

Effects of Upper and Lower Cervical Spinal Manipulative Therapy
on Blood Pressure and Heart Rate Variability in Volunteers
and Patients With Neck Pain: A Randomized Controlled,
Cross-Over, Preliminary StudyThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Chiropractic Medicine 2015 (Mar); 14 (1): 1–9 ~ FULL TEXT
OPEN ACCESS Ni Ni Win, MBBS, PhD, Anna Maria S. Jorgensen, PhD,
Yu Sui Chen, PhD, and Michael T. Haneline, DC, MPH
Senior Lecturer, International Medical University,
Chiropractic, School of Health Sciences,
Kuala Lumpur, Malaysia.OBJECTIVE: The aims of this study were to examine autonomic nervous system responses by using heart rate variability analysis (HRV), hemodynamic parameters and numeric pain scale (NPS) when either upper (C1 and C2) or lower (C6 and C7) cervical segments were manipulated in volunteers, and whether such response would be altered in acute mechanical neck pain patients after spinal manipulative therapy (SMT).
METHODS: A randomized controlled, cross-over, preliminary study was conducted on 10 asymptomatic normotensive volunteers and 10 normotensive patients complaining of acute neck pain. HRV, blood pressure (BP) and heart rate (HR), and NPS were recorded after upper cervical and lower cervical segments SMT in volunteer and patient groups.
RESULTS: The standard deviation of average normal to normal R-R intervals (SDNN) increased (83.54 ± 22 vs. 105.41 ± 20; P = .02) after upper cervical SMT. The normalized unit of high frequency (nuHF), which shows parasympathetic activity, was predominant (40.18 ± 9 vs. 46.08 ± 14) after upper cervical SMT (P = .03) with a significant decrease (109 ± 10 vs. 98 ± 5) in systolic BP (P = .002). Low frequency to high frequency (LF/HF) ratio, which shows predominance of sympathetic activity increased (1.05 ± 0.7 vs. 1.51 ± 0.5; P = .02) after lower cervical SMT in the healthy volunteers group. However, there was an increase in SDNN (70.48 ± 18 vs. 90.23 ± 20; P = .02 and 75.19 ± 16 vs 97.52 ± 22; P = .01), a decrease in LF/HF ratio (1.33 ± 0.3 vs. 0.81 ± 0.2; P = .001 and 1.22 ± 0.4 vs. 0.86 ± 0.3; P = .02), which was associated with decreased systolic BP (105 ± 10 vs. 95 ± 9; P = .01 and 102 ± 9 vs. 91 ± 10; P = .02) and NPS scores (3 ± 1 vs. 0; P = .01 and 3 ± 1 vs. 1 ± 1; P = .03) following both upper and lower cervical SMT in the patient's group. The baseline HR was 67 ± 9 vs 64 ± 5 (upper cervical) and 65 ± 7 vs 69 ± 11 (lower cervical) in both the healthy volunteer' and patient' groups.
CONCLUSION: Upper cervical SMT enhances dominance of parasympathetic and lower cervical SMT enhances dominance of sympathetic activity in this young volunteer group. However, dominance of parasympathetic activity was found in patients with neck pain that received both upper and lower cervical SMT.
KEYWORDS: Blood pressure; Heart rate; Manipulation; Spinal
From the FULL TEXT Article:
Introduction
Hemodynamic parameters (blood pressure [BP] and heart/pulse rate) have been studied pre and post cervical [1–5] and thoracic manipulation. [2, 6–8] Hemodynamic changes have also been reported following atlas SMT, although the results are somewhat controversial with no significant changes in BP [1] or decrease in BP. [9–11] The results of studies concerning SMT to treat hypertension have not been clinically concluded [12–14] with the bias of hypotensive complication [12] and decreased diastolic BP. [13] The possible underlying mechanisms of spinal manipulative therapy (SMT) and hemodynamic changes, such as autonomic regulation, the effects of the pressor reflex and anatomical abnormal positions, are still poorly understood. [2, 3, 10, 11]
Heart rate variability (HRV) analysis is a noninvasive and widely used technique [15–21] which can provide important clinical information on the autonomic nervous system (ANS) and central nervous system because cyclical variation in heart rate is mediated by central neural mechanisms via baroreceptors and chemoreceptors. [17] HRV is used mostly to predict heart conditions, such as myocardial infarction [22] and to hypothesize the underlying mechanism of anesthetic drugs on hemodynamic changes. [20, 21]
The sympathetic and parasympathetic components of HRV are active over different frequency ranges. The low-frequency component (LF, 0.05– 0.15 Hz) is influenced by both cardiac sympathetic and parasympathetic activity [16] and the high-frequency component (HF, > 0.15 Hz) originates from cardiac parasympathetic activity. [18] Therefore, the LF/HF ratio reflects dominance of cardiac sympathetic activity. [19, 20]
Clinical information on the effect of SMT on the ANS and central nervous system using HRV analysis is sparse. HRV analysis showing cardiac autonomic functions has been conducted following spinal manipulation in the cervical [2, 5] and thoracic regions. [2, 6] As well, HRV analysis was examined in pain-free patients vs patients with lower back pain who received one chiropractic treatment at L5. [23] However, information on hemodynamic parameters and HRV analysis is still lacking in acute mechanical neck pain patients after receiving SMT in the upper (C1 and C2) and lower (C6 and C7) cervical regions.
In this study, two hypotheses were tested: 1) parasympathetic response would be predominant after SMT of an upper cervical segment (C1 and C2 vertebrae) and sympathetic response would be predominant after SMT of a lower cervical segment (C6 and C7) in painless healthy volunteers, and 2) this predominance of parasympathetic or sympathetic responses would be altered in acute mechanical neck pain patients after SMT. The aims of this study are therefore to investigate whether there is a relationship between SMT of the upper versus lower cervical spine and autonomic response in pain free subjects and acute mechanical neck pain patients.
Methods
This study was approved by the International Medical University's (IMU) Joint-Committee of the Research and Ethics Committee with IRB number; IMUJC 181110. Ten asymptomatic normotensive volunteers and ten normotensive patients presenting to an academic chiropractic clinic complaining of acute neck pain (American Society of Anesthesiologist physical status I and II) were recruited with written informed consent in this study. A computer based random number generator produced a list used to assign the participants to receive lower and upper SMT. Acute mechanical neck pain was defined as pain in the anatomic region of the neck for which it is not possible to identify a specific pathological cause of pain and the duration of pain is not over 6 weeks. It generally includes neck pain, with or without pain in the upper limbs, which might or might not interfere with activities of daily living. [24] Participants who had disorders such as diabetes mellitus or an autonomic neuropathy known to affect ANS function were excluded. Participants with a history of cervical fracture or dislocation, anatomical cervical spine abnormality, a history of cervical spine trauma, or a history of benign paroxysmal positional vertigo were also excluded from the study. Five patients in the acute neck pain group were taking B complex vitamins to support their nervous system.
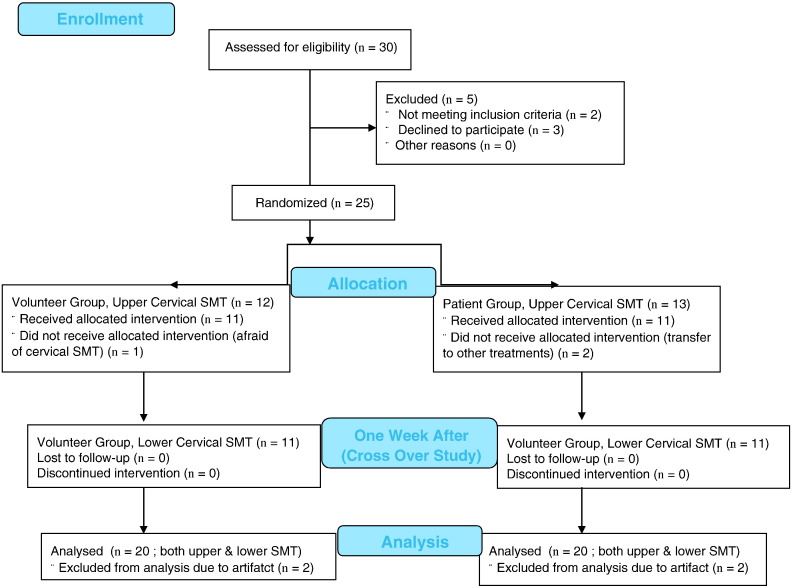
Figure 1 The research assistant, who was blinded to the study participants’ group designation, performed data collection. The demographic data such as age, sex, body weight, basic blood pressure and heart rate measurement were performed on the first visit (week 1). The hemodynamics and HRV data collection started on the participants’ second visit (week 2) after a one week wash out period from the first visit to become familiar with the chiropractic setting and cervical spine SMT. The participants were randomly assigned to receive an upper cervical SMT (C1 or C2) or a lower cervical SMT (C6 or C7) on week 2. As a cross-over study design, after another one week wash out period which followed the second visit (week 3), participants were given upper or lower cervical SMT alternatively, depending on the location of their previous cervical SMT. A summary of the study protocol is shown in Figure 1.
The patient or volunteer rested in a supine position on a chiropractic therapy table for 5 minutes while an automatic blood pressure measuring device was applied. Electrocardiogram (ECG) electrodes (leads I, II, and V5) were attached for HRV analysis. ECG was noninvasively and continuously recorded throughout the study and sampled at 400 Hz using the PowerLab device of ADInstruments (Castle Hill, Australia) and stored on a computer for further offline processing. BP was measured using a digital BP device (Omron digital automatic blood pressure monitor, model HEM-7080, Omron healthcare CO., LTD, Kyoto, Japan) on the left arm of the participant, measured one time pre-SMT and one time post-SMT.
The participants were asked to concentrate on breathing normally (breath in and breath out) during the experiment. The blinded participants’ respiration rate (RR) was counted by a research assistant during hemodynamic and HRV data collection. Experimental room temperature was maintained at 25 °C to provide a comfortable environment. Participants were assessed at approximately the same time of day. The risk of influencing HRV was kept to a minimum by ensuring that the interaction between the participant and the chiropractor was limited to the application of the intervention.
Pain measures include the patients’ perceived level of pain, measured using a Numeric Pain Scale (NPS). Baseline BP, heart rate (HR), HRV and NPS measurements were taken from the participants 5 minutes after resting quietly in a supine position while breathing normally. After taking the control data, a chiropractor who did not participate in data analysis, performed upper or lower cervical spine manipulation using a high velocity, low amplitude, cervical break SMT technique in a supine position. Procedures used to determine where to apply spinal manipulation included observation of the subjects’ head and neck posture in a sitting position, and static and motion palpation. The chiropractor who performed SMT in this study had 30 years of experience in both private practice and clinical teaching. The ECG recording obtained during the period of the manipulation was excluded from the analysis. BP and HRV were recorded for 5 minutes after SMT was completed, while the participant was left undisturbed. NPS was assessed after hemodynamic and HRV data were recorded.
Frequency-domain analysis of HRV using Fast Fourier Transform (FFT) method was carried out using the MLS310/7 HRV module for beat-to-beat interval variation in ECG recordings, which was developed on the LabChartPro, ADInstruments (Castle Hill, Australia). The HRV Module uses a threshold detector to detect the R component from each raw ECG waveform and generate RR interval data. Beats are automatically distinguished by the software and classified into three groups: normal, ectopic or artifact. Artifacts were excluded and included beats in the analysis as real time. 1024–point FFT with Welch window and weighted averaging was used for analysis of each segment to increase accuracy.
The power of the R wave to R wave interval (RRI) (ms2) with normalized unit (nu) of nuLF (0.04–0.15 Hz) and nuHF (0.15–0.4 Hz) bands were calculated to confirm as normal distribution and LF and HF ratio was calculated by using MLU260M/7 LabChartPro b7 multi version upgrade (Win & Mac) software. Time-related components of HRV, including the standard deviation of average normal to normal R-R intervals (SDNN) was also calculated. Data were analyzed and averaged at 5–minute intervals at baseline (before SMT) and after SMT in both lower and upper cervical SMT groups.
The descriptive categorical variable gender was compared between groups by means of the χ2 test. The variables BP, HR, NPS score and normalized units of HRV were compared using paired-samples t-tests for within-group pre and post SMT measures of upper or lower cervical groups, whereas independent-samples t tests were used to assess differences of pre or post SMT data between volunteer and patient groups. Statistical significance was determined as P < .05. The SPSS for Windows (Version 16.0, Chicago, released 2007) was used for statistical analysis.
Results

Table 1

Table 2A

Table 2B

Table 3A

Table 3B The demographic and anthropometric characteristics of the participants (gender, age, body weight and height) are shown in Table 1. The ages ranged from 19 to 23 years and from 18 to 23 years in the volunteer group and in the patient group respectively. Baseline HR and BP were comparable in the volunteer and patient groups (Table 2A, Table 2B), but a statistically significant difference (P = .002) in BP was observed after upper cervical SMT in the volunteer group (– 11 ± 6 mmHg) and patient group (– 13 ± 7 mmHg in post-upper cervical SMT (P = .01) and – 12 ± 8 mmHg in post-lower cervical SMT (P = .02)). NPS was significantly reduced (P = .01) in post-upper cervical SMT and (P = .03) in post-lower cervical SMT in the patient’s group.
In the volunteer group, SDNN was increased (P = .02) after upper cervical SMT and no significant changes after lower cervical SMT (P = .07). nuLF was significantly decreased (P = .01) and nuHF was significantly increased (P = .03) after upper cervical SMT leading to a decrease in LF/HF ratio showing a larger increase in parasympathetic activity when compared with sympathetic activity. In contrast, nuLF was significantly increased (P = .02) and nuHF was significantly decreased (P = .03) after lower cervical SMT leading to an increase in LF/HF ratio showing a large increase in sympathetic activity when compared with parasympathetic activity.
The baseline SDNN in the patient group significantly decreased (83.54 ± 22 vs 70.48 ± 18 in upper cervical (P = .03) and 85.89 ± 22 vs 75.19 ± 16 in the lower cervical (P = .02). Oppositely, LF/HF ratio in the patient group significantly increased (1.33 ± 0.3 vs 1.04 ± 0.6 in upper cervical (P = .02) and 1.22 ± 0.4 vs 1.05 ± 0.7 in lower cervical (P = .01)) compared to the volunteer group (Table 3A, Table 3B). SDNN significantly increased (70.48 ± 18 vs 90.23 ± 20 after upper cervical (P = .02) and 75.19 ± 16 vs 97.52 ± 22 after lower cervical SMT (P = .01).
However, a decrease in LF/HF ratio showing a larger increase in parasympathetic activity when compared with sympathetic activity was found in patients that received both upper and lower cervical SMT.
Discussion
The results of this study demonstrate that parasympathetic response was predominant after SMT applied to an upper cervical segment with a significant decrease in systolic BP. As well, a sympathetic response was predominant after SMT of a lower cervical segment in the healthy volunteer group. However, there was decreased sympathetic action with parasympathetic predominance, which was associated with decreased systolic BP and NPS, following both upper and lower cervical SMT in the patient’s group.
In spite of our findings, hemodynamic and HRV changes after cervical SMT are somewhat controversial. Knutson [5] stated that palpation and vectored atlas SMT caused a significant decrease in systolic BP in patients with putative upper cervical subluxation/joint dysfunction in comparison with resting controls. McKnight and DeBoer [4] reported that both systolic and diastolic BP were statistically significantly lowered after cervical SMT in student volunteer subjects that had subluxations in the cervical spine. Welch and Boone [2] reported that diastolic BP dropped significantly after SMT applied to the C1 and C2 segments.
A lowering of BP has been reported after a single mechanical impulse to the transverse process of atlas in both normotensive [9] and stage 1 hypertensive patients. [10] A significant reduction in BP occurred after application of the mechanical stimulus in the supine posture. [9] However, Ward reported there was no significant BP difference in normotensive college students immediately after atlas manipulation. [1] This contradictory result may have been due to a different manipulative technique used which involved mechanical stimulation using an Activator [11] and a high-velocity low-amplitude spinal manipulation [1]. The timing of data collection in the post-intervention period was 1–min, 10–min, 24–hour post-intervention1 as opposed to 20 and 30 seconds after intervention [9]. In addition, the reduction in BP peaked at 20–second post-stimulation1 and stabilized to pre-intervention level within the 60–second measurement period. In our study, BP was measured 5 minutes after SMT.
In a study in which the SMT group showed a – 17 ± 9 mm Hg reduction in systolic BP compared with a – 3.2 ± 11 mmHg reduction in the control group, Bakris et al. [10] concluded that restoration of atlas alignment was associated with marked and sustained reduction in BP similar to the use of two-drug combination therapy. However, realignment of the atlas does not lower the BP, but may also be part of a systemic homeostatic mechanism not yet understood because the same adjustment that decreased in BP measurements in hypertensive patients also increased in BP measurements to more normal levels in hypotensive patients. [11]
In a study by Holt et al. [25], the direction of BP change was not dependent on the region of the spine manipulated and manipulation to any segment (C1–C7, T1–L2 and L3–S1) in the spine resulted in statically significant average decrease in systolic BP of – 3.9 ± 10.3 mmHg. [25] Dimmick et al. [26] showed that SMT appears to affect the difference in systolic BP between the left and right arms in normotensive subjects. In our study, BP was uniformly measured only in the left arm in both groups.
Yates et al. [8] examined the effects of SMT of the thoracic spine (T1–T5) on BP and the level of anxiety in 21 patients with elevated BP. Blood pressure (both systolic and diastolic) and the level of anxiety significantly decreased post-SMT. [9] A single session of head-neck manipulative manual therapy resulted in decreased tension, anger status, and perceived pain in patients with chronic tension type headache. [27] SMT has been shown to produce concurrent hypoalgesic effects, as revealed by decreased resting pain scores (P = .049). [28] Similarly, NPS scores were decreased after cervical SMT in neck pain patients in our study.
There is controversy among studies concerning SMT to treat hypertension. Specific contact short lever SMT caused a hypotensive effect in a medicated hypertensive patient that led to hypotension complication. [12] The preliminary findings of a study on BP changes in prehypertension or hypertensive stage I African American patients receiving chiropractic care showed that a statistically significant decrease in diastolic BP was observed upon exclusion of four BMI outliers. [13]
There is insufficient low bias evidence to support the use of SMT as a therapy to treat hypertension. [14] There are several theories about the biologic plausibility of SMT lowering BP, including an autonomic nervous system response, particularly its sympathetic component, vascular decompression of cranial nerves, the reduction of aldosterone levels to reduce sodium reuptake in the kidney. [2, 10, 29, 30]
Our result of a decrease in systolic BP follows the results of previous studies [2, 3, 4, 9, 25] and these decreases were observed after SMT in normal normotensive volunteers, those with cervical segmental dysfunction, and mechanical neck pain patients. There are other possible underlying mechanisms for the decreases in BP, such as stimulation of the cervicosympathetic reflex, moderation of muscle tone and elimination of the effects of the pressor reflex. [3] In our study, the mechanism why this lowering of BP occurred after upper cervical SMT in the volunteer group, and after both upper and lower cervical SMT in the patient group was not clear; however a strong increase in vagal tone had overcome the sympathetic tone and associated stress or pain releasing effect.
In addition, the baseline LF/HF ratio in the patient group significantly increased compared to the volunteer group suggesting that acute pain can increase sympathetic nervous system activity. Also, a decrease in LF/HF ratio indicating decreased sympathetic activity followed decreased NPS following SMT. Griffis et al. [31] suggested that acute pain may affect proinflammatory pathways, possibly through mechanisms related to sympathetic activation. The reason for pain alleviation may be due to wider neurological ramifications rather than localized joint area disturbance. [32, 33]
HRV parameters are strongly associated with changes in cardiac autonomic functions following SMT [2, 5, 34] which are also related to the presence or the absence of pain. [23] Welch and Boone [2] reported decreased LF/HF ratio reflecting parasympathetic activity dominance after manipulation of C1. [2] There were significant decreases in BP and pulse pressure, but no changes of heart rate post-manipulation. Welch hypothesized that if an upper cervical segment was manipulated, a parasympathetic response would be elicited because of the proximity of the upper cervical vertebrae to the brainstem where motor nuclei of the cranial nerves III, VII, IX, X, and XI are located.
If an upper thoracic or lower cervical segment was manipulated, a sympathetic response would be elicited because of the involvement of the stellate ganglion which stimulates the sympathetic chain ganglia. Knutson hypothesized that the decrease in systolic BP after upper cervical SMT was likely due to activation of the cervicosympathetic reflexes which responded to signals from the muscle spindles/Golgi tendon organs of the suboccipital spine to counteract vestibulosympathetic reflexes. [3] Parasympathetic activity dominance after SMT of an upper cervical segment in our study supported those hypotheses. Budgell and Hirano [5] found there was an increase in the ratio of LF/HF, reflecting a shift in balance between sympathetic dominance over parasympathetic output to the heart, after manipulation of C1 and C2.There was a significant decrease in heart rate post manipulation.
In our study, a significant increase in SDNN activity with decrease in LF/HF ratio was noted after upper cervical SMT in the volunteer group and after upper and lower cervical SMT in the patient group. However, both the volunteer and patient groups’ SDNN were within the reference range of healthy young subjects [35]; therefore, these changes were less likely to be clinically significant. Increased mean RR with decreased LF/HF ratio has been reported in pain following SMT at L5 [23] and decreases in both SDNN and LF/HF ratios were reported after SMT applied at the level of C1. [2] The relationship between HRV with physical and mental factors and psychological disease are still not well understood. [35–37] The SDNN was significantly higher in athletes than active young subjects (both male and female) and sportswomen displayed significantly lower LF/HF ratio values (P = .009) than active women. [35] Furthermore, lower SDNN with elevated LF/HF ratio was noted in bipolar disorder patients [36] and decreased RR interval with elevated LF/HF ratio was reported as an effect of physical and mental stress. [37]
A large multi-site randomized clinical study of the effect of chiropractic care on HRV and pain showed that VAS was reduced significantly from 3.7 ± 2.2 to 2.1 ± 2.0 (P < .001) after one chiropractic adjustment. [34] The HF and LF components significantly increased. However, the study was not clear about which spinal segment was manipulated and HRV data was collected in a seated position.
Roy et al. reported that both HF (– 0.76%), LH (– 7.82%), and LF/HF ratio (0.46) decreased in the treatment pain group following SMT using diversified technique at L5. [23] However, HF decreased (– 10.36%) more than LH increased (0.34%) in the treatment pain-free group, which led to a decreased LF/HF ratio (0.34). Those findings concluded that SMT applied to the lumbar vertebrae affected lumbar parasympathetic nervous system output and therefore the modulation of HRV would seem related to the presence or absence of pain.
According to our knowledge, no previous reports have compared the effect of upper and lower cervical SMT on ANS activities with pain-free and pain patients. In our study, after upper cervical SMT, an increase in parasympathetic activity was noted in both the pain-free volunteer and acute neck pain patient groups. An increase in sympathetic activity was noted in pain-free volunteers and a decrease in sympathetic activity was noted in the acute neck pain patient group after lower cervical SMT.
In a study by Roy et al participants could breathe at their own pace, though they did not measure the respiration rate. Therefore, their conclusion on the effect of lumbar manipulation on HRV is speculative, as the reported significant results could represent a variation in respiration frequency that may be different between groups. [38] In our study, RR was 13–18 bpm in both groups before and after SMT. In addition, parasympathetic output after L5 SMT was not a possible mechanism according to the anatomical organization of the autonomic nervous system, the parasympathetic nervous system originating from the craniosacral division and the sympathetic nervous system originating from the thoracolumbar division of the spinal cord. [39]
Our results are congruent with Welch [2], who reported there was parasympathetic dominance with decreased BP after upper cervical (C1 and C2) SMT. The inconsistency of our results with Budgell and Hirano [5] wherein sympathetic action dominance did not correlate with a decrease in heart rate may be due to different age groups. Also, it was difficult to assess subjects’ stress after SMT in that study because there was no measurement of BP following SMT.
Finally, our study may be criticized for changes in respiratory pattern during SMT which may alter the power of HRV. HF values vary with tidal volume and respiratory rate (RR) if RR is less than 8 bpm. [8] However, RR was 13–18 bpm in both groups before and after SMT, meaning that HF was unaffected by RR. Tidal volume was not monitored in this study to avoid unnecessary disturbance to the patient.
Limitations
Limitations of the study include: The sample of patients was not large enough to discover clinically significant changes in HRV, blood pressure and pain measurements. Because of the small sample size, a type II error is possible. Future research should therefore involve a randomized clinical trial with a larger sample size. Another limitation is that no control or sham group was utilized in this study. There was also a lack of control over variables that could have affected cardiovascular parameters such as diet (caffeine) and exercise. Further studies involving the effects of upper and lower cervical SMT on hemodynamic and HRV changes in sub-acute and chronic pain groups are recommended.
Conclusion
Our report provides early evidence that HRV analysis is a noninvasive method that applies to the assessment of changes in sympathovagal regulation associated with hemodynamic and NPS score changes. These preliminary findings suggest that upper cervical SMT may enhance dominance of parasympathetic and lower cervical SMT may enhance dominance of sympathetic activity in a young volunteer group. However, dominance of parasympathetic activity was found in patients with neck pain that received both upper and lower cervical SMT. This preliminary study sets a baseline for future studies using other populations, such as those who are older or with hypertension.
Funding Sources and Conflicts of Interest
This study was supported by research endorsement funds of the International Medical University. No conflicts of interest were reported for this study.
References:
Ward J., Tyer K., Coats J.
Immediate Effects of Atlas Manipulation on Cardiovascular Physiology
Clinical Chiropractic 2012 (Dec); 15 (3-4): 147–157Welch A., Boone R.
Sympathetic and Parasympathetic Responses to Specific Diversified Adjustments to Chiropractic
Vertebral Subluxations of the Cervical and Thoracic Spine
Journal of Chiropractic Medicine 2008 (Sep); 7 (3): 86–93Knutson G.A.
Significant Changes in Systolic Blood Pressure Post Vectored Upper Cervical Adjustment vs Resting
Control Groups: A Possible Effect of the Cervicosympathetic and/or Pressor Reflex
J Manipulative Physiol Ther 2001 (Feb); 24 (2): 101–109McKnight M.E., DeBoer K.F.
Preliminary Study of Blood Pressure Changes in Normotensive Subjects Undergoing Chiropractic Care
J Manipulative Physiol Ther 1988 (Aug); 11 (4): 261–266Budgell B., Hirano F.
Innocuous mechanical stimulation of the neck and alterations in heart-rate variability in healthy young adults.
Auton Neurosci. 2001;91(1–2):96–99Budgell B., Polus B.
The effects of thoracic manipulation on heart rate variability: a controlled crossover trial.
J Manipulative Physiol Ther. 2006;29(8):603–610Ward J., Coats J., Tyer K.
Immediate effects of anterior upper thoracic spine manipulation on cardiovascular response.
J Manipulative Physiol Ther. 2013;36(2):101–110McKnight M.E., DeBoer K.F.
Preliminary Study of Blood Pressure Changes in Normotensive Subjects Undergoing Chiropractic Care
J Manipulative Physiol Ther 1988 (Aug); 11 (4): 261–266Watanabe N., Polus B.
A single mechanical impulse to the neck: does it influence autonomic regulation of cardiovascular function?
Chiropr J Aust. 2007;37(2):42–48Bakris G., Dickholtz M., Meyer P.
Atlas Vertebra Realignment and Achievement of Arterial Pressure Goal in Hypertensive Patients:
A Pilot Study
Journal of Human Hypertension 2007 (May); 21 (5): 347–352Bakris G., Dickholtz M., Meyer P.
Atlas Vertebra Realignment and Achievement of Arterial Pressure Goal in Hypertensive Patients:
A Pilot Study
Journal of Human Hypertension 2007 (May); 21 (5): 347–352Plaugher G., Bachman T.
Chiropractic Management of a Hypertensive Patient
J Manipulative Physiol Ther 1993 (Oct); 16 (8): 544–549McMasters Kim L., Wang Joe, York Jennifer.
Blood Pressure Changes in African American Patients Receiving Chiropractic Care in a Teaching Clinic:
A Preliminary Study
Journal of Chiropractic Medicine 2013 (Jun); 12 (2): 55–59Mangum K., Partna L., Vavrek D.
Spinal manipulation to treat hypertension: a systematic qualitative literature review.
J Manipulative Physiol Ther. 2012;35(3):235–243Akselrod S., Gordon D., Ubel F.A.
Power spectral analysis of HR fluctuation: a quantitative probe of beat-to-beat cardiovascular control.
Science. 1981;213:220–222Pomeranz B., Macaulay R.J., Caudill M.A.
Assessment of autonomic function in human by HR spectral analysis.
Am J Physiol. 1985;248(1Pt 2):H151–H153Malliani A., Pagani M., Lombardi F.
Cardiovascular neural regulation explored in the frequency domain.
Circulation. 1991;84:482–492Kunze D.L.
Reflex discharge patterns of cardiac vagal efferent fibres.
J Physiol Lond. 1972;222:1–15Huang H.H., Chan H.L., Lin P.L.
Time frequency spectral analysis of HR variability during induction of general anaesthesia.
Br J Anaesth. 1997;79:754–758Win N.N., Fukayama H., Kohase H.
The different effects of intravenous propofol and midazolam sedation on hemodynamic and heart rate variability.
Anesth Analg. 2005;101:97–102Win N.N., Kohase H., Umino M.
Haemodynamic changes and heart rate variability during midazolam-propofol co-induction.
Anaesthesia. 2007;62:561–568Kjellgren O., Gomes J.A.
Heart rate variability and baroreflex sensitivity in myocardial infarction.
Am Heart J. 1993;125:204–214Roy R.A., Boucher J.P., Comtois A.S.
Heart rate variability modulation after manipulation in pain-free patients vs patients in pain.
J Manipulative Physiol Ther. 2009;32(4):277–286Haldeman S, Carroll L, Cassidy JD, Schubert J, Nygren A.
The Bone and Joint Decade 2000–2010 Task Force on Neck Pain
and Its Associated Disorders: Executive Summary
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S5–7Holt K., Beck R., Sexton S., Haavik T.
Reflex effects of a spinal adjustment on blood pressure.
Chiropr J Aust. 2010;40(3):95–99Dimmick K., Young M., Newell D.
Chiropractic manipulation affects the differences between arterial systolic blood pressures on the left and right in normotensive subjects.
J Manipulative Physiol Ther. 2006;29(1):46–50Toro-Velasco C, Arroyo-Morales M, Fernandez-de-Las-Penas C, Cleland JA.
Short-Term Effects of Manual Therapy on Heart Rate Variability, Mood State, and
Pressure Pain Sensitivity in Patients With Chronic Tension-Type Headache:
A Pilot Study
J Manipulative Physiol Ther. 2009 (Sep); 32 (7): 527–535Sterling M., Jull G., Wright A.
Cervical mobilisation: concurrent effects on pain, sympathetic nervous system activity and motor activity.
Man Ther. 2001;6:72–81Ratliff C.R., Sandefur R.M., Wagnon R.J.
Serum aldosterone changes after specific chiropractic manipulation.
Am J Chiropr Med. 1988;1:66–70Crawford J., Hickson G., Wiles M.
The Management of Hypertensive Disease: A Review of Spinal Manipulation and the Efficacy of Conservative Therapeusis
J Manipulative Physiol Ther 1986 (Mar); 9 (1): 27–32Griffis C.A., Crabb Breen E., Compton P.
Acute Painful Stress and Inflammatory Mediator Production.
Neuroimmunomodulation. 2013;20(3):127–133Baliki M.N., Geha P.Y., Apkarian A.V.
Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics.
J Neurosci. 2008;28(6):1398–1403Apkarian A.V., Sosa Y., Sonty S.
Chronic back pain is associated with decreased prefrontal and thalamic gray matter density.
J Neurosci. 2004;24(46):10410–10415Zhang J., Dean D., Nosco D., Strathopulos D., Floros M.
Effect of chiropractic care on heart rate variability and pain in a multisite clinical study.
J Manipulative Physiol Ther. 2006;29(4):267–274Corrales M.M., Torres B.C., Esquivel A.G.,
Salazar M.A.G., Orellana J.N.
Normal values of heart rate variability at rest in a young, healthy and active Mexican population.
Health. 2012;4(7):337–385Levy B.
Illness severity, trait anxiety, cognitive impairment and heart rate variability in bipolar disorder.
Psychiatry Res. 2014 [S0165-1781(14)00638-6]Kaur S., Bhalla P., Bajaj S.K., Sanyal S., Babber R.
Effects of physical and mental stress on heart rate variability in type-A and type-B personalities.
Indian J Appl Basic Med Sci. 2013;15(20):59Piché M., Descarreaux M.
Heart rate variability modulation after manipulation in pain-free patients vs patients in pain? The importance of controlling for respiration rate changes.
J Manipulative Physiol Ther. 2010;33(7):554–555Charles R.N., Norman L.S., Robert J.D., David A.R.
Human Nervous System. 6th ed. Humana Press Inc.;
Totowa New Jersey: 2005. Chapter 20;
Autonomic Nervous System; pp. 349–369.
Return to BLOOD PRESSURE
Since 1-14-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |