

Resveratrol Requires Red Wine Polyphenols
for Optimum Antioxidant ActivityThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Journal of Nutrition, Health & Aging 2016; 20 (5): 540–545 ~ FULL TEXT
Aires V, Limagne E, Cotte AK, Latruffe N, Ghiringhelli F, Delmas D.
Department of Medicine,
Center for Diabetes, Metabolism and Endocrinology,
Karolinska Institute,
Karolinska University Hospital,
Huddinge, 141 86
Stockholm, Sweden
sara.straniero@ki.se
OBJECTIVE: There is substantial evidence that a diet rich in fruit and vegetables may reduce the risk of aging and stress oxidative associated diseases. It has been suggested that benefits associated with fruit and red wine consumption could be due to pooled antioxidant microcomponents in diet. The aim of this study was to investigate the antioxidant activities of pure resveratrol (a well known phytoalexin, RSV) and red wine polyphenols (RWP), using UV-B radiated isolated rat hepatocytes as a model of oxidative stress.
METHODS: Rat hepatocytes were isolated by the collagenase method. The cells were loaded with resveratrol and/or polyphenols at different concentrations. The production of thiobarbituric acid reactive substances (TBARS) released by UV-B radiated cells and the levels of lipid-soluble antioxidants (Dolichol, Vitamin E, Coenzyme Q9 and Q10) were measured.
RESULTS: Resveratrol had pro-oxidant or antioxidant effects depending on (lower or higher) dosage. RWP protection from photolipoperoxidation was dose-dependent and increased with dosage. Combination of the two compounds exhibited synergistic antioxidant effect, and made resveratrol effective both at lower and higher dosages.
CONCLUSIONS: These results suggest that resveratrol requires red wine polyphenols for optimum antioxidant activity.
Key words: Resveratrol, red wine polyphenols, oxidative stress, lipid-soluble antioxidants.
From the FULL TEXT Article:
Introduction
The so called civilisation diseases have numerous causes (1); the oxidative stress (OS) more or less contributes to their development. OS results from an imbalance between formation and neutralization of antioxidants. It is initiated by free radicals like hydroxyl, peroxyl and superoxide radicals, which become stable through electron pairing with biological macromolecules such as proteins, lipids and DNA in cells causing protein and DNA damage along with lipid peroxidation. The damage caused by OS has been implicated as a potential contributor to the pathogenesis of cancer, diabetes, atherosclerosis, cardiovascular diseases, inflammatory diseases and aging (2,3). The damage can become more widespread due to weakened cellular antioxidant defense systems. All biological systems have antioxidant defense mechanisms that protects against oxidative damages and repairs enzymes to remove damaged molecules. However, these natural antioxidant defense mechanisms may not be fully effective, maybe because of their overload, and dietary intake of antioxidant may be required. Fruits and vegetables contain different antioxidant compounds, such as Vitamin C, Vitamin E, carotenoids or polyphenol molecules which interact with various biological systems (4-6).
Red wine contains a rich source of a large number of antioxidants, namely the phenolic acids and polyphenols, which provide it with its protective redox potential (7,8). Studies on squamous carcinoma cells showed an inhibitory effect of red wine polyphenols on cell growth (9) and studies on the antimutagenic activity of grape extracts suggested that the protective effect of many compounds in the extract was synergistic (11). An important role in the antioxidant activity of red wine, has been attributed to the presence of resveratrol, a triphenolic stilbene present in the black skin of grapes and proanthocyanidins (11). Mounting evidence suggests that resveratrol could act as a powerful anti-cancer (12), anti-inflammatory (13), anti-diabetes (14) and anti-oxidation agent (15). Most strikingly, resveratrol can exert a powerful antioxidant effect in organisms: resveratrol scavenges cellular reactive oxygen species (ROS) and corrects radical-induced responses such as DNA damage (16), imbalance of mitochondria redox state (17) and interfering cellular signal transductions (18). Furthermore, resveratrol increases yeast cell survival (19), and subsequent studies on worms, fruit flies, fish and mice have also linked resveratrol effects on longevity (20,21). The beneficial effects of RSV on aging have been suggested to resemble and potentially mimic caloric restriction (CR) in Caenorhabditis elegans (22), Drosophila melanogaster (20) and mice (21).
However, both the anti-aging benefits and the counteraction of all aging-related diseases were observed only at much higher doses of resveratrol (tens or hundreds mg per kg/body weight) than those that may be involved in the so-called ”French paradox”: the strikingly low incidences of coronary heart diseases in France, despite intake of a high-fat diet, have been attributed to the consumption of red wine (23). Furthermore, unlike the case with functional foods, resveratrol were said to have both favorable and harmful dose-dependent effects (24).
These observations, the incomplete knowledge of the mechanisms of action, the growing public interest and the vast (not always well-controlled) consumption of resveratrol, prompted us to further investigate its action as antioxidant compound. Recalling that resveratrol is a naturally occurring phytoalexin (“defender of the plant”) that is produced in response to injury, such as mechanical trauma, UV light and infection by fungi and pathogenic microorganisms, providing means for defense together with others constitutive or inducible compounds (25), in this research the antioxidant action of resveratrol was studied in vitro in the absence or presence of the polyphenols normally present in red wine, using UV-B radiated isolated rat hepatocytes as a model of oxidative stress (26). Perhaps it may be worthwhile to mention here that phenolic compounds usually exist as conjugated forms in the blood and that in vivo resveratrol may not reach hepatocytes as aglycone form. In order to evaluate effects on lipid peroxidation, the production of thiobarbituric acid reactive substances (TBARS) released by UV-B radiated cells was assayed. Furthermore, the levels of natural fat-soluble antioxidants (Dolichol, Vitamin E, Coenzyme Q9 and Q10), which protect membrane lipids from oxidative damage (27,28), were measured.
Materials and Methods
Materials
All reagents were of analytical and HPLC grade. Solvents were purchased from Panreac Química S.L.U. (Barcelona, Spain). Standard molecules and chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Milli-Q (Millipore-Lab, Bedford, MA) purified water was used for all analyses.
Animals
All of the experiments were conducted following the Official Italian Regulation n. 116/92 for the care and use of laboratory animals and were approved by the Independent Ethics Committee of the University of Pisa (Approval number: 2A/42155). The liver samples were obtained from 3-month-old Sprague-Dawley rats raised at the Pisa University Interdepartmental research Centre on Biology and Pathology of Aging vivarium. The animals were kept in a controlled environment (22 °C; 12/12 h light/dark cycle) and were maintained on a standard laboratory diet (Teklad, Harlan, Italy). All rats had free access to water.
Animals were randomly assigned to experimental groups: resveratrol (n = 6), red wine polyphenols (n = 6), resveratrol and red wine polyphenols (n = 6).
Rats were anaesthetized by an intraperitoneal injection of pentobarbital (50 mg/kg body weight) and the liver was removed.
Preparation of isolated liver cells
Isolated hepatocytes were prepared by the method of collagenase perfusion (29). Cell viability was tested with the trypan-blue exclusion and was always higher than 90%. The obtained cells were divided in different aliquots and antioxidants loaded by the incubation with 20 volumes of washing buffer (29) containing resveratrol and/or polyphenols for 10 minutes, centrifuged, washed once with washing buffer and diluted with suspension buffer fortified with pyruvate (15 mM) (29). An aliquot without incubation with resveratrol and/or polyphenols was used as control.
Antioxidant solution
Resveratrol and polyphenols was dissolved in pure ethanol and then diluted with washing buffer.
Ultraviolet-B (UV-B) radiation
UV-B radiation of hepatocytes was performed as described by Parentini et al. (26). A cell monolayer was obtained by pouring 50 mL of a 6 mg/mL cell suspension in a Petri dish with a surface of 150 cm2 (no cover top). The dish was placed under the radiation source for 40 minutes. At the end of radiation, hepatocytes viability was 70% and incubation with resveratrol and/or polyphenols had no additional effect on cell viability.
The radiation source was a flat-bed chamber for cell irradiation, designed and built by the Laboratory of Biophysics Institute of the National Research Council (IFB-CNR), Pisa. UV-B sources were two UV-B-313 fluorescent tubes, mounted about 7.0 cm above the sample holder. UV-B irradiance was about 1.7 W/m2, slightly lower than the UV-B present in a natural environment. Light irradiance was measured by means of a United Detector Technology radiometer (UDT instruments, San Diego, CA, USA). After centrifugation of the cell suspension, the supernatant was used to assay the release of thiobarbituric acid reactive substances (TBARS) in the medium and cells were used to study the effects of radiation on Dolichol, Vitamin E, Coenzyme Q9 and Q10 levels.
Thiobarbituric acid reactive substances (TBARS) assay
TBARS were assayed by taking 1 mL aliquot of the medium at the end of radiation. Assay was performed substantially according to Cavallini et al. (30). The quantification of TBARS production was analyzed by high performance liquid chromatography-VIS detection (532 nm) using a reverse-phase column and eluted as described by Grotto et al. (31). The calibration curve was prepared with malondialdehyde (MDA) and the results were reported as MDA
Extraction of Dolichol, Vitamin E and Coenzyme Q
. Dolichol (a long-chain polyisoprenoid broadly distributed in all tissues and membranes), Vitamin E and Coenzyme Q (CoQ) were extracted simultaneously into hexane from a sodium dodecyl sulphate-treated homogenate (32). Aliquots of the extract were taken for Dolichol, Vitamin E and CoQ assay, dried under nitrogen and redissolved in isopropanol, methanol and methanol/reagent alcohol solution, respectively.
High-pressure liquid chromatography (HPLC) assay
. Dolichol was assayed by an HPLC-UV detection (210 nm) using a reverse-phase column and eluted as described by Maltese et al. (33). Coenzyme Q9 and Q10 was assayed by an HPLC-UV detection (275 nm) using a reverse-phase column and eluted as described by Lang et al. (32). Vitamin E was assayed by an HPLC-fluorometric detection (excitation at 295 nm, emission at 350 nm) using a reverse-phase column and eluted as described by Ruperez et al. (34).
Statistical analyses
. The analysis of variance (ANOVA) test was used to evaluate differences among multiple conditions. If positive, the Tukey test was used to test for their statistical significance. Student’s t test was used to evaluate differences between two conditions. Values of p < 0.05 were considered to be statistically significant.
Results
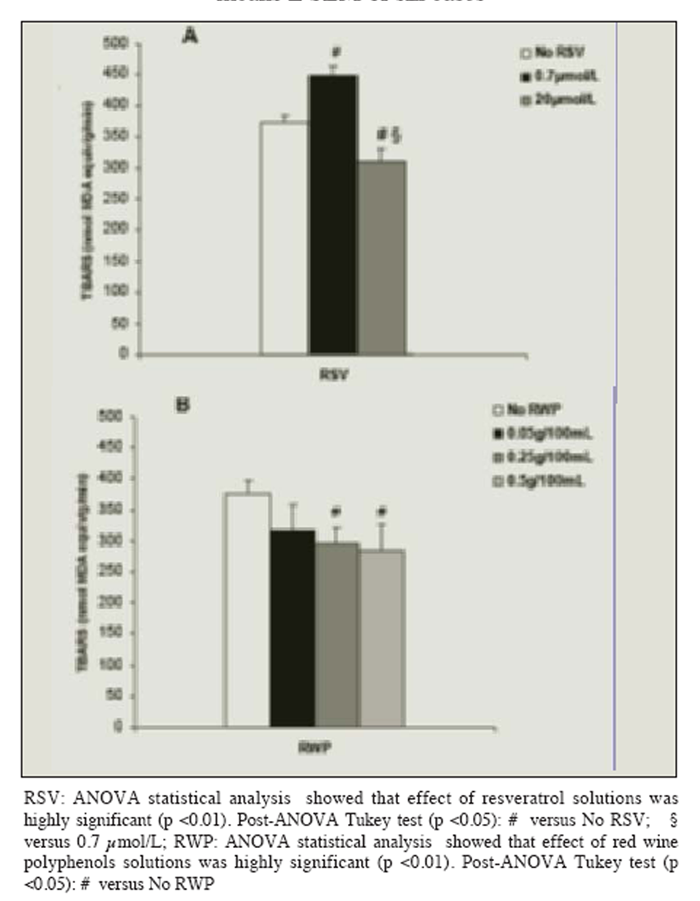
Figure 1 The production of TBARS, released by UV-B radiated cells without resveratrol or polyphenols, increased in a linear manner for at least 40 min (20 min: 214 ± 24 nmol MDA equivalent/g/min; 40 min: 394 ± 25 nmol MDA equivalent/g/min; p < 0.01). The release of TBARS from the radiated liver cells loading with solutions containing resveratrol alone was dose-dependent: resveratrol had a pro-oxidant effect at a concentration similar to that found in vivo after a moderate consumption of red wine (0.7 ?mol/L), and an antioxidant effect at a higher concentration (20 ?mol/L), (Figure 1A).
Loading cells with polyphenols reduced lipid peroxidation in a dose-dependent manner after the 40 min exposure to UV-B radiation: maximum protection was attained by the use of a polyphenol concentration equal to or higher than 0.25 g/100 mL (Figure 1B). Figure 2 shows that loading cells with both resveratrol and polyphenols had synergistic effects (i.e. protection was much higher than that given by resveratrol or polyphenols alone): protection by resveratrol was seen at any tested concentrations (including 0.7 ?mol/L), and increased with the resveratrol concentration in the loading solution up to 20 ?mol/L.
Figure 3 shows the effects of UV-B radiation and resveratrol solutions on lipid-soluble antioxidants isolated rat hepatocytes. UV-B radiation caused a 65% decrease in alpha-tocopherol and CoQ9, 40% in CoQ10 and 25% in dolichol levels, in keeping with previous observations (26). Loading cells with the lower resveratrol solution (but not with a 20 ?M solution), caused a bigger loss of vitamin E, CoQ9 and CoQ10 in UV-B radiated cells, but had no significant effect on dolichol.
Figure 4 shows that pre-loading with red wine polyphenols had no effect on the UV-B induced depletion in fat-soluble antioxidants, but prevented the decrease observed with lower resveratrol solution. Polyphenols together with increasing concentrations of resveratrol counteracted in part (but significantly: p <0.05) the effects of radiation on CoQ9 and Vitamin E levels.
Figure 1
Effect of the pre-incubation with solutions containing resveratrol (RSV) (A) or red wine polyphenols (RWP) (B) on photolipoperoxidation of UV-B radiated rat hepatocytes. Results are given as changes with respect to not-radiated cells. No changes in the levels of the TBARS production were observed in absence of radiation regardless of the concentration of RSV or RWP in the loading-solution. Results represent the means ± SEM of six cases
Discussion
UV-induced peroxidation of fatty acids containing more than two methylene-linked double bonds and lipid peroxidation is a fundamental parameter of oxidative stress. Furthermore, UV-B radiation is a method to increase oxidative stress from extracellular environment and may offer several advantages over classical chemical tests (e.g. oxidation with FeSO4 or H2O2 might interfere with antioxidant activity of phytochemicals). A recent study show that the effects UV-B radiation can be used to evaluate the antioxidant activity of plant extract and their interactions on red blood cell ghosts (30).
Figure 2
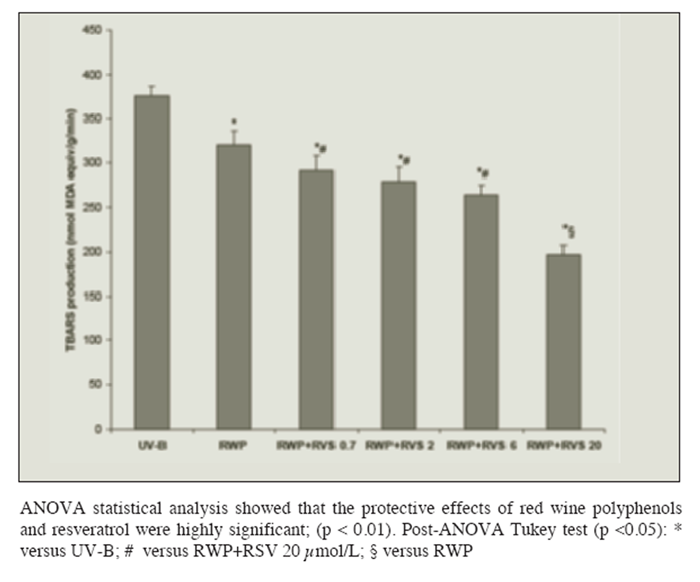
Figure 2 Protection of rat hepatocytes from photolipoperoxidation after UV-B radiation (UV-B) by pre-incubation with red wine polyphenols (RWP) 0.25 g/100mL and increasing amounts of resveratrol ( RSV: 0.7, 2, 6 or 20 ?mol/L). Results are given as changes with respect to not-radiated cells. No changes in the levels of the TBARS production were observed in absence of radiation regardless of the concentration of RWP and RSV in the loading-solution. Results represent the means ± SEM of six cases
Natural products are widely used as complementary and alternative medications for the prevention and treatment of various human diseases (35). Resveratrol, a naturally occurring phytoalexin found abundance in grapes, red wines and other edible plants, has been reported to exert a variety of pharmacological effects. In recent years, resveratrol has received considerable attention for its antioxidation and free radical scavenging properties.
In this research, attention was focused on the antioxidant action of resveratrol in the absence or presence of the polyphenols normally present in red wine, using UV-B radiated isolated rat hepatocytes as a model of oxidative stress. The routine use of TBARS determination in a wide array of sample types was criticized (31,36), but assay proved to be a satisfactory index of the occurrence/extent of lipid peroxidation with UV-B radiated isolated rat hepatocytes (27).
A surprising finding was that loading cells with a solution containing resveratrol alone, at a concentration similar to that found in vivo after a moderate consumption of red wine, increased the release of TBARS and led to a decrease in Vitamin E, Coenzyme Q9 and Q10 levels in the radiated liver cells. This pro-oxidant effect was not seen at a higher resveratrol concentration. The presence of resveratrol and polyphenols had synergistic antioxidant effect, and made resveratrol effective both at lower and higher dosages. These results might provide answer to the “French paradox”, i.e., the apparent compatibility of a high-fat diet rich in cholesterol and saturated (and low in unsaturated) fatty acids with a low incidence of coronary atherosclerosis (37): the antioxidant and anti-inflammatory activities of red wine might derive not only from the mere chemical composition but also from the interaction occurring within the bulk of different molecules (38). Even though the antioxidant activities of the wines vary over a factor of 2, the ratios of the activities to the total phenol content are approximately the same (about a factor of 10), indicating the direct relationship between the two (38).
Figure 3
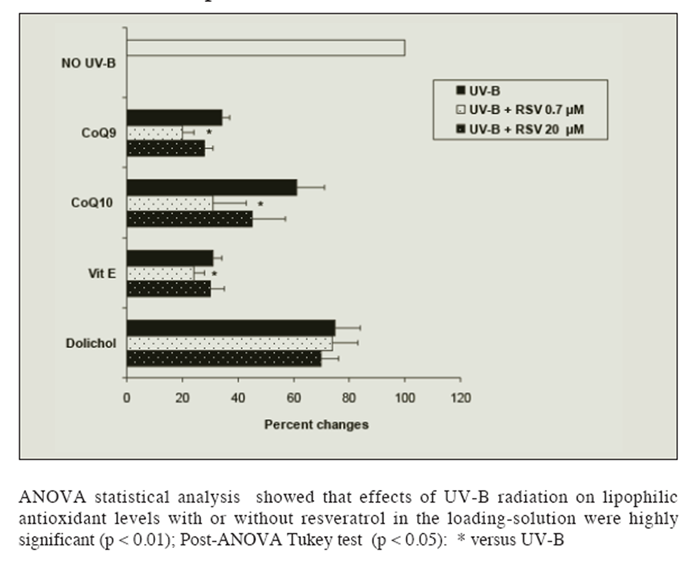
Figure 3 Effect of UV-B radiation on the level of lipid-soluble antioxidants (Coenzyme Q9 and Q10, Vitamin E and Dolichol) in isolated liver cells pre-loaded or not with resveratrol (RSV: 0.7 or 20 ?mol/L). Results are given as percent changes with respect to not-radiated cells. No changes in the levels of the lipophilic antioxidants were observed in absence of radiation regardless of RSV concentration in the loading-solution. Results represent the means ± SEM of six cases
An interesting hypothesis is that loading the membranes of mammalian cells with a small number of resveratrol molecules (and no polyphenols) may produced a structural perturbation on the membrane bilayer and change the metabolism of free radicals in lipophilic compartment of cytomembranes (27). More recently, data demonstrate that resveratrol produce a moderate but significant structural perturbation of the lipid bilayer and induce echinocytosis in human erythrocytes: the extent of these changes is dependent on resveratrol concentration (39). Cell membrane is a diffusion barrier which protects the interior of the cell. Therefore, its structure and functions are susceptible to alterations as a consequence of interactions with chemical species. Preceding studies show an earlier loss of Vitamin E than the others membrane lipid-soluble antioxidants in isolated rat hepatocytes treated with NADPH-ADP-Fe system generating free radicals from hydrophilic, extracellular environment (40). Also UV-B radiation is a method to increase oxidative stress from extracellular environment and the effect of polyphenols together with increasing concentrations of resveratrol may be protective because preserve an innate antioxidant membrane machinery.
Figure 4
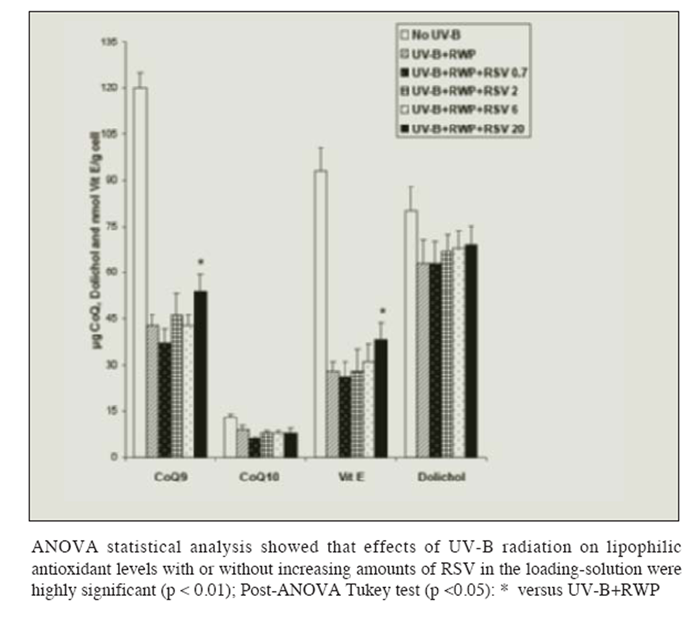
Figure 4 Effect of UV-B radiation on the level of lipid-soluble antioxidants (Coenzyme Q9 and Q10, Vitamin E and Dolichol) in isolated liver cells pre-loaded with red wine polyphenols (RWP) 0.25 g/100mL and increasing amounts of resveratrol ( RSV: 0.7, 2, 6 or 20 ?mol/L). Results are given as ?g Coenzyme Q9/g cell, ?g Coenzyme Q10/g cell, nmol Vitamin E/g cell and ?g Dolichol/g cell. No changes in the levels of the TBARS production were observed in absence of radiation regardless of the concentration of RWP and RSV in the loading-solution. Results represent the means ± SEM of six cases
Overall, our results demonstrate that resveratrol and red wine polyphenols together can protect mammalian cells from oxidative stress, whereas resveratrol alone may have pro-oxidant or anti-oxidant effects depending on concentration. This conclusion is in line with the hypothesis that the benefits of diets rich in fruits and vegetables are attributable to the uptake of phytocomplexes rather than to individual micro-compounds. Several authors have hypothesized that some health effects of plant polyphenols may not require their efficient absorption through the gut and may be due to the direct, protective effects on the intestinal mucosa against oxidative stress or the action of carcinogens (41). Studies on human cells show that polyphenols can protect skin fibroblasts and keratinocytes from photo-radiation damage (42); that resveratrol protects cells from an oxidative stress by tert-butylhydroperoxide only at high concentrations (in the micromolar range (43)); and that the protection is enhanced if resveratrol is used in combination with polyphenols such as quercetina and pterostilbene (44).
Furthermore, recent data demonstrate that low dose resveratrol treatment causes premature senescence in lung cancer cells via ROS-mediated DNA damage (45). Finally, our results may contribute to move forward in the further studies on the antioxidants contained in food matrices and their potential synergistic combinations, since non-physiological, excessive exogenous supply of dietary antioxidants could therefore even interfere with the delicate redox balance of the cell, especially in elderly individuals.
Conflict of interest:
The authors Dr. Straniero, Dr. Cavallini and Prof. Bergamini has a patent 10798587.1 pending.
Ethical Standards:
All handling and management procedures were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals of the University of Pisa.
References:
Kesse-Guyot E, Andreeva VA, Touvier M, Jeandel C, Ferry M, Hercberg S, Galan P
Overall and abdominal adiposity in midlife and subsequent cognitive function.
J Nutr Health Aging 2015;19:183-189Maxwell SR.
Prospects for the use of antioxidant therapies.
Drugs 49:345-361Willett WC.
Balancing life-style and genomics research for disease prevention.
Science 2002;296:695-698Kelewala NS, Ananthanarayan L.
Antioxidant acitivity of selected food-stuffs.
Int J Food Sci Nutr 2004;55:511-516Berger RG, Lunkenbein S, Strohle A, Hahn A.
Antioxidants in food: Mere myth or magic medicine?
Crit Rev Food Sci Nutr 2012;52:162-171Potenza L, Calcabrini C, De Bellis R, Mancini U, Cucchiarini L, Dachà M.
Effect of quercetin on oxidative nuclear and mitochondrial DNA damage
BioFactors 2008;33: 33-48Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB.
The relative antioxidant activities of plant-derived polyphenolic flavonoids.
Free Radic Res 1995;22:375-383.Morton LW, Abu-Amsha Caccetta R, Puddey IB, Croft KD.
Chemistry and biological effects of dietary phenolic compounds: relevance to cardiovascular disease.
Clin Exp Pharmacol Physiol 2000;27:152-159.Elattar TM, Virji AS.
The effect of red wine and its components on growth and proliferation of human oral squamous carcinoma cells.
Anticancer Res 1999;19:5407-5414Stagos D, Kazantzoglou G, Theofanidou D, Kakalopoulou G, Magiatis P, Mitaku S.
Activity of grape extracts from Greek varieties of Vitis vinifera against mutagenicity induced by
bleomycin and hydrogen peroxide in Salmonella typhimurium strain TA102.
Mutat Res 2006;609:165-175Liu Y, He XQ, Huang X, Ding L, Xu L, Shen YT, Zhang F, Zhu MB, Xu BH, Qi ZQ,Wang HL.
Resveratrol protects mouse oocytes from methylglyoxal-induced oxidative damage.
PLoS One 2013;8:e77960Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB, Yang K, Shen HF, Xie LP.
Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro
and inhibits tumor growth in vivo.
Cancer Sci 2010;101:488-493Baek SJ, Wilson LC, Eling TE.
Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1)
by increasing the expression of p53.
Carcinogenesis 2002;23:425-434Su HC, Hung LM, Chen JK.
Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats.
Am J Physiol Endocrinol Metab 2006;290:E1339-1346? G?lçin ?.
Antioxidant properties of resveratrol: a structure-activity insight.
Innov Food Sci Emerg Tech 2010;11:210-218De Salvia R, Festa F, Ricordy R, Perticone P, Cozzi R.
Resveratrol affects in a different way primary versus fixed DNA damage induced by H(2)O(2)
in mammalian cells in vitro.
Toxicol Lett 2002;135:1-9Sareen D, Darjatmoko SR, Albert DM, Polans AS.
Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer.
Mol Pharmacol 2007;72:1466-1475Pirola L, Frojdo S.
Resveratrol: one molecule, many targets.
IUBMB Life 2008;60:323-332Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P.
Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan.
Nature 2003;425:191-196Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D.
Sirtuin activators mimic caloric restriction and delay ageing in metazoans.
Nature 2004;430:686-689Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS.
Resveratrol improves health and survival of mice on a high-calorie diet.
Nature 2006;444:337-342Collins JJ, Evason K, Kornfeld K.
Pharmacology of delayed aging and extended lifespan of Caenorhabditis elegans.
Exp Gerontol 2006;41:1032-1039Renaud S, de Lorgeril M.
Wine, alcohol, platelets, and the French paradox for coronary heart disease.
Lancet1992; 339:1523-1526Scott E, Steward WP, Gescher AJ, Brown K.
Resveratrol in human cancer chemoprevention-choosing the ‘right’ dose.
Mol Nutr Food Res 2012;56:7-13Dixon RA.
Natural products and plant disease resistance.
Nature 2001;411:843-847Parentini I, Bergamini E, Cecchi L, Cavallini G, Donati A, Maccheroni M.
The effect of carbon tetrachloride and ultraviolet radiation on dolichol levels in liver cells
isolated from 3- and 24-month old male Sprague-Dawley rats.
Biogerontology 2003;4:365-370Bergamini E, Bizzarri R, Cavallini G, Cerbai B, Chiellini E, Donati A, Gori Z, Manfrini A.
Ageing and oxidative stress: a role for dolichol in the antioxidant machinery of cell membranes?
J Alzheimers Dis 2004;6:129-135Afri M, Ehrenberg B, Talmon Y, Schmidt J, Cohen Y, Frimer AA.
Active oxygen chemistry within the liposomal bilayer. Part III: Locating Vitamin E, ubiquinol and ubiquinone
and their derivatives in the lipid bilayer.
Chem Phys Lipids 2004;13:107-121Seglen PO.
Preparation of isolated rat liver cells.
Methods Cell Biol 1976;13:29-83Cavallini G, Dachà M, Potenza L, Ranieri A, Scattino C, Castagna A, Bergamini E.
Use of red blood cell membranes to evaluate the antioxidant potential of plant extracts.
Plant Foods Hum Nutr 2014;69:108-114Grotto D, Santa Maria LD, Boeira S, Valentini J, Charão MF, Moro AM, Nascimento PC.
Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. VJ Pharm Biomed Anal 2007;43:619-624Lang JK, Gohil K, Packer L.
Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma,
tissue homogenates, and subcellular fractions.
Anal Biochem 1986;157:106-116Maltese WA, Erdman RA.
Characterization of isoprenoid involved in the post-traslational modification of mammalian cell proteins.
J Biol Chem 1989;264:18168-18172Ruperez FJ, Barbas C, Castro M, Martinez S, Herrera E.
Simplified method for Vitamin E determination in rat adipose tissue and mammary glands by
high-performance liquid chromatography.
J Chromatogr A 1998;823:483-487Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB.
Cancer prevention with natural compounds.
Semin Oncol 2010;37:258-281Janero DR.
Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation
and peroxidative tissue injury.
Free Radic Biol Med 9:515-540Sun AY, Simonyi A, Sun GY.
The “French Paradox” and beyond: the neuroprotective effects of polyphenols.
Free Radic Biol Med 2002;32:314-318Rice-Evans CA, Miller NJ, Paganga G.
Antioxidant properties of phenolic compounds.
Trends Plant Sci Rev 1997;2:152-159Suwalsky M, F Villena, Gallardo MJ.
In vitro protective effects of resveratrol against oxidative damage in human erythrocytes.
Biochim Biophys Acta 2015;1848:76-82Guarini M, Stabile A, Cavallini G, Donati A, Bergamini E.
Effects of oxidative stress on the Dolichol content of isolated rat liver cells.
Free Radic Res 2007;41:1283-1288Manach C, Williamson G, Morand C, Scalbert A, Rémésy C.
Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies.
Am J Clin Nutr 2005;81: 230S-242SPacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST, Mertens-Talcott SU.
Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated
human skin fibroblasts.
J Agric Food Chem 2008;56:8434-8441Pandey KB, Rizvi SI.
Protective effect of resveratrol on formation of membrane protein carbonyls and lipid peroxidation
in erythrocytes subjected to oxidative stress.
Appl Physiol Nutr Metab 2009;34:1093-1097Mikstacka R, Rimando AM, Ignatowicz E.
Antioxidant effect of trans-resveratrol, pterostilbene, quercetin and their combinations in
human erythrocytes in vitro.
Plant Foods Hum Nutr 2010;65:57-63Luo H, Yang A, Schulte BA, Wargovich MJ, Wang GY.
Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage.
PLoS One 2013;8:e60065
Return to RESVERATROL
Return to POLYPHENOLS
Return to PHYTOALEXINS
Since 8-07-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |