

Outcomes of Usual Chiropractic.
The OUCH Randomized Controlled Trial of Adverse EventsThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Spine (Phila Pa 1976). 2013 (Sep 15); 38 (20): 1723–1729 ~ FULL TEXT
Bruce F Walker, Jeffrey J Hebert, Norman J Stomski, Brenton R Clarke,
Ross S Bowden, Barrett Losco, Simon D French
School of Health Professions
Murdoch University, Murdoch, Australia
STUDY DESIGN: Blinded parallel-group randomized controlled trial.
OBJECTIVE: Establish the frequency and severity of adverse effects from short-term usual chiropractic treatment of the spine when compared with a sham treatment group.
SUMMARY OF BACKGROUND DATA: Previous studies have demonstrated that adverse events occur during chiropractic treatment. However, as a result of design limitations in previous studies, particularly the lack of sham-controlled randomized trials, understanding of these adverse events and their relation with chiropractic treatment is suboptimal.
METHODS: We conducted a trial to examine the occurrence of adverse events resulting from chiropractic treatment. It was conducted across 12 chiropractic clinics in Perth, Western Australia. The participants comprised 183 adults, aged 20 to 85 years, with spinal pain. Ninety-two participants received individualized care consistent with the chiropractors' usual treatment approach; 91 participants received a sham intervention. Each participant received 2 treatments.
RESULTS: Completed adverse questionnaires were returned by 94.5% of the participants after appointment 1 and 91.3% after appointment 2. Thirty-three percent of the sham group and 42% of the usual care group reported at least 1 adverse event. Common adverse events were:
increased pain sham 29% usual care 36% muscle stiffness sham 29% usual care 37% headache sham 17% usual care 9% The relative risk (RR) was not significant for adverse event occurrence (RR = 1.24; 95% CI: 0.85-1.81), occurrence of severe adverse events (RR = 1.9; 95% CI: 0.98-3.99), adverse event onset (RR = 0.16; 95% CI: 0.02-1.34), or adverse event duration (RR = 1.13; 95% CI: 0.59-2.18). No serious adverse events were reported.
CONCLUSION: A substantial proportion of adverse events after chiropractic treatment may result from natural history variation and nonspecific effects.
Key words: back pain, neck pain, adverse effects, chiropractic, manipulation, spinal, randomized controlled trial, clinical trial, placebo effects, nocebo effects.
Level of Evidence: 2 Level of Evidence: 2
This paper reports the results of the proposed study:
Outcomes of Usual Chiropractic, Harm & Efficacy, the OUCH Study:
Study Protocol for a Randomized Controlled Trial
Trials. 2011 (Oct 31); 12: 235
From the FULL TEXT Article:
Background
Chiropractic therapy is commonly used to manage musculoskeletal conditions in high-income countries. [1, 2] The occurrence of adverse events resulting from chiropractic treatment is of considerable interest to chiropractors and the general public. Most adverse events associated with chiropractic treatment are mild, short lasting, and typical of musculoskeletal condition symptoms. [3–11] However, due to a lack of appropriately designed studies, particularly sham-controlled trials, there are differences in views about what constitutes a chiropractic treatment-related adverse event.
The occurrence of adverse events after chiropractic treatment has been examined in 1 randomized controlled trial, [3] 5 prospective single-arm studies, [4–8] and 3 retrospective studies. [9–11] These studies reported that 34% to 61% of participants experienced at least 1 adverse event. [4–8] Most events were benign, transient, and typically consisted of increased pain, muscle stiffness, tiredness, headache, and radiating discomfort. [4–8] Less common events were dizziness, nausea, tinnitus, and impaired vision. More serious adverse events associated with chiropractic treatment, including disc injury, cauda equina syndrome, fracture, and stroke, have been reported but the rate has not been robustly established. [9, 11–13]
Predictors of adverse events have been identified in 4 previous studies of chiropractic treatment. [6–8, 14] These predictors included female sex, [6, 8] age (27–46 yr), [14] high-velocity manipulation (compared with low-velocity mobilization), [14] first treatment session, [6] medication use, [14] more than 1 region treated or [14] only thoracic spine treated, and" treatment including cervical rotation, [8] work status, [8] and general practitioner visit in previous 6 months. [8] Notably, none of the identified predictors have been found to influence adverse events consistently across studies.
Several limitations constrain the findings of previous studies. In the prospective studies, the chiropractors providing treatment also administered the questionnaires, which possibly resulted in underreporting of adverse events. [4–8] In addition, recall bias may also have led to an underestimation of adverse events in the retrospective studies. [15] Moreover, all previous studies lacked a sham intervention, which may have resulted in an overestimation of adverse events as some events could have been associated either with natural history or with nonspecific effects. Therefore, the estimates of adverse events resulting from the specific effect of treatment are not known. What is known is that adverse events after chiropractic treatment of spinal pain range from trivial to catastrophic. Given these facts plus the limitations of previous studies, additional research is required to develop a more accurate safety profile of chiropractic treatment.
We assessed whether common adverse events differed between participants who received usual chiropractic treatment or a sham intervention for spinal pain. We also captured information about the types, severity, onset, and duration of adverse events.
MATERIALS AND METHODS
Study Design
The complete protocol for this study has been published elsewhere and it was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12611000542998). [16] We conducted this parallel-group randomized controlled trial during 3–month period between August 2012 and October 2012 in 12 Western Australia metropolitan chiropractic centers. Participants were allocated to either(1) a sham group including typical interaction with the practitioner or
(2) a usual care group providing individualized chiropractic treatment.Participants and outcome assessors were blinded to group allocation. All participants provided demographic and clinical characteristics at entry to the trial, adverse events were evaluated 2 days after each of the 2 treatments, and blinding was assessed at 2–week follow-up.
Recruitment
Participants were recruited from newspaper advertisements. All included participants were 18 years of age or older, English literate, had nonspecific spinal pain (neck, midback, or low back pain) of at least 1–week duration, and scored at least 3 on the Numerical Rating Scale (NRS) for pain [17] and 12 on the Functional Rating Index. [18]
We excluded participants who thought that they would be unable to tolerate any intervention potentially delivered in usual chiropractic, including: manipulation, mobilization, traction, soft tissue massage, and physical modalities.
We also excluded participants who had spinal pain related to cancer or infection, spinal fracture, spondyloarthropathy, known osteoporosis, progressive upper or lower limb weakness, symptoms of cauda equina syndrome or other significant neurological condition, disc herniation, cardiovascular disease, uncontrolled hypertension, cognitive impairment, blood coagulation disorder, previous spinal surgery, previous history of stroke or transient ischémie attacks, pacemaker or other electrical device implanted, and a current compensation claim.
Intervention Components
Each participant was assigned to either a sham group or a usual chiropractic group, whereupon 2 treatments were delivered with approximately 1 week between treatments. The chiropractors delivering either the sham or usual chiropractic treatment attended a training session that provided instruction about the trial and how to undertake their respective treatments. To be eligible, all chiropractors need to practice within the Western Australian metropolitan region and were required to be registered with the Chiropractic Board of Australia.Sham group: the practitioners in this group comprised 4 registered chiropractors who administered at each visit (a) detuned ultrasound [19]; (b) an Activator instrument, [20] a handheld device that delivers a low impulse wound to lowest output and administered on the back randomly through a tongue depressor to disperse any remaining force; and (c) a randomly placed hand on the spine while ultrasound was administered to give a "hands-on" experience.
Usual care: the practitioners in this group consisted of 8 registered chiropractors who provided individualized chiropractic care consistent with their usual treatment approach. The only condition that may have influenced the chiropractors' usual treatment approach was a request to adhere to Australian imaging guidelines. [21]Randomization
A statistician used a random number generator to create a permuted block randomization list with variable block sizes of 8 to 12. The group assignment was placed in sequentially numbered, opaque, sealed, envelopes. Staff not administering baseline or outcomes measures opened the envelope and allocated participants to the groups.
Informed Consent and Blinding
Murdoch University's Human Research Committee granted ethics approval for the study (2011/109). All participants provided written informed consent. Research staff administering baseline measures and outcome measures were also blinded to group allocation.
Outcome Assessment
Our primary outcome was adverse event occurrence. We inquired about occurrence of adverse events by using a questionnaire that was informed by previous research. [4–8] The occurrence of an adverse event was assessed by an item stating "Did you experience any new unwelcome symptoms or an increase of your presenting symptoms during the first 48 hours (2 d) after treatment?"(yes/no). Further details about any adverse events were obtained by 4 open and 4 close-ended questions about increased pain, muscular stiffness, headache, and radiating discomfort. Each question inquired about the intensity (11–point NRS), onset (5 categorical responses ranging from <10 min to >24 hr), and duration (5 categorical responses ranging from <1 hr to >2 d). Participants completed the adverse event questionnaire 2 days after each appointment and returned it by postal mail. The period between the appointment and completion of the questionnaire was selected to allow sufficient time for the manifestation of adverse events.
Statistical Analysis
Our a priori sample size estimation was based on detecting a 20% difference in the occurrence of adverse events between the 2 treatment groups. Assuming an a level of 0.05 and a 2–tailed hypothesis, recruiting 180 participants (90 per group) would provide 80% power to detect a difference of at least this magnitude. We thought a 20% difference to be a conservative estimate, because previous studies have shown that about half of those who receive chiropractic treatment experience adverse events, [4–8] whereas in a previous study, the de-tuiied ultrasound adverse event rate was less than 10%. [19]
All data were reported descriptively. We classified adverse event intensity as NRS score of 1 to 3 = mild; NRS score of 4 to 6 = moderate; and NRS score of 7 to 10 = severe. Missing data were handled with multiple imputation. [22] We undertook intention to treat and available case analyses. We calculated relative risk (RR) statistics with 95% confidence intervals (CIs) for the following outcomes: adverse event occurrence, severe adverse event occurrence (severe or not severe), adverse event duration (>24 hr or <24 hr), and time to onset of adverse event (>24 hr or <24 hr). When more than 1 adverse event was reported, the most intense adverse event was identified by the highest NRS score and used for all analyses. Blinding was evaluated by using the Bang Index. [23]
RESULTS
Study Population
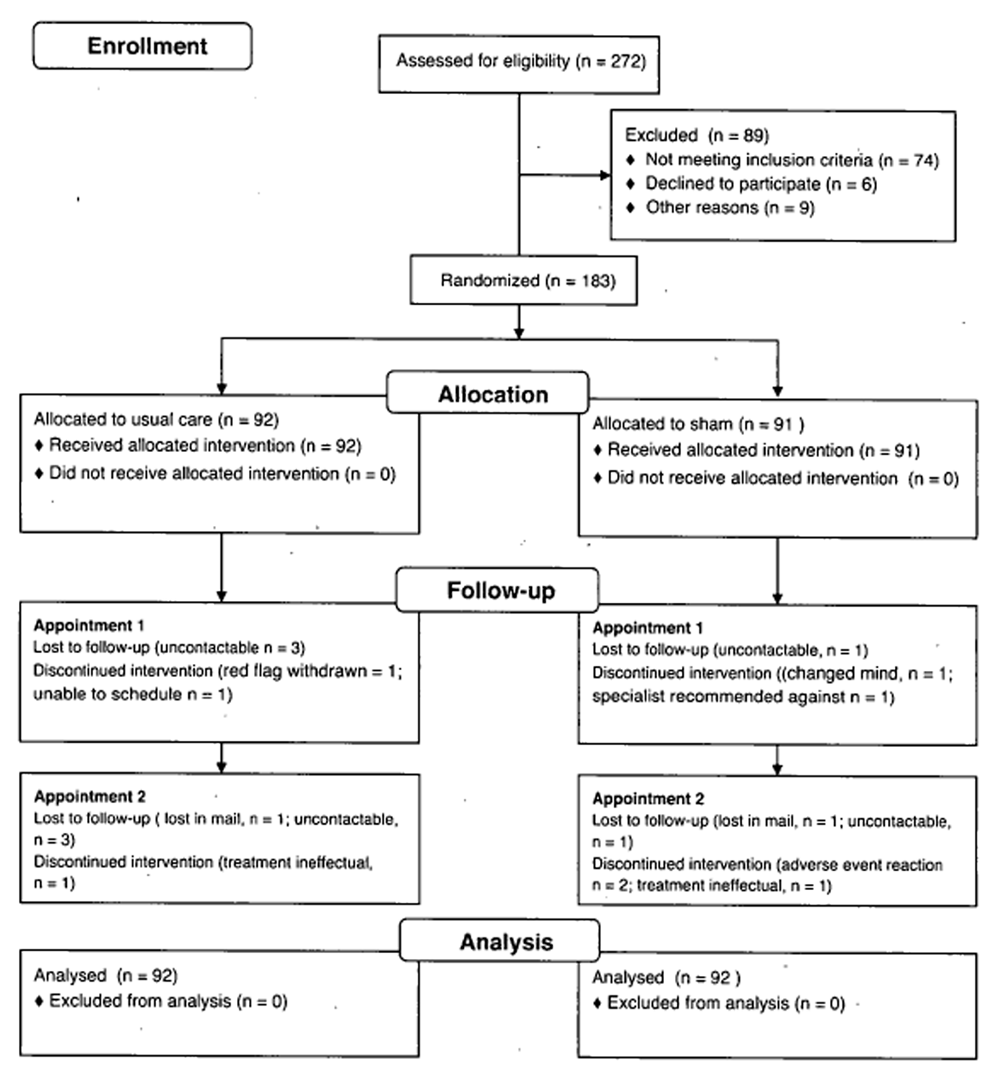
Figure 1

Table 1 Participants We screened 272 potential participants, of whom 198 satisfied selection criteria. Between August to September 2012, 183 participants were randomized to either a sham intervention group (n = 91) or a usual care group (n = 92). Participant flow through the study is displayed in Figure 1.
At baseline, there were no important differences in demographic details or clinical characteristics, apart from the higher percentage of females in the usual care group (42% compared with 31%). Participant baseline demographic details and clinical characteristics are displayed in Table 1.
The vast majority of patients (98%) had experienced spinal pain for more than 3 months. Three quarters had experienced spinal pain for more than 5 years (75% in sham group; 73% in usual care). The overwhelming majority indicated that it had been more than 1 year since last experiencing a 4–week pain-free period (89% in sham group; 98% in usual care group), and more than two-thirds indicated that it had been more than 1 year since their last 1–week pain-free period (71% in sham group; 68% in usual care group).
Chiropractors
In total, 8 chiropractors (5 males) delivered usual care and 4 chiropractors (2 males) provided the sham intervention. Chiropractors had on average 12.6 (SD, 2.3) years' clinical experience, whereas chiropractors in the sham group had on average 3.6 (SD, 1.1) years' clinical experience. About 3 quarters of the chiropractors had obtained their qualifications from Australian universities (8/11). All chiropractors were registered and practiced full time.
Type of Therapies Used in Usual Care Group

Table 2

Table 3

Table 4

Table 5 Details about therapies used are displayed in Table 2.
Adverse Events: Types, Severity, Onset, and Duration
Adverse Events In total, 33% of the sham group and 42% of the usual care group reported at least 1 adverse event after either appointment.
The types, onset, and duration of adverse events are displayed in Tables 3 to 5. Most participants who experienced an adverse event reported more than 1 event (71% in sham group; 77% in usual care group). In total, 198 adverse events were reported (92 in sham group; 106 in usual care group). Common adverse events were increased pain (29% in sham group; 36% in usual care group), muscle stiffness (29% in sham group; 37% in usual care group), headache (17% in sham group; 9% in usual care group), and radiating discomfort (15% in sham group; 15% in usual care group). Less common adverse events, each of which accounted for less than 5% of adverse events in the respective groups, included dizziness, muscle spasm, fatigue, sleeplessness, and joint swelling. The RR for experiencing an adverse event was not significant (RR = 1.24; 95% CI: 0.85–1.81).
Across both appointments, adverse event intensity was most comrrionly moderate in the sham group (50%; n = 46/92) and either moderate (37%; n = 39/106) or severe (37%; n = 39/106) in the usual care group. The rate of severe adverse events was not different between the groups (RR = 1.9; 95% CI: 0.98–3.99).
Across both appointments, 79% of the adverse events reported in the sham group (73/92) and 84% in the usual care group (89/106) occurred within 24 hours. The RR for adverse event onset was not significant (RR = 0.16; 95% CI: 0.02–1.34).
For duration and across both appointments, 51% of the adverse events in the sham group (47/92) and 41% in the usual care group persisted for less than 24 hours (44/106). The RR for the duration of adverse events was not significant (RR = 1.13; 95% CI: 0.59–2.18).
The intention to treat and available case analyses provided consistent results with 1 exception. Regarding the occurrence of serious adverse events, the RR estimates were 2.02 (95% CI: 1.01–4.07) in the available case analysis and 1.97 (95% CI: 0.98–4.0) in the intention-to-treat analysis.
Blinding
The proportion of participants who identified the assigned treatment was 67% for the sham group and 85% for the usual care group. Bang Index values showed that 25% of the sham group (95% CI: 10%^0%) and 61% of the usual care group (95% CI: 48%–74%) guessed correctly beyond what would be expected by chance. [23]
DISCUSSION
This was the first study to use a sham-controlled design to examine adverse events after chiropractic treatment. A substantial proportion of adverse events experienced during chiropractic care for spinal pain may be the result of natural symptom ñuctuation or from nonspecific effects. Adverse events were common in both the usual chiropractic care and sham groups, but no important differences were seen between the groups and no serious adverse events were reported. However, although very similar, the estimates of severe adverse event risk arising from the intention to treat and available case analyses resulted in conflicting conclusions. Although the intention-to-treat approach was our primary analysis, we cannot rule out the possibility of increased risk of severe adverse event occurrence with chiropractic treatment compared with sham therapy. The adverse event rate reported by the usual care group in this study was consistent with rates reported by previous studies (42% compared with 34%–61 %). [4–8] Moreover, the finding that most adverse events were benign and transitory is also consistent with other chiropractic studies. [4–8] The proportion of adverse events in these previous studies due to other effects such as natural history or nonspecific effects remains indeterminable because none of the studies used a sham arm. However, the results of our study suggest that many adverse events experienced after chiropractic treatment result from either natural history variation or nonspecific effects. Some may view these results as evidence that chiropractic treatment is essentially an entirely benign intervention, but it more likely reflects that our study was underpowered to detect a statistically significant difference between groups.
Studies of interventions other than manual therapies have also associated nonspecific effects with adverse events. [24–26] Interestingly, some studies demonstrated that the adverse events reported by participants in either the placebo or the sham arm mirror the adverse events in the active intervention arm. [27–31] This association has been attributed in part to the effect of patient expectancy, which typically depends on details about possible adverse events conveyed through information sheets, consent forms, or the investigators' behavior. [24–31] Other studies have shown that a strong aversion to experience an adverse event, coupled with a sense of helplessness about avoiding it, may evoke negative emotions and subsequent reductions in beneficial nonspecific effects. [32] It then seems likely that an expectation of adverse events coupled with not wanting to experience adverse events may promote nonspecific effects that contribute to adverse events. [32]
Careful consideration should be given to how the information from this study is presented to patients. As numerous studies have shown, disclosing information about the risks of adverse events increases the likelihood of adverse events occurring. [24–31] Conversely, framing information about adverse events in positive terms (noting that most patients did not experience an adverse event), rather than negative terms (detailing the minority who experienced an adverse event), can lead to a lower adverse event rate. [33, 34] In light of this, we recommend that a form of words be developed for chiropractic patients that accurately reflects the results of our study about potential common adverse events without unnecessarily engendering fear.
Conducting this study under typical clinical conditions enhances the external validity of our findings. However, this study was powered to detect a 20% difference in adverse events; therefore, we were underpowered to detect the magnitude of between-group differences observed in our sample of participants. In terms of internal validity, we did not measure either anxiety or depression, and it should be noted that it is possible that differing levels of anxiety or depression may have influenced the participants' experience of adverse events in either group. However, there were no important differences between groups in the psychosocial characteristics we assessed at baseline including the Pain Catastrophizing Scale and Fear-Avoidance Beliefs Questionnaire. Nevertheless, it should be noted that it is possible that differing levels of anxiety or depression may have influenced the participants' experience of adverse events in either group.
We endeavored to blind study participants to group allocation but were unsuccessful. This was probably due to inherent difficulties in finding an adequate sham intervention to use in a chiropractic trial or indeed any type of randomized controlled trial. [35] The lack of blinding success may have influenced the reporting of adverse events. In particular, the adverse event rate may have been underreported in the sham group as the majority thought they were receiving an inactive intervention.
Finally, the chiropractors providing usual care were more experienced than chiropractors delivering the sham intervention. However, experience is unlikely to affect an inert sham intervention such as that delivered in this trial. It may be the case that the more experienced chiropractors had better interpersonal skills, which may have reduced the number of adverse events attributable to nonspecific effects.
CONCLUSION
Additional studies of larger and more diverse populations are warranted. Such studies should be powered to detect the magnitude of between-group differences observed in this trial and include a wait list arm to account for natural variation in spinal pain.
Key Points
Adverse events resulting from chiropractic are common.
Most adverse events resulting from chiropractic are benign and transitory.
A substantial proportion of adverse events resulting from chiropractic
seem to be due to nonspecific effects.Acknowledgments
The authors gratefully acknowledge the assistance of Professor Charlotte Leboeuf-Yde in reviewing the draft article, Christine Losco for assistance with recruitment and data collection, and the participating chiropractors.
References:
Walker BF, Müller R, Grant WD.
Low back pain in Australian adults. Health provider utilization and care seeking.
J Manipulative Physiol Ther 2004;27:327-35.Lambeek LC, van Tulder MW, Swinkels IC, et al.
The trend in total cost of back pain in The Netherlands in the period 2002 to 2007.
Spine 2011;36:1050-8.Hurwitz EL, Morgenstern H, Vassilaki M, et al.
Adverse reactions to chiropractic treatment and their effects on satisfaction and clinical outcomes among patients enrolled in the UCLA Neck Pain Study.
J Manipulative Physiol Ther 2004;27:16-25.Barrett AJ, Breen AC.
Adverse effects of spinal manipulation.
J Royal Soc Med 2000;93:258-9.Cagnie B, Vinck E, Beernaert A, et al.
How Common Are Side Effects of Spinal Manipulation And Can These Side Effects Be Predicted?
Manual Therapy 2004 (Aug); 9 (3): 151–156Leboeuf-Yde C, Hennius B, Rudberg E, et al.
Side effects of chiropractic treatment: a prospective study.
J Manipulative Physiol Ther 1997;20:511-5.Rubinstein S, Leboeuf-Yde C, Knol D, et al.
Predictors of adverse events following chiropractic care for patients with neck pain.
J Manipulative Physiol Ther 2008;31:94-103.Senstad O, Leboeuf-Yde C, Borchgrevink C.
Frequency and characteristics of side effects of spinal manipulative therapy.
Spine 1996;22:435-40.Carey PF.
A report on the occurrence of cerebral vascular accidents in chiropractic practice.
J Can Chiropr Assoc 1993;37:104-6.Klougart N, Leboeuf-Yde C, Rasmussen LR.
Safety in Chiropractic Practice Part II: Treatment to the Upper Neck
and the Rate of Cerebrovascular Incidents
J Manipulative Physiol Ther 1996 (Nov); 19 (9): 563–569Rezai M, Cote P, Cassidy JD, et al.
The association between prevalent neck pain and health-related quality of life: a cross-sectional analysis.
Eur Spine J 2009;18:371-81.Coulter, I, Hurwitz, E, Adams, A et al.
The Appropriateness of Manipulation and Mobilization
of the Cervical Spine PDF
Santa Monica, CA: RAND Corporation; 1996 Document No. MR-781-CR.Hebert JJ, Stomski NJ, French SD, et al.
Serious Adverse Events and Spinal Manipulative Therapy
of the Low Back Region: A Systematic Review of Cases
J Manipulative Physiol Ther 2015 (Nov); 38 (9): 677–691Hurwitz EL, Morgenstern H, Vassilaki M, et al.
Frequency and clinical predictors of adverse reactions to chiropractic care in the UCLA neck pain study.
Spine (Phila Pa 1976) 2005;30: 1477-84.Hassan E.
Recall bias can be a threat to retrospective and prospective research designs.
Intern J Epidemiol 2006. doi:10.5580/2732.Walker B, Losco B, Clarke B, et al.
Outcomes of usual chiropractic, harm &c efficacy, the ouch study: study protocol for a randomized controlled trial.
Trials 2011;12:235.Katz J, Melzack R.
Measurement of pain.
Surg Clin North Am 2010;79:231-52.Feise RJ, Michael Menke J.
Functional rating index: a new valid and reliable instrument to measure the magnitude of clinical change in spinal conditions.
Spine (Phila Pa 1976) 2001;26:78-86; discussion 87.Costa LO, Maher CG, Latimer J, et al.
Motor control exercise for chronic low back pain: a randomized placebo-controlled trial.
Phys Ther 2009;89:1275-86.Activator Methods,
http://www.activator.com. Accessed July 6, 2011.Australian Acute Musculoskeletal Pain Guidelines Group.
Evidence-Based Management of Acute Musculoskeletal Pain.
Canberra: National Library of Australia; 2003.Rubin DB.
Multiple Imputation for Nonresponse in Surveys.
New York, NY: John Wiley & Sons; 1997.Bang N, Ni L, Davis C.
Assessment of blinding in clinical trials.
Control Clin Trials 2004;25:143-56.Amanzio M, Corazzini LL, Vase L, et al.
A systematic review of adverse events in placebo groups of anti-migraine clinical trials.
Pain 2009;146:261-9.Barsky AJ, Saintfort R, Rogers MP, et al.
Nonspecific medication side effects and the nocebo phenomenon.
JAMA 2002;287: 622-7.Colloca L, Miller FG.
The nocebo effect and its relevance for clinical practice.
Psychosom Med 2011;73:598-603.Myers MG, Cairns JA, Singer J.
The consent form as a possible cause of side effects.
Clin Pharmacol Ther 1987;42:250-3.Lancman ME, Asconape JJ, Craven WJ, et al.
Predictive value of induction of psychogenic seizures by suggestion.
Ann Neurol 1994;3S:359-61.Flaten MA, Simonsen T, Olsen H.
Drug-related information generates placebo and nocebo responses that modify the drug response.
Psychosom Med 1999;61:250-5.Luparello TJ, Leist N, Lourie CH, et al.
The interaction of psychologic stimuli and pharmacologie agents on airway reactivity in asthmatic subjects.
Psychosom Med 1970;32:509-13.Mondaini N, Gontero P, Giubilei G, et al.
Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon?
J Sex Med 2007;4:1708-12.Vase L, Robinson ME, Verne GN, et al.
Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms.
Pain 2005;115:338-47.Miller FG, Colloca L.
The placebo phenomenon and medical ethics: rethinking the relationship between informed consent and risk-benefit assessment.
Theor Med Bioeth 2011; 32: 219-43.Edwards A, Elwyn G, Covey J, et al.
Presenting risk information—a review of the effects of "framing" and other manipulations on patient outcomes.
J Health Commun 2001;6:61-82.Boutron I, Estellat C, Guittet L, et al.
Methods of blinding in reports of randomized controlled trials assessing pharmacologie treatments: a systematic review.
PLoS Med 2006;3:e425.
Return to ADVERSE EVENTS
Return to CHRONIC NECK PAIN
Since 2-24-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |