

A Randomized Controlled Trial Comparing a Multimodal
Intervention and Standard Obstetrics Care for
Low Back and Pelvic Pain in PregnancyThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Am J Obstet Gynecol. 2013 (Apr); 208 (4): 295.e1-7 ~ FULL TEXT
OPEN ACCESS James W. George, DC, Clayton D. Skaggs, DC, Paul A. Thompson, PhD,
D. Michael Nelson, MD, PhD, Jeffrey A. Gavard, PhD, Gilad A. Gross, MD
Chiropractic Science Division,
College of Chiropractic,
Logan University,
Chesterfield, MO, USA.
james.george@logan.eduOBJECTIVE: Women commonly experience low back pain during pregnancy. We examined whether a multimodal approach of musculoskeletal and obstetric management (MOM) was superior to standard obstetric care to reduce pain, impairment, and disability in the antepartum period.
STUDY DESIGN: A prospective, randomized trial of 169 women was conducted. Baseline evaluation occurred at 2428 weeks' gestation, with follow-up at 33 weeks' gestation. Primary outcomes were the Numerical Rating Scale (NRS) for pain and the Quebec Disability Questionnaire (QDQ). Both groups received routine obstetric care. Chiropractic specialists provided manual therapy, stabilization exercises, and patient education to MOM participants.
RESULTS: The MOM group demonstrated significant mean reductions in Numerical Rating Scale scores (5.8 ± 2.2 vs 2.9 ± 2.5; P < .001) and Quebec Disability Questionnaire scores (4.9 ± 2.2 vs 3.9 ± 2.4; P < .001) from baseline to follow-up evaluation. The group that received standard obstetric care demonstrated no significant improvements.
CONCLUSION: A multimodal approach to low back and pelvic pain in mid pregnancy benefits patients more than standard obstetric care.
From the Full-Text Article:
Introduction
Musculoskeletal pain in pregnant women commonly is viewed as transient, physiologic, and self-limited. However, most women report either low back pain (LBP) or pelvic pain (PP) during pregnancy [16] and the morbidity that is associated with such complaints. [7, 8] Moreover, up to 40% of patients report musculoskeletal pain during the 18 months after delivery, [2, 7, 9, 10] and one-fifth of these women have severe LBP that leads to major personal, social, or economic problems. [7, 9, 11] Pregnancy-related LBP contributes substantially to health care costs. For example, one-fifth of pregnant women in Scandinavian countries experience back pain as an indication for up to 7 weeks of sick leave in the perinatal period. [7, 9] Ninety-four percent of women who experienced LBP in an index pregnancy have recurrent symptoms with subsequent pregnancy, and two-thirds of these patients experience disability and require sick leave during pregnancy. Notably, 19% of women with pain in an initial pregnancy report avoidance of a future pregnancy out of fear of recurrence of the musculoskeletal symptoms. [11]
Most past investigations that have evaluated interventions to reduce morbidity in women with LBP/PP during pregnancy have used modalities that have included prescription exercise, [12] manual manipulation, [13] education, [14] acupuncture, [15] or pelvic belts. [16] Recently, a multimodal randomized trial compared osteopathic manipulation to usual obstetric care and sham ultrasonic therapy on 144 participants. [13] Importantly, this trial did not include behavioral and exercise therapies. We conducted a prospective, randomized, masked clinical trial to test the hypothesis that a multimodal approach of manual therapy, exercise, and education for LBP/PP in pregnant women is superior to standard obstetric care (STOB) for the reduction of pain, impairment, and disability in the antepartum period.
Materials and Methods
The institutional review boards of Logan University, College of Chiropractic, St. Louis, MO, and Washington University School of Medicine, St. Louis, MO, approved this study. Subjects were recruited from 3 clinical settings. The Women's Health Center included a state-approved collaborative practice of Washington University attending physicians and nurse practitioners who worked with residents in obstetrics and gynecology and maternal fetal medicine fellows to serve both high- and low-risk patients, regardless of payer status. The 2 additional sites were university-affiliated private practices that were staffed by nurse practitioners, board-certified or board-eligible obstetrician-gynecologists, perinatologists, or a combination of these.
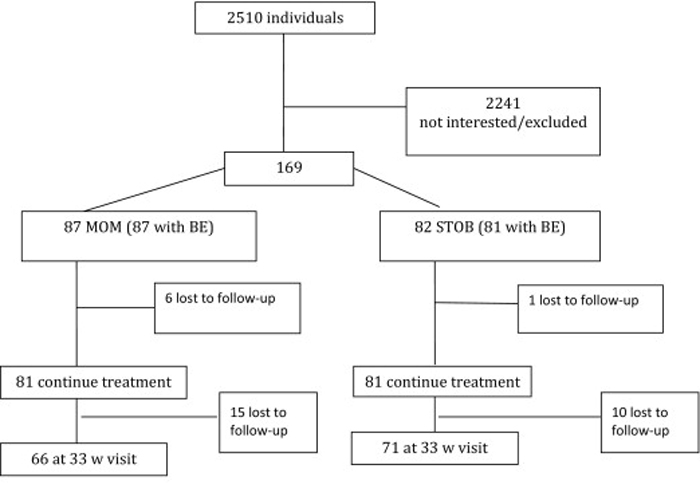
Figure 1 The study design is outlined in the Figure. Patients 1545 years old with a single fetus from 2428 weeks' gestation were evaluated by their obstetric provider for LBP, PP or both. Gestational age was calculated with a last menstrual period that corroborated with a first- or second-trimester ultrasound evaluation. Candidate patients with symptoms were screened by a dedicated study coordinator to identify exclusion criteria that included acute inflammatory disease, acute infectious disease, chronic back pain for >8 weeks before pregnancy, a mental health disorder, back pain from visceral disease, ongoing treatment for previous back pain, peripheral vascular disease, substance abuse, or litigation pending from back pain. Patients were not excluded if they had lower extremity neurologic symptoms or radiculopathy. A single, masked chiropractic specialist conducted the baseline evaluation (BE) with eligible volunteers before randomization. A blocked-randomization scheme was used across the 3 locations. With the use of an online Web Data Entry System that uses a computer-generated list of randomized numbers, subjects were allocated to the STOB group or the STOB plus multimodal musculoskeletal and obstetric treatment (MOM) group. [17, 18]
Three subjective questionnaires and 4 physical tests were used to quantify pain, disability, and physical function at the 24 to 28week BE. Current pain levels were assessed by the numeric rating scale (NRS), which is a subjective pain assessment tool that uses a rating of zero for no pain to a rating of 10 for a maximum level of pain. [19] The Quebec task force disability questionnaire (QDQ) assessed the impact of pain. The personal pain history (PPH) detailed the previous course and features of pain complaints. [20] The physical assessments to identify the origin of pain included the straight leg raise (SLR), posterior PP provocation test, active SLR, and long dorsal ligament test. [2124] These assessment tests commonly are used for lumbar and pelvic examinations.
Patients in the STOB group received total care from a self-chosen obstetric provider who had the discretion to recommend ≥1 of the following remedies: rest, aerobic exercise, heating pad application for a maximum length of 10 minutes, use of acetaminophen for mild pain, or narcotics for discomfort unrelieved by other measures. Referral to orthopedic or neurologic services was used for cases in which pain was debilitating or unresponsive to standard modalities.
Like the STOB group, the frequency of obstetrics visits for patients in the MOM group was also dictated by their self-chosen obstetrics providers. The MOM group additionally had weekly visits with a chiropractic specialist who provided education, manual therapy, and stabilization exercises, based on the biopsychosocial model. [14] The biopsychosocial model explains that a patient's pain syndrome is not comprised solely of the injured body structure but also includes psychologic and social components, such as fear of movement and high pain expectancy. Patients were reassured the pain experienced was unlikely pathologic and that reactivation of joint and muscle mobility by exercise would likely improve symptoms and signs without posing risk to the patient or her fetus. The goal of manual therapy was to restore joint motion and reduce muscle tension. Hypomobile joints were assessed with the long dorsal ligament test, posterior PP provocation test, and clinical palpation and were treated with routine joint mobilization. Joint mobilization techniques were performed by gently moving hypomobile joints in their restricted directions to help restore proper range of motion. Muscle tension was evaluated by clinical palpation and was treated with postisometric relaxation and myofascial release. [25] The stabilization exercises were targeted to strengthen the muscles that supported the low back and pelvis, because these muscles maintain the spine and hip stability that are important for the increased load that is created by pregnancy. The gluteus maximus, gluteus medius, quadratus lumborum, abdominal wall, and intrinsic spine muscles were targeted in the quadruped, supine, or side-lying positions. Patients were instructed to perform their home exercise program twice daily. Patient compliance with their home exercise program was not tracked but was encouraged with each follow-up visit.
Self-administered exercises initially were selected from a standardized protocol that is used for low back and pelvic stabilization. [26] Sacroiliac belts were reserved for cases with significant hypermobility or when a patient's pain restricted exercise performance. [16] Patients who were assigned to the MOM group had weekly appointments with the chiropractic specialist until 33 weeks' gestation.
Each participant in the STOB and MOM groups was reevaluated at 33 weeks' gestation to allow participants 46 treatments after the BE, which is an amount of treatment that is typical in clinical practice. The actual number of treatment visits for individual participants was not recorded. Reevaluation was done by the initial masked provider who was unaware of patient group allocation and who used the self-reported measures and the same physical tests that were used before random assignment, with the following modifications. The PPH was edited to exclude redundant questions and to include queries about routine visits, unscheduled provider visits in the office, or in an urgent care facility, the pain medication used, and days absent from work. An additional test, the Patient's Global Impression of Change, was included to assess the participant's perception of clinical improvement. [27] The self-reported measures were the primary outcome variables and were used in the a priori power analysis to determine sample size. The physical assessment measures were secondary outcome variables between the 2 groups. Patients in both the STOB and MOM groups received care only from their obstetrics providers after the visit at 33 weeks' gestation.
Power analysis used the NRS and the QDQ as the primary outcome variables. Sample size for the QDQ was computed in the following manner: power, 0.9; standard deviation, 1.0; change of 1 for the treatment group; change of 0.4 for the control group; alpha, .05; 2sided test; correlation between measurements, 0.5; resulting in a number of 120. For the NRS, a clinically meaningful difference was defined as 2 units. Assuming a standard deviation of 2 for each group, a correlation of 0.5 between measurements, and a STOB group change of 0.5 units, 120 patients would yield a power estimate for the NRS to detect the listed changes of 0.96. When we allowed for a 20% drop out rate, a sample size of 144 women was indicated as having adequate power.
Differences in demographic and obstetric characteristics and pain indices at BE between women in the MOM group and the STOB group were assessed with either the χ2 test (for categoric variables) or the t test (for continuous variables). Mixed models repeated measures of analysis was used to assess a 2level fixed group effect (MOM vs STOB), a 2level random time period effect (BE at 2428 weeks' gestation vs follow-up examination at 33 weeks' gestation), and an interaction effect of pain indices. Additional within-group contrasts between time points were performed for each of the MOM and STOB groups; between-group contrasts were performed between the MOM and STOB groups at each time point. We report the within-group and between-group findings between BE and 33 weeks' gestation to examine the effectiveness of the intervention at relieving LBP and PP during pregnancy. The primary outcome measures were the NRS and QDQ variables. Secondary outcomes included improvements in all other pain indices and reductions in medication use, trouble sleeping, and work absenteeism. Generalized estimating equations techniques were used to model discrete secondary outcome variables over time. The Bonferroni correction was used to adjust for multiple comparisons (P < .05/10 = P < .005). All analyses were performed using SAS software (version 9.2 for Windows [Microsoft, Redmond, WA]; SAS Institute Inc, Cary, NC).
Results

Table 1

Table 2

Table 3 Demographic and obstetric characteristics at BE were similar between the intervention (MOM) and control (STOB) groups (Table 1). One hundred sixty-nine patients were recruited, which represented 25 more than the original power calculations allotted. Recruitment occurred with a targeted dropout rate, and data for all patients who were recruited were analyzed. Despite the extra patients, the 2 groups remained similar. No significant differences in any pain index were found between the 2 groups at BE (Table 2). A significant reduction from BE to 33 weeks' gestation was found on 7 pain indices in the MOM group (NRS, QDQ, SLR [left], active SLR, long dorsal ligament test, PPH [leg and shoulders]) but on only 1 pain index in the STOB group (PPH [leg]). Women in the STOB group reported a significant increase from BE to 33 weeks' gestation on 5 pain indices (QDQ, SLR [left and right], active SLR, and PPH [pubic/groin]). Interactions that indicated that the change from BE to 33 weeks' gestation was significantly different for the MOM and STOB groups were found for NRS, QDQ, SLR [left and right], posterior PP provocation test [left], active SLR, and long dorsal ligament test. The 2 groups did not differ on the use of prescription or over-the-counter pain medications, trouble sleeping, or absenteeism from work at BE (Table 3). The MOM group reported significantly less trouble sleeping at 33 weeks' gestation than the STOB group.
Comment
Our data reject the null hypothesis that the effects of a multimodal approach to treating LBP/PP that is specific to pregnancy are not different from standard obstetric care. We have shown that a combination of manual therapy, exercise, and patient education reduces pain and disability when applied at 2433 weeks' gestation. The benefits derived are both subjective and objective. Patients perceived less pain and disability and an overall global improvement in daily activities. Their physical examinations revealed improved range of motion, stability, and less irritation at the lumbar and pelvic joints. Notably, no adverse events were reported in either group. We conclude that a multimodal approach to musculoskeletal LBP/PP that is instituted in the late second and early third trimesters of pregnancy benefits patients above and beyond standard obstetrics provider care.
Disability with pregnancy-related LBP causes some patients to restrict normal activities and to seek sick leave. [1, 8, 9] Contributing factors to LBP and PP in pregnancy include increased spine load from body habitus changes and joint hypermobility from the hormonal environment characteristic of pregnancy. [4] Importantly, there is little relationship between pregnancy-related back pain and structural disease, such as disc disease or spondylolisthesis. [28] Women with LBP/PP during pregnancy often report pain that progresses in severity throughout the day, which is a presentation that is consistent with overuse during the activities of daily living. [29, 30] This contrasts with the localized inflammation of a herniated disc, for which pain is highest in the morning and improves with mobility. [31] These findings are reassuring that LBP/PP during pregnancy are unlikely linked to a structural source; although without imaging, lumbar structural disease cannot be ruled out completely. The cause of the symptoms instead reflects a combination of biomechanical factors that yield abnormal loading on muscles and joints and behavioral factors that are related to inadequate patient coping strategies. [3234] The foundation for the multimodal approach to back pain was based, in part, on these premises.
Chiropractic interventions and education, meshed with standard prenatal practice, led to an improvement in the MOM group that were not observed in the STOB group between 24 and 33 weeks' gestation, as assessed by the self-report NRS and QDQ questionnaires. The 33 weeks' gestation assessment of the patient global improvement change and most, but not all, tests of physical assessment at 33 weeks' gestation were better in the MOM group, compared with the STOB group. These results suggest that the multimodal approach in the MOM treatment in pregnancy reduces pain and discomfort, while improving the quality of daily activities for pregnant women who experience LBP/PP. We cannot state which of the 3 components of the multimodal approach was most influential in the outcome that was observed because there is support for each component; manual therapy, exercise, and education.
observational study of chiropractic manipulation as part of a diagnosis-based clinical decision rule to treat pregnancy-related lumbar-PP. Clinically significant improvements in disability were found in 73% of patients; 82% of patients demonstrated clinically significant reductions in pain. This was not a randomized trial, nor was there a control group. Recently, osteopathic manipulation was found to slow the progression of deterioration of back-specific functioning when compared with standard obstetrics care and sham ultrasound evaluation, although a reduction in pain was not different between the groups. [13] The authors used a similar combination of manual techniques and also gave discretion to the treating physician to select procedures for specific patients based on the physical findings.
Exercises such as stabilization training, pelvic tilting, and water gymnastics benefit some pregnant women with LBP/PP. [36] An intense program of 15 different exercises that were performed in 60 minutes 3 times a week under the supervision of a midwife improved strength and reduced pain intensity of women between 17 and 22 weeks' gestation, compared with control subjects who did not exercise. [12] Our exercise intervention, which was individualized for each patient by the chiropractic provider, consisted of ≤4 exercises that were performed at home. This suggests that a less intense, self-administered exercise program may provide an equal benefit to reduce pain and to allow the patient to conduct the exercises in her home.
Patient education has long been a staple of care for LBP. Patients with poor coping strategies, such as catastrophizing and fear-avoidance, have poorer outcomes compared with those who show insights into symptoms and greater self-efficacy. [14] Notably, fear avoidance behaviors decondition and weaken muscle tissue, which in turn leads to less spine stability. Our education component emphasized these issues and yielded improved perceptions. Biopsychosocial education or education on self-treatment and decreasing fear avoidance are alternative approaches for improving symptoms of LBP/PP in pregnant women. [37]
Major strengths of our study included the randomized clinical trial design and the application to a population with a diverse socioeconomic status, which commonly is observed in general obstetrics practice. An additional strength was the use of a comprehensive multimodal approach. Our study had limitations. We screened >2500 patients and enrolled only 169 women who met inclusion criteria and were interested in participation. Our exclusion criteria were necessarily numerous, and we likely enrolled patients who were motivated to achieve successful results. Responses to complaints of LBP/PP may vary among obstetrics providers, which could not be controlled completely in either group. Our data also could not discern which specific treatment or combination of treatments provided the most clinical benefit. Some benefits are reported for each modality in the nonrandomized trials cited earlier. In addition, our trial did not use sham treatments, nor was placebo controlled; thus, placebo effect could have contributed to patients' improvement. Last, this trial did not evaluate the use of prophylactic treatment, which is a potentially important component to therapy that warrants future study in pregnant populations.
References
Kristiansson, P., Svarsudd, K., and von Schoultz, B.
Back pain during pregnancy: a prospective study.
Spine. 1996; 21: 702709Stapleton, D.B., MacLennan, A.H., and Kristiansson, P.
The prevalence of recalled low back pain during and after pregnancy:
a south Australian population survey.
Aust N Z J Obstet Gynaecol. 2002; 42: 482485Wang, S.M., Dezinno, P., Maranets, I., Berman, M.R.
Low back pain during pregnancy: prevalence, risk factors and outcomes.
Obstet Gynecol. 2004; 104: 6570Mogren, I.M. and Pohjanen, A.I.
Low back pain and pelvic pain during pregnancy: prevalence and risk factors.
Spine. 2005; 30: 983991Haugland, K.S., Rasmussen, S., and Daltveit, A.K.
Group intervention for women with pelvic girdle pain in pregnancy:
a randomized controlled trial.
Acta Obstet Gynecol Scand. 2006; 85: 13201326Nilsson-Wikmar, L., Holm, K., Oijerstedt, R.
Effect of three different physical therapy treatments on pain and activity in
pregnant women with pelvic girdle pain: a randomized clinical trial with
3, 6, and 12 months follow-up postpartum.
Spine. 2005; 30: 850856Noren, L., Ostgaard, S., Johansson, G., and Ostgaard, H.C.
Lumbar back and posterior pelvic pain during pregnancy: a 3-year follow-up.
Eur Spine J. 2002; 11: 267271Wu, W., Meijer, O.G., Jutte, P.C. et al.
Gait in patients with pregnancy-related pain in the pelvis: an emphasis on
the coordination of transverse pelvic and thoracic rotations.
Clin Biomech. 2002; 17: 678686Owens, K., Pearson, A., and Mason, G.
Symphysis pubis dysfunction: a cause of significant obstetric morbidity.
Eur J Obstet Gynecol Reprod Biol. 2002; 105: 143146Ostgaard, H.C., Zetherstrom, G., and Roos-Hansson, E.
Back pain in relation to pregnancy.
Spine. 1997; 22: 29452950Brynhildsen, J., Hansson, A., Persson, A., and Hammard, M.
Follow-up of patients with low back pain during pregnancy.
Obstet Gynecol. 1998; 91: 182186Garshasbi, A. and Faghih Zadeh, S.
The effect of exercise on the intensity of low back pain in pregnant women.
Int J Gynaecol Obstet. 2005; 88: 271275Licciardone, J.C., Buchanan, S., Hensel, K., King, H.H.
Osteopathic manipulative treatment of back pain and related symptoms
during pregnancy: a randomized controlled trial.
Am J Obstet Gynecol. 2010; 202: 43.e143.e8Murphy DR, Hurwitz EL, McGovern EE:
Outcome of Pregnancy-Related Lumbopelvic Pain Treated According to a Diagnosis-Based Decision Rule:
A Prospective Observational Cohort Study
J Manipulative Physiol Ther 2009 (Oct); 32 (8): 616624Ee, C.C., Manheimer, E., Pirotta, M.V., and White, A.R.
Acupuncture for pelvic and back pain in pregnancy: a systematic review.
Am J Obstet Gynecol. 2008; 198: 254259Mens, J.M., Damen, L., Snijders, C.J., and Stam, H.J.
The mechanical effect of a pelvic belt in patients with
pregnancy-related pelvic pain.
Clin Biomech. 2006; 21: 122127Thompson, P.A.
The web data entry system: reference guide, version 2.5.
Division of Biostatistics,
Washington University, St. Louis, MO; 2003Thompson, P.A.
The web data entry system: reference guide, version 2.5.
Division of Biostatistics,
Washington University, St. Louis, MO; 2003Willamson, A. and Hoggart, B.
Pain: a review of three commonly used pain rating scales.
J Clin Nurs. 2005; 14: 798804Demoulin C, Ostelo R, Knotterus JA, Smeets RJ.
Quebec back pain disability scale was responsive and showed reasonable
interpretability after a multidisciplinary treatment.
J Clin Epidemiol;63:1249-55.Rabin, A., Gerszten, P.C., Karausky, P., Bunker.
The sensitivity of the seated straight-leg raise test compared with the
supine straight-leg raise test in patients presenting with magnetic
resonance imaging evidence of lumbar nerve root compression.
Arch Phys Med Rehabil. 2007; 88: 840843Ostgaard, H.C., Zetherstrom, G., and Roos-Hansson, E.
The posterior pelvic pain provocation test in pregnant women.
Eur Spine J. 1994; 3: 258260de Groot, M., Pool-Goudzwaard, A.L., Spoor, C.W.
The active straight leg raising test (ASLR) in pregnant women: differences in
muscle activity and force between patients and healthy subjects.
Man Ther. 2008; 13: 6874Vleeming, A., Albert, H.B., Ostgaard, H.C..
European guidelines for the diagnosis and treatment of pelvic girdle pain.
Eur Spine J. 2008; 17: 794819Lewit, K.
Manipulative therapy in rehabilitation of the motor system.
London: Butterworths. 2002; : 261275McGill, S.
Low back disorders: evidenced-based prevention and rehabilitation.
in: Human Kinetics,
Champaign, IL; 2002: 213229Farrar, J.T., Young, J.P. Jr, La Mareaux, L.
Clinical importance of changes in chronic pain intensity measured on an
11-point numerical pain rating scale.
Pain. 2001; 94: 149158Chan, Y.L., Lam, W.W.M., Lau, T.K., Metreweli, C.
Back pain in pregnancy: magnetic resonance imaging correlation.
Clin Radiol. 2002; 57: 11091112Mantle, M.J., Greenwood, R.M., and Currey, H.L.F.
Backache in pregnancy.
Rheumatol Rehabil. 1977; 16: 95101Nwuga, V.C.B.
Pregnancy and back pain among upper class Nigerian women.
Aust J Physiother. 1982; 28: 811Snook, S.H., Webster, B.S., McGorry, R.W.
The reduction of chronic non-specific low back pain through the control
of early morning lumbar flexion: a randomized controlled trial.
Spine. 1998; 23: 26012607Waddell, G., Somerville, D., Henderson, I., and Newton, M.
Objective clinical evaluation of physical impairment in chronic low back pain.
Spine. 1992; 17: 617628Cherkin, D.C., Deyo, R.A., Street, J.H., and Barlow, W.
Predicting poor outcomes for back pain seen in primary care using
patients' own criteria.
Spine. 1996; 21: 29002907Bronfort, G. and Bouter, L.M.
Responsiveness of general health status in chronic low back pain:
a comparison of the COOP charts and the SF-36.
Pain. 1999; 83: 201209Lisi, A.J.
Chiropractic Spinal Manipulation for Low Back Pain of Pregnancy:
A Retrospective Case Series
J Midwifery Womens Health 2006 (Jan); 51 (1): e7-10Pennick, V.E. and Young, G.
Interventions for preventing and treating pelvic and back pain in pregnancy.
Cochrane Database Syst Rev. 2007; (CD001139)Bastiaenen, C.H., de Bie, R.A., Wolters, P.M. et al.
Effectiveness of a tailor-made intervention for pregnancy-related pelvic girdle
and/or low back pain after delivery: short-term results of a randomized clinical trial.
BMC Musculoskeletal Disord. 2006; 27: 719
Return to PEDIATRICS
Return to PREGNANCY AND CHIROPRACTIC
Since 1312015


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |