily spread to the olfactory bulb and from there to the meninges to establish meningitis. In this context, it should be noted that the blood vessels of the nasal mucosa are supplied by sympathetic postganglionic fibers originating in the superior cervical ganglion, whose preganglionic fibers stem from T1–T4 segments. Thus, upper cervical and upper thoracic subluxations may cause or encourage such nasal-focus syndromes in their initial stages.
The Optic Nerve (Cranial II)
The optic nerve is a pure sensory nerve that provides the perception of vision. A large number of visual defects, however, may exist that are due to mechanical defects anterior to the optic nerve (eg, lens and accommodation defects).
Injury to the optic nerve is not uncommon. Loss of vision often accompanies a fracture through the sphenoid involving the orbital foramen. Complete blindness of one eye invariably indicates a lesion of the optic nerve. Optic neuritis and tumors compressing the optic nerve are the most common lesions causing loss of the direct light reflex. Lesions of the optic nerve are especially noted with optic atrophy, optic neuritis, papilledema, and visual impairment. Thus, the optic nerves should be tested for visual acuity and fields of vision, and directly inspected by observing the condition of the fundi.
Fields of Vision. Visual fields are measured most accurately with a mechanical perimeter or tangent screen by one skilled in its use. A rough estimate of the fields can be obtained in the following manner: With the patient and examiner facing each other about 2 feet apart, the patient is asked to close one eye and fix the open eye on the examiner's eye that is opposite (examiner's right, patient's left). A small object, preferably red, is moved on a line equidistant from the eyes of the two individuals, from the periphery toward the center of the field. This procedure is carried out in the four quadrants, and the patient mentions when he first sees the object enter his field of vision. A comparison is thus made between the extent of the patient's fields and those of the examiner. Obviously, the examiner's fields must be normal; and if so, gross quadrantic or hemianopic defects are often found in this manner.
The Trigeminal Nerve (Sensory Fibers of Cranial V)
The trigeminal is a mixed sensory-motor nerve that serves as the chief sensory nerve of the face. The three sensory bundles of the trigeminal nerve (which travel in separate foramina) and their distribution are the: (1) mandibular branch chin, lower lip, cheek, upper ear, and lateral scalp; (2) maxillary branch cheek, upper lip, lateral nose, and lower eyelid; and (3) ophthalmic branch anterolateral nose, eye, forehead, and anterior scalp to the midpoint of the crown. These areas should be tested for touch, pain, and sometimes for temperature. A wisp of cotton is used for touch, a pin for pain, and warm and cool tubes for temperature sensation. Pain perception is tested over the nasal and buccal mucous membrane as well as the face.
The Sneeze Reflex. Sneezing when the nasal membrane is irritated is absent or greatly diminished in lesions of the cranial nerves V to X and the upper cervical nerves.
Visceral Reflexes. Pottenger attributed the tooth decay commonly associated with pulmonary tuberculosis and the unilateral hectic flush associated with pulmonary disorders to vagal afferent impulses mediating in the medulla with sensory efferent fibers of the trigeminus. It should also be noted that most of the dilator fibers contained within the cervical sympathetics unite in their course with the trigeminus, which has some dilator fibers of cerebral origin. Pottenger also felt strongly that the herpes associated with pneumonia, the common cold, and some gastrointestinal disorders are common parasympathetic viscerovisceral reflexes mediated by the vagus and trigeminal nerves.
The Facial Nerve (Sensory Fibers of Cranial VII)
The facial nerve is a mixed sensory-motor nerve. Its sensory fibers supply the taste buds in the anterior two-thirds of the tongue.
Taste perception is tested with solutions of sour (vinegar), sweet (sugar), and salty (salt) substances. Certain signals are arranged with the patient before the test is made. For instance, holding up two fingers may mean that no taste is recognized and three fingers may mean that a taste is recognized. Then the applicator is dipped in the solution, and the patient is asked to protrude the tongue. It is kept protruded with the aid of a sterile pad, and a solution is applied to one side of the tongue. After the patient has signaled that a taste is perceived or not, a request is made to name the flavor. Each side of the tongue should be tested separately. It is best to have the patient rinse the mouth thoroughly with warm water after each solution is used to avoid confusion between the solutions.
The Cochlear Division of the Auditory Nerve (Cranial VIII)
The cochlear division of Cranial VIII is a pure sensory nerve that provides the sense of hearing. To screen hearing, sound perception can be evaluated on both sides by using a watch. During examination, widespread and conversational tones can be compared. Cochlear involvement is commonly seen in otitis media, skull fractures, otosclerosis, and basal tumors.
If an abnormality is noted during a screening procedure, the integrity of the cochlear nerve can accurately be tested by audiometry. However, using a tuning fork is common procedure in general practice. Bone conduction (with the base of the fork held against the vertex or occiput) should be equal in both ears. Covering one ear should shift the sound to the closed ear (Weber's lateralization test). When sound is no longer heard with bone conduction, bringing the fork near the ear without setting it in vibration again should again be heard by air conduction (Rinne's test). Air conduction should be about twice the duration of bone conduction.
Weber's Test. To test for deafness and lateralization, the base of a vibrating tuning fork is placed in the center of the forehead at the hairline and the patient is asked if the tone is heard better in one ear than the other. Normally, the tone is heard equally in both ears.
Nerve Deafness vs Conduction Deafness. Deafness resulting from cochlear division impairment is called nerve deafness, as opposed to deafness resulting from middle ear pathology (conduction deafness). Weber's and Rinne's tests differentiate. In Weber's test, the sound heard best when the fork is on the vertex is by the unaffected ear if the deafness is due to disease of the auditory apparatus. If the deafness is due to obstruction of the air passages, the vibration will be heard by the affected ear. A positive Rinne's test, if the sound is heard better by bone conduction, points toward a blockage of the middle ear. In nerve deafness, both air and bone conduction will be impaired. Tinnitus is often associated, but it also may be found also in middle ear disease or due to wax in the external meatus. Nerve deafness is the result of cochlear neuropathy; eg, extension of otitis media, skull fracture (especially temporal), otosclerosis, tumors, syphilis, congenital syphilis, and genetic defects.
The Vestibular Division of the Auditory Nerve (Cranial VIII)
The vestibular division of cranial VIII is a pure sensory nerve that serves as the primary mechanism for balance and equilibrium via afferents from the semicircular canals. The complete system provides the nervous system with information concerning gravity, rotation, and acceleration and is located primarily at the posterior fossa level.
Vestibular function is ordinarily not evaluated unless vertigo, nausea, or disturbances of postural coordination on the affected side are reported or observed. These symptoms and signs suggest a lesion of cranial VIII, sensory ataxia, or brain-stem disease.
Stein states that vertigo from whatever cause means a disturbance of the vestibular apparatus and can be analyzed by ear tests. In vestibular dysfunction, a clinical clue of nystagmus is often a finding of the caloric test. This procedure is sometimes called Barany's test or the thermal test.
The classic origins of vertigo and imbalance syndromes are seen in Meniere's syndrome and vestibular neuritis. Meniere's disease is a syndrome of deafness, tinnitus, and dizziness in association with nonsuppurative disease of the labyrinth. In vestibular neuritis, which often occurs in epidemics, there is severe vertigo without a hearing impairment. The dizziness passes off in a few days but may recur at times during sudden movements or quick turns of the head. Positional vertigo is usually the result of otolith disease, a lesion of the posterior fossa, a vertebral or basilar artery deficit, or an upper cervical subluxation complex.
Lewit writes of the relationship of the cervical spine to Meniere's disease (Review in Czechoslovak Medicine, VII:2, 1961) after 120 cases of Meniere's disease and similar forms of vertigo were sent by leading ear departments of Prague for manipulative treatment. The conclusions reached were: "(1) The results of manipulative treatment in Meniere's disease whether there is any noticeable symptomatology from the cervical spine at the same time or not and in cases of vertigo (Barre-Lieu’s syndrome) are practically the same. (2) There are gradual transitions between all the groups of vertigo, sometimes even in one and the same patient. (3) The vertebral origin of Meniere's disease can frequently be directly proved by the aid of tests; ie, experimentally. Thus, cases of cervical origin can be diagnosed from the start. And (4) although Meniere's disease (labyrinthine vertigo) may be provoked by other ways than that of the cervical spine, by labyrinthitis for example, it appears that we have to cope with a syndrome that in the large majority of cases is connected with functional disturbances of the cervical spine; therefore, it should be indicated for manipulative treatment."
Vestibulospinal Postural Reflexes. Molina-Negro and associates report that, through its activation from the utricule in response to displacement of the center of gravity, the vestibular system seems to provoke an increase of the excitability of gamma-static motor neurons of extensor muscles of the trunk and legs and to a lesser degree of the gamma-dynamic and alpha motor neurons. This contributes to the adaptation of muscle tone for the maintenance of the standing static posture and for the alternative change from the extensor to flexor phase during gait. Temporary vestibular hyperfunction provoked by central or peripheral stimulation is manifested clinically by vertigo. The presence of adequate gamma-static innervation on one side seems contradictory to complete suppression of labyrinthine function ipsilaterally. On the other hand, the absence of response to caloric stimulation in one or both labyrinths does not exclude the possibility of vestibular hyperfunction. These researchers conclude that the study of phasic stretch reflexes appears to be a valid method of measurement of the functional state of the static labyrinth and vestibular apparatus.
The Glossopharyngeal Nerve (Sensory Fibers of Cranial IX)
The glossopharyngeal is a mixed sensory-motor nerve. Its sensory fibers supply the posterior third taste buds of the tongue, the pharynx, parotid gland, and middle ear.
Taste sensation of the posterior third of the tongue is tested in the same manner described for the VII nerve. In practice, testing the integrity of the sensory fibers of cranial nerves VII and IX is usually conducted at the same time.
The Vagus Nerve (Sensory Fibers of Cranial X)
The vagus is a mixed sensory-motor nerve. Its sensory fibers supply the pharynx, larynx, heart, lungs, and alimentary tract.
Upper vagal integrity is screened by the motor response to the gag reflex, and the oculocardiac reflex (Aschner's sign). Gag reflexes can be tested by irritating the posterior pharyngeal wall. A laryngoscope examination should be performed if there is any sign of involvement of the pneumogastric nerve. To evaluate the oculocardiac reflex, note any irregularities or changes in respiratory rhythm or cardiac action via carotid sinus sensitivity.
Carotid Sinus Syndrome
The carotid sinus syndrome of vasovagal syncope is the result of pressure on a hypersensitive carotid sinus. Pressure causes marked bradycardia, vasodilation, a lowered blood pressure, and associated vertigo, syncope, and convulsive seizures. The associated fainting is called carotid sinus syncope. This reflex is especially irritable in hypertension, arteriosclerosis, and vasomotor instability. The response is often absent in lesions of the cranial nerves IX and X. When testing the sensitivity of this reflex, pressure should be put on only one side of the neck, while monitoring pulse and blood pressure. Bilateral testing in the prone position is usually avoided as it may provoke a convulsion.
The Salivary-Taste Phenomenon
Worthy of note here is the salivary-taste phenomenon, which is considered a reflex of the vagus. When a drop of weak acid such as vinegar is placed on the tongue, the salivary glands will normally increase their output of saliva that can be observed. The sensation is carried by afferent facial nerve fibers with efferent motor action by vagal parasympathetics to the salivary glands.
Tests to determine the possibility of parasympathicotonia (vagotonia) and sympathicotonia will be described in Chapter 10.
BIBLIOGRAPHY
Chicago, American Hospital Association, 1977.
American Orthopaedic Association: Manual of Orthopaedic Surgery.
Chicago, American Orthopaedic Association, 1972, pp 27-38.
Anderson KV:
Endogenous opioid peptides and the control of pain.
In Brena SF, Chapman SL (eds): Management of Patients with Chronic Pain.
New York, Spectrum, 1983, pp 33-46.
Arbuckle BE:
Reflexes.
Journal of the American Osteopathic Association, 46(7), July 12, 1946.
Balduc HA: Overview of contemporary chiropractic science for the Chiropractic Association of Oklahoma.
Northwestern College of Chiropractic, Convention notes, April 24, 1983.
Bauch K, Hartig W:
Electrodermatographic examination of segmental sweat secretion in the diagnosis and differential diagnosis of internal diseases.
Acta Neurovegetativa, 30(4):536-545, 1973.
Beck JW (ed):
The Management of Pain.
Amsterdam, Elsevier, 1979, pp 306-316.
Beecher HK:
Measurement of Subjective Responses. New York,
Oxford University Press, 1959.
Berthold CH:
Morphology of normal peripheral axons.
In Waxman SG (ed): Physiology and Pathobiology of Axons.
New York, Raven Press, 1978.
Bishop B:
Pain: Its physiology and rationale for management. Part I, neuroanatomical substrate of pain.
Physical Therapy, 60:13-23, 1980; Part II, analgesia systems of the CNS, 60:21-23, 1980.
Black RG:
The chronic pain syndrome.
Surgical Clinics of North America, 55:999-1011, 1975.
Bonica JJ:
Neurophysiologic and pathologic aspects of acute and chronic pain.
Archives of Surgery, 112:750-761, 1977.
Brena SF, Chapman SL:
Chronic pain: Physiology, diagnosis, management.
In Leek JC, Gershwin ME, Fowler WM Jr: Principles of Physical Medicine and Rehabilitation in the Musculoskeletal Diseases.
Orlando, FL, Grune & Stratton, 1986, pp 189-216.
Brown AG:
The termination of cutaneous nerve fibers in the spinal cord of man.
TINS, 4(3):64-67, 1981.
Burns L, Chandler LC, Rice RW:
Pathogenesis of Visceral Disease Following Vertebral Lesions.
Chicago, American Osteopathic Association, 1948, pp 3-20.
Burns R:
The cervical spine in manipulative medicine.
Practitioner, 222:803-806, 1979.
Cabot RC:
Physical Diagnosis.
New York, William Wood, 1919, p 484.
Canadian Memorial Chiropractic College:
Segmental Neuropathy.
Toronto, Canadian Memorial Chiropractic College, date of publication not shown. Monograph, pp 12-21.
Cervero F:
Deep and visceral pain.
In Kosterlitz HW, Terenius LY (eds): Pain and Society.
Weinheim, Verlag Chemic, 1980, pp 263-282.
Chatton MJ:
General symptoms.
In Krupp MA, Chatton MJ, Werdegar D: Current Medical Diagnosis & Treatment 1985.
Los Altos, CA, Lange Medical, 1985, p 1.
Chusid JG:
Correlative Neuroanatomy & Functional Neurology, ed 19.
Los Altos, CA, Lange Medical, 1985, pp 222-239, 241-243.
Clark WC, Hunt HF:
Pain.
In Downey J, Darling R (eds): Physiological Basis of Rehabilitation Medicine.
Philadelphia, W.B. Saunders, 1971, pp 373-401.
Coote JH, et al:
Reflex discharges into thoracic white rami elicited by somatic and visceral afferent excitation.
Journal of Physiology, 202:141-159, 1969.
Crue BB, et al:
Neurophysiology of pain peripheral aspects.
Bulletin of the Los Angeles Neurological Society, 41(1):13-42, 1976.
Curtis DR, Eccles JC:
Synaptic action during and after repetitive stimulation.
Journal of Physiology (British), 150:374, 1960.
Cyriax J:
Textbook of Orthopaedic Medicine: Vol One, Diagnosis of Soft Tissue Lesions, ed 8.
London, Bailliere Tindall, 1982, pp 22-42, 65-66.
de Reuck AVS, Knight J (eds):
Myotactic, Kinesthetic, and Vestibular Mechanisms.
Ciba Foundation Symposium. Boston, Little, Brown, 1967.
De Rusha JL:
Upper cervical technic correlated with neurodiagnosis.
ACA Journal of Chiropractic, September 1961.
Dee R:
Articular neurology.
In Sokoloff L (ed): The Joints and Synovial Fluid.
New York, Academic Press, 1978, p 177.
De Sterno CV:
The pathophysiology of TMJ dysfunction and related pain.
In Gelb, H (ed): Clinical Management of Head, Neck and TMJ Pain and Dysfunction.
Philadelphia, W.B. Saunders, 1977.
Ditmar E:
Cutaneo-visceral neural pathways.
Journal of Physical Medicine (British), 15:208, 1952.
Elton D, Burrows GD, Stanley GV:
Clinical measurement of pain. Medical
Journal of Australia, 1:109-111. 1979.
Fordyce WE:
Behavioral Methods for Chronic Pain and Illness.
St. Louis, C.V. Mosby, 1976.
Fowler WM Jr, Taylor RG:
Differential diagnosis of muscle disease.
In D'Ambrosia RD: Musculoskeletal Disorders: Regional Examination and Differential Diagnosis.
Philadelphia, J.B. Lippincott, 1977, pp 93-95.
Friedman AP (ed):
Symposium on headache and related pain syndromes.
Medical Clinics of North America, 62:427-623, 1978.
Friedman HH:
Problem-Oriented Medical Diagnosis, ed 2.
Boston, Little, Brown, 1979, pp 316-341.
Frogley R:
Neurological observations of a subluxation.
The Journal of Clinical Chiropractic, 1(3), 1973.
Gandhavadi B, et al:
Autonomic pain: Features and methods of assessment.
Postgraduate Medicine, 7(1):85-90, 1982.
Giles LG:
Spinal fixation and viscera.
Journal of Clinical Chiropractic, 1(3): 144-145, 1973.
Glover JR:
Back pain and hyperesthesia.
Lancet, 1(7135):1165-1169, 1960.
Goldstein M (ed):
The Research Status of Spinal Manipulative Therapy.
Bethesda, MD, National Institutes of Health, DHEW Publication No. (NIH) 76-998, 1975, pp 173-180, 185-187.
Goodheart GJ:
Collected Published Articles and Reprints.
Montpellier, OH, Williams County Publishing, 1969, pp 23-24.
Goodman CE:
Pathophysiology of pain.
Archives of Internal Medicine, 143:527, 1983.
Granit R:
Receptors and Sensory Perception.
New Haven, CT, Yale University Press, 1955.
Grieve GP:
Common Vertebral Joint Problems.
London, Churchill Livingstone, 1981, pp 69-73, 161-196.
Guilliam N:
Evolution of the nervous system.
ACA Journal of Chiropractic, May 1970.
Guyton AC:
Textbook of Medical Physiology, ed 6.
Philadelphia, W.B. Saunders, 1981, pp 588-625.
Haldeman S:
Observations made under test conditions with synchrotherme.
Journal of the Canadian Chiropractic Association, 14(1):9-12, 1970.
Hardy JR, et al:
Pain Sensations and Reactions.
Baltimore, Williams & Wilkins, 1952.
Harris W, Wagnon RJ:
The effects of chiropractic adjustments on distal skin temperature.
Journal of Manipulative and Physiological Therapeutics, 10(2):57-60, April 1987.
Hart FD (ed): The Treatment of Chronic Pain. Philadelphia, F.A. Davis, 1974, pp 1, 4, 8, 15, 64, 113.
Hendler N, Uematesu S, Long D: Thermographic validation of physical complaints in "psychogenic pain" patients. Psychosomatics, 23(3):283-287, 1982.
Hertz AF: On the sensibility of the alimentary canal in health and disease. Lancet, 1:1051, 1911.
Homewood AE: The Neurodynamics of the Vertebral Subluxation, ed 3. Place of publication not shown, published by author, 1981, pp 112-125, 132-146, 250-260, 267-281.
Hoppenfeld S: Orthopaedic Neurology. Philadelphia, J.B. Lippincott, 1977, pp 1-3.
House EL, Pansky B, Siegel A: A Systematic Approach to Neuroscience, ed 3. New York, McGraw-Hill, 1979, pp 215-216.
Huskisson EC: Measurement of pain. Journal of Rheumatology, 9(5):768-769, 1982.
Igneizi RJ, Atkinson JH: Pain and its modulation: Part I. Neurosurgery, 6(5): 577-583, 1980.
Inman VT, Saunders JB: Referred pain from skeletal structures. Journal of Nervous and Mental Disease, 99:660-667, 1944.
Ivanov BN: Changes in electrical skin sensitivity and conductivity in the lumbago syndrome after manipulative therapy. In Lewit K, Gutmann G (eds): Rehabilitcia. Proceedings of the IVth Congress. Prague, International Federation of Manual Medicine, supplement 10-11:171-176, 1975.
Janse J: A concept: The neurological element as it relates to clinical chiropractic. The Chirogram, December 1975.
Janse J: The integrative purpose and function of the nervous system: A review of classical literature. Journal of Manipulative and Physiological Therapeutics, 1(3), September 1978.
Janse J: Principles and Practice of Chiropractic. Lombard, IL, National College of Chiropractic, 1976, pp 48-50, 73-74, 243-250, 310-312.
Jaskoviak PA, Schafer RC: Applied Physiotherapy: Practical Applications Within Clinical Chiropractic. Arlington, VA, American Chiropractic Association, 1986, pp 15-44.
Judge RD, Zuidema GD (eds): Methods of Clinical Examination: A Physiological Approach, ed 3. Boston, Little, Brown, 1968, pp 341-366.
Karvinen E, Komi PV: Neuromuscular performance. In Larson LA (ed): Fitness, Health, and Work Capacity. New York, Macmillan, 1974, pp 73-78, 81-86.
Keegan JJ, Garrett FD: The segmental distribution of the cutaneous nerves in the limbs of man. Anatomical Record, 102:409-439, 1948.
Kellgren JH: Observations on referred pain arising from muscle. Clinical Science, 3:175, 1938.
Kellgren JH: On the distribution of pain arising from deep somatic structures with charts of segmental pain areas. Clinical Science, 4:35-46, 1939.
Kellgren JH, Samuel EP: The sensitivity and innervation of the articular capsule. Journal of Bone & Joint Surgery, 32(B):84-92, 1950.
Kelso AF, Grant RG, Johnston WL: Use of thermograms to support assessment of somatic dysfunction or effects of osteopathic manipulative treatment: Preliminary report. Journal of the American Osteopathic Association, 82(3):182-185, 1982.
Kessler RM, Hertling D (eds): Management of Common Musculoskeletal Disorders. Philadelphia, Harper & Row, 1983, pp 27-30, 91-98.
Kirby JD: The technique of nerve tracing. The Chirogram, June 1976.
Kitzinger E: Vertebrogenic syndromes and nondrug treatment. International Journal of Chinese Medicine, 1(3):3-7, September 1984.
Korr IM: The spinal cord as organizer of disease processes: Some preliminary perspectives. Journal of the American Osteopathic Association, September 1976.
Kraus H: Muscular aspects of oral dysfunction. In Gelb H (ed): Clinical Management of Head, Neck and TMJ Pain and Dysfunction. Philadelphia, W.B. Saunders, 1977.
Krupp MA, Chatton MJ, Werdegar D: Current Medical Diagnosis & Treatment 1985. Los Altos, CA, Lange Medical, 1985, 581-630.
Krupp MA, Tierney LM Jr, Jawetz E, Roe RL, Camargo CA: Physician's Handbook, ed 21. Los Altos, CA, Lange Medical, 1985, pp 34-53.
Kunert W: Functional disorders of internal organs due to vertebral lesions. Ciba Symposium, 13(3), 1965.
Kuntz A: Anatomic and physiologic properties of cutaneo-visceral vasomotor reflex arcs. Journal of Neurophysiology, 8:421-429, 1943.
Kuntz A: The Autonomic Nervous System. Philadelphia, Lea & Febiger, 1949, pp 22, 486.
Lamb DW: The neurology of spinal pain. Physical Therapy, 59:971-973, 1979.
Laufenberg PC: New dimensions in neurologic evaluation. The Digest of Chiropractic Economics, pp 10-12, 105-106, May/June 1979.
Livingston MC: Spinal manipulation in medical practice: A century of ignorance. Medical Journal of Australia, 1:552-554, 1968.
Lundeberg T: Long-term results of vibratory stimulation as a pain relieving measure for chronic pain. Pain, 20(1):13-23, 1984.
Lundeberg T: Naloxone does not reverse the pain-reducing effect of vibratory stimulation. Acta Anaesthesiologica Scandinavica, 29:212-216, 1985.
Lundeberg T, Nordemar R, Ottoson D: Pain alleviation by vibratory stimulation. Pain, 20(1):25-44, 1984.
Learmouth JR: Neurosurgery in the treatment of diseases of the urinary bladder, II: treatment of visceral pain. Journal of Urology, 26:13, 1931.
Leavitt F, Garron DC, Whisler WW, Sheinkop MB: Affective and sensory dimensions of back pain. Pain, 4:273-281, 1978.
Lewis T: Suggestions relating to the study of somatic pain. British Medical Journal, 1:321-327, 1938.
Lynch MK, Kessler RM: Pain. In Kessler RM, Hertling D (eds): Management of Common Musculoskeletal Disorders. Philadelphia, Harper & Row, 1983, pp 51-72.
MacDonald ML, Stanish WD: Neuromuscular system. In Scott WN, Nisonson B, Nicholas JA: Principles of Sports Medicine. Baltimore, Williams & Wilkins, 1984, pp 15-24.
Mayer DJ, Price DD: Central nervous system mechanisms of analgesia. Pain, 2:379-404, 1976.
McCullough JW: An overview of medical thermographic imaging. ACA Journal of Chiropractic, 21(4):22-23, 1984.
McKenzie RA: The Lumbar Spine. Upper Hutt, New Zealand, Spinal Publications Ltd, 1981, pp 9-14.
McNaught AB, Callander R: Illustrated Physiology, ed 4. New York, Churchill Livingstone, 1983, pp 244-245.
Melzack R, et al: Neurophysiological foundations of pain. In Sternbach RA (ed): The Psychology of Pain. New York, Raven Press, 1978, pp 1-26.
Melzack R: The gate theory revisited. In LeRoy PL (ed): Current Concepts in the Management of Chronic Pain. Miami, Sympopsia Specialists, 1977.
Melzack R, Stillwell DM, Fox EJ: Trigger points and acupuncture points for pain: Correlations and implications. Pain, 3:3-23, 1977
Melzack R, Wall PD: On the nature of cutaneous sensory mechanisms. Brain, 85: 331-356, 1962.
Melzack R, Wall PD: Pain mechanisms: A new theory. Science, 150:971-979, 1965.
Merskey H, et al: The lateralization of pain. Pain, 7:271-280, 1979.
Mitchell GAG: Cardiovascular Innervation. London, Livingstone, 1956, p 356.
Molina-Negro P, Bertrand RA, Martin E, Gioani Y: The role of the vestibular system in relation to muscle and postural reflexes in man. Acta Oto-Laryngologica, 89:524-533, 1980.
Nathan PW: The gate-control theory of pain A critical review. Brain, 99:123-158, 1976.
Normell LA, Wallin BG: Sympathetic skin nerve activity and skin temperature changes in man. Acta Physiologica Scandinavica, 9(3):417-426, 1974.
Olson WH, Brumback RA, Gascon G, Christoferson LA: Practical Neurology for the Primary Care Physician. Springfield, IL, Charles C. Thomas, 1981, pp 1-32, 98-114, 157-178, 236-269.
Pastan RS: Diffuse musculoskeletal pain. Diagnosis, 5(3):42-46, 1983.
Perl ER: Mode of action of nociceptors. In Hirsch C, et al (eds): Cervical Pain. Oxford, England, Pergamon, 1972, pp 157-163.
Phillips RB: The irritable reflex mechanism. ACA Journal of Chiropractic, January 1974.
Pierce WV, Stillwagon G: Charting and interpreting skin temperature differential patterns. Digest of Chiropractic Economics, 12(5):37-39, 1970.
Pleet AB, Massey EW: Notalgia paresthetica. Neurology, 28:1310-1311, 1978.
Poole PB: Considerations of neurogenic pain. Ortho-Briefs, Council on Chiropractic Orthopedics of the American Chiropractic Association, Fall 1982.
Pottenger FM: Symptoms of Visceral Disease, ed 6. St. Louis, C.V. Mosby, 1944, pp 183, 187, 189, 266.
Prichard JW: Nerve. In Albright JA, Brand RA (eds): The Scientific Basis of Orthopaedics. New York, Appleton-Century-Crofts, 1979, pp 396-399, 405-408.
Raphael R: personal correspondence. Portland, Oregon, 1987.
Rask MR: Thermography of the human spine: Study of 150 cases with back pain and sciatica. Orthopaedic Review, 8(11):73-82, 1979.
Reiter L: Apophyseal joint functional anatomy and experimental findings: A literature review. PCC Research Form, 1(2):49-52, Winter 1985.
Research Group of Acupuncture Anesthesia; Peking Medical College: Effect of acupuncture on pain threshold on human skin. Chinese Medical Journal, 3:151-157, 1973.
Riley LH Jr, Richter CP: Uses of the electrical skin resistance method in the study of patients with neck and upper extremity pain. John Hopkins Medical Journal, 137(2):69-74, 1975.
Sandman KB: Myofascial pain syndromes: their mechanism, diagnosis and treatment. Journal of Manipulative and Physiological Therapeutics, 4(3):135-139, September 1981.
Schafer RC: Basic Chiropractic ParaprofessionaL Manual. Des Moines, IA, American Chiropractic Association, 1978, pp XV:39-42.
Schafer RC: Chiropractic Physical and Spinal Diagnosis. Oklahoma City, American Chiropractic Academic Press, 1980, pp II:20, 33; III:4-14; V:1-17, 26-27, 34-54.
Schafer RC: Chiropractic Management of Sports and Recreational Injuries. Baltimore, Williams & Wilkins, 1982, pp 91, 170-171, 231-252, 262-265, 281.
Schafer RC: Clinical Biomechanics: Musculoskeletal Actions and Reactions. Baltimore, Williams & Wilkins, 1983, pp 137, 140-185, 221-223, 240-243, 246-249, 397-401, 405-408, 427-428.
Schafer RC (ed): Basic Chiropractic Procedural Manual, ed 4. Arlington, VA, American Chiropractic Association, 1984, pp 39-40, 73-80.
Schafer RC: Physical Diagnosis: Procedures and Methodology in Chiropractic Practice. Arlington, VA, American Chiropractic Association. Released in 1987. Chapter 4, The Clinical Interpretation of Pain; Chapter 7, Basic Neurologic Considerations.
Schafer RC: Symptomatology and Differential Diagnosis. Arlington, VA, American Chiropractic Association, 1986, pp 8-9, 203-288, 326-340.
Shepard RS: Human Physiology Examination Review. New York, Arco, 1975, pp 321-322.
Simons DG: Myofascial trigger points: a need for understanding. Archives of Physical Medicine and Rehabilitation, Vol 62, March 1981.
Sinclair DC, Weddell G, Feindel WH: Referred pain and associated phenomena. Brain, 7:184-211, 1948.
Sites JS: Meralgia paresthetica: A case report. Research Forum, 2(2):55-58, 1986.
Sola AE: Myofascial trigger point therapy. Medical Times, January 1982, pp 70-77.
Stein HI: Diagnosis and treatment of disturbances of the vestibular system. Journal of the American Medical Association, 5(8):407-412, 1952.
Stejskal L: Five suggestions for manipulative treatment based upon a study of postural reflexes. In Lewit K, Gutmann G (eds): Rehabilitcia. Proceedings of the IVth Congress. Prague, International Federation of Manual Medicine, supplement 10-11:171-176, 1975.
Stoddard A: Manual of Osteopathic Technique, ed 2. London, Hutchinson, 1962.
Sutton DC, Lueth HC: Pain. AMA Archives of Internal Medicine, 45:827, 1930.
Szasz TS: The psychology of persistent pain. In Soulairac A, et al: Pain. London, Academic Press, 1968, pp 93-113.
Terrett ACJ, Vernon H: Manipulation and pain tolerance: A controlled study of the effect of spinal manipulation on paraspinal cutaneous pain tolerance levels. American Journal of Physical Medicine, 63(5):217-224.
Thomas AH: The anatomy, physiology, and psychology of pain. Journal of Occupational Medicine, 7:526-534, 1965.
Tobis JS, Hoebler FK: Musculoskeletal manipulation in the treatment of low back pain. Bulletin of the New York Academy of Medicine, 59(7):600-608, 1983.
Tran TA: Essentials of the neurological examination: Tests for cerebral function. The Chirogram, July 1975.
Travell JG, Bigelow NH: Referred somatic pain does not follow a simple segmental pattern. Federation Proceedings, 5:106, 1946.
Travell JG: Myofascial trigger points: clinical view. In Bonica JJ, Albe-Fessard D (eds): Advances in Pain Research and Therapy. New York, Raven Press, 1976.
Travell JG: Referred pain from skeletal muscle. New York State Journal of Medicine, February 1, 1955.
Travell JG, Rinzler SH: The myofascial genesis of pain. Postgraduate Medicine, 11:425-434, 1952.
Travell JG, Simons DG: Myofascial Pain and Dysfunction: The Trigger Point Manual. Baltimore, Williams & Wilkins, 1983, p 663.
Vallbona C: Bodily responses to immobilization. In Kottke FJ, Stillwell GK, Lehman JF (eds): Krusen's Handbook of Physical Medicine and Rehabilitation. London, W.B. Saunders, 1982.
Ufberg SH: Muscular nociception. ACA Journal of Chiropractic, 14:93-96, September 1980.
Varni JG, Doerr HO, Franklin JR: Bilateral differences in skin resistance and vasomotor activity. Psychophysiology, 8(3):390-400, 1971.
Vazuka FA: Essentials of the Neurological Examination. Philadelphia, Smith, Kline, & French Laboratories, 1962.
Wall PD, Sweet WH: Temporary abolition of pain in man. Science, 155:108, 1967.
Wall PD: Gate control theory of pain mechanisms: a re-examination and re-statement. Brain, 101:1-18, 1978.
White JC, et al: Cardiac innervation: Experimental and clinical studies. AMA Archives of Surgery, 26:765, 1933.
Willer JC, Boureau F, Albe-Fessard D: Role of large diameter cutaneous afferents in transmission of nociceptive messages: Electrophysiological study in man. Brain Research, 152:358-364, 1978.
Wyke BD: Articular neurology and manipulative therapy. In Aspects of Manipulative Therapy. Proceedings of Multidisciplinary International Conference on Manipulative Therapy. Melbourne, Lincoln Institute of Health Sciences, Carlton, Victoria, Australia, August 1979, pp 67-72.
Wyke BD: Neurological aspects of pain therapy: A review of some current concepts. In Swerdlow M (ed): The Therapy of Pain. Lancaster, England, MTP Press, 1981.
Wyke BD: Neurological mechanisms in the experience of pain. Acupuncture and Electro-Therapeutical Research International Journal, 4:27-35, 1979.
Wyke BD: Neurological mechanisms in spasticity: A brief review of some current concepts. Physiotherapy, 62(10):316-319, 1976.
Zimmermann M: Physiological mechanisms in chronic pain. In Pain and Society, report of Dahlem Workshop, Berlin, November 1979. Deerfield Park, FL, Basle, 1980, pp 283-298.