Association Between Use of Acid-Suppressive Medications and
Antibiotics During Infancy and Allergic Diseases in Early ChildhoodThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: JAMA Pediatrics 2018 (Jun 4); 172 (6): e180315 ~ FULL TEXT
OPEN ACCESS Edward Mitre, MD; Apryl Susi, MS; Laura E. Kropp, MPH
Department of Microbiology and Immunology,
F. Edward Hébert School of Medicine,
Uniformed Services University of the Health Sciences,
Bethesda, Maryland
A new Study reports: Infants who are given antacids like Zantac or Pepcid
are more likely to develop childhood allergies
FROM: TIME Magazine ~ April 2, 2018 ~ FULL TEXT
Infants who are given antacids like Zantac or Pepcid are more likely to develop childhood allergies, perhaps because these drugs may alter their gut bacteria, a new large study suggests.
Early use of antibiotics also raised the chances of allergies in the study of nearly 800,000 children.
Researchers combed the health records of kids born between 2001 and 2013 and covered by Tricare, an insurance program for active duty and retired military personnel and their families. A surprising 9 percent of the babies received antacids, reflecting the popularity of treating reflux in infancy.
Over four years, more than half of all the children developed allergies to foods or medications, rashes, asthma, hay fever or other allergic diseases. The study couldn’t prove causes, but the connection with antacids and antibiotics was striking.
For children who received an antacid during their first six months, the chances of developing a food allergy doubled; the chances of developing a severe allergic reaction called anaphylaxis or hay fever were about 50 percent higher. For babies who received antibiotics, the chances doubled for asthma and were at least 50 percent higher for hay fever and anaphylaxis.
The results were published Monday in JAMA Pediatrics.
The Abstract:Importance Allergic diseases are prevalent in childhood. Early exposure to medications that can alter the microbiome, including acid-suppressive medications and antibiotics, may influence the likelihood of allergy.
Objective To determine whether there is an association between the use of acid-suppressive medications or antibiotics in the first 6 months of infancy and development of allergic diseases in early childhood.
Design, Setting, and Participants A retrospective cohort study was conducted in 792,130 children who were Department of Defense TRICARE beneficiaries with a birth medical record in the Military Health System database between October 1, 2001, and September 30, 2013, with continued enrollment from within 35 days of birth until at least age 1 year. Children who had an initial birth stay of greater than 7 days or were diagnosed with any of the outcome allergic conditions within the first 6 months of life were excluded from the study. Data analysis was performed from April 15, 2015, to January 4, 2018.
Exposures Exposures were defined as having any dispensed prescription for a histamine-2 receptor antagonist (H2RA), proton pump inhibitor (PPI), or antibiotic.
Main Outcomes and Measures The main outcome was allergic disease, defined as the presence of food allergy, anaphylaxis, asthma, atopic dermatitis, allergic rhinitis, allergic conjunctivitis, urticaria, contact dermatitis, medication allergy, or other allergy.
Results Of 792,130 children (395,215 [49.9%] girls) included for analysis, 60,209 (7.6%) were prescribed an H2RA, 13,687 (1.7%) were prescribed a PPI, and 131,708 (16.6%) were prescribed an antibiotic during the first 6 months of life. Data for each child were available for a median of 4.6 years. Adjusted hazard ratios (aHRs) in children prescribed H2RAs and PPIs, respectively, were 2.18 (95% CI, 2.04–2.33) and 2.59 (95% CI, 2.25–3.00) for food allergy, 1.70 (95% CI, 1.60–1.80) and 1.84 (95% CI, 1.56–2.17) for medication allergy, 1.51 (95% CI, 1.38–1.66) and 1.45 (95% CI, 1.22–1.73) for anaphylaxis, 1.50 (95% CI, 1.46–1.54) and 1.44 (95% CI, 1.36–1.52) for allergic rhinitis, and 1.25 (95% CI, 1.21–1.29) and 1.41 (95% CI, 1.31–1.52) for asthma. The aHRs after antibiotic prescription in the first 6 months of life were 2.09 (95% CI, 2.05–2.13) for asthma, 1.75 (95% CI, 1.72–1.78) for allergic rhinitis, 1.51 (95% CI, 1.38–1.66) for anaphylaxis, and 1.42 (95% CI, 1.34–1.50) for allergic conjunctivitis.
Conclusions and Relevance This study found associations between the use of acid-suppressive medications and antibiotics during the first 6 months of infancy and subsequent development of allergic disease. Acid-suppressive medications and antibiotics should be used during infancy only in situations of clear clinical benefit.
Key Points
Question Does use of medications that disturb the microbiome in infancy increase subsequent risk of developing allergic diseases?
Findings In this cohort study of 792,130 children, the hazard of developing an allergic disease was significantly increased in those who had received acid-suppressive medications or antibiotics during the first 6 months of life.
Meaning Exposure to acid-suppressive medications or antibiotics in the first 6 months of life may increase risk of allergic disease development.
From the FULL TEXT Article:
Introduction
The cumulative prevalence of allergic diseases and asthma has risen over the past several decades. In particular, food allergy has exhibited a particularly brisk rise in children [1–4] Furthermore, studies in the United States and other countries have demonstrated increases in allergic rhinoconjunctivitis, asthma, skin prick test positivity to common allergens, and hospitalizations due to anaphylaxis. [5–9]
An area of environmental change that may be contributing to the rise of allergic diseases is the increasing use of medications that alter the development of the human microbiome. Both gastric acid–suppressive medications and antibiotics have been implicated as factors that may enhance the development of allergic diseases. [10–13] Use of these medications, which can directly cause intestinal dysbiosis, is of concern in light of increasing evidence that alterations in the human microbiome can increase the risk for allergy development. [11, 14, 15] Furthermore, acid-suppressive medications, which reduce protein digestion, can affect how ingested antigens are processed in the intestinal tract. Acid suppression has been shown to increase immunoglobulin (Ig) E production in response to orally ingested antigens in animal studies [16, 17] and has been associated with food and medication allergy in humans. [18–20]
The objective of this study was to evaluate the hypothesis that exposure to either acid-suppressive medications or antibiotics during infancy is associated with an increased risk of childhood allergic diseases. We tested this hypothesis by conducting a retrospective cohort analysis investigating the development of allergy in children in the TRICARE Management Activity Military Health System (MHS) database.
Methods
Data Source and Inclusion Criteria
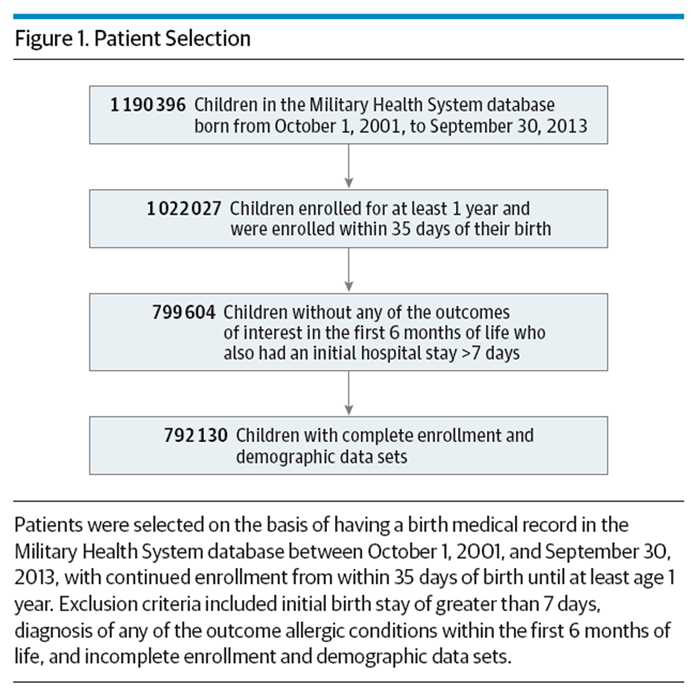
Figure 1 A retrospective cohort study was performed using the TRICARE Management Activity MHS database. TRICARE Management Activity oversees health care delivery for US uniformed service members and their dependents. The MHS database includes all outpatient and inpatient billing records as well as outpatient pharmacy use for all eligible uniformed services members and their dependents in both military and civilian facilities. The database includes billing data for military patients seen outside the direct care military system. The MHS database was queried to identify all children eligible for health care starting at birth. To be included in the study, children must have had a birth medical record in the database between October 1, 2001, and September 30, 2013, and continued enrollment from within 35 days of birth until at least age 1 year. Also, children who were diagnosed with any of the outcome allergic conditions within the first 6 months of life were excluded from the study, as were children who had incomplete enrollment or demographic data and children who had an initial birth stay of greater than 7 days (Figure 1).
). [21] The name of the medication, the number of days supplied, and the age at which the prescription was dispensed were extracted for each eligible child. Exposures for the primary analysis were defined as binary time-fixed variables, categorically defined as any dispensed H2RA, PPI, or antibiotic in the first 6 months of life.
The study was reviewed and approved by the institutional review board of the Uniformed Services University with waiver of informed consent. Data were deidentified.
Variable Definitions
The MHS database was used to identify children prescribed an outpatient histamine-2 receptor antagonist (H2RA), proton pump inhibitor (PPI), or antibiotic at any time prior to age 6 months using the American Hospital Formulary Service classification (listed in eTable 1 in the Supplement
Allergic disease outcomes were defined using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes. For each child, the outcomes were evaluated from age 6 months and censored at time of health care disenrollment or the end of the study (September 30, 2014). Each child’s outpatient and inpatient medical visits were extracted using MHS claim files from October 1, 2001, to September 30, 2014. An event was defined as the first medical encounter with the specific allergic condition categorized by ICD-9-CM diagnostic codes listed in eTable 2 in the Supplement). Additional variables obtained using ICD-9-CM codes included prematurity and birth by cesarean delivery (eTable 2 in the Supplement).
Statistical Analysis
We summarized continuous data with medians and interquartile ranges (IQRs) and categorical data with numbers and percentages. Because the medication groups were not mutually exclusive, comparison of demographics and other variables for children receiving H2RAs, PPIs, antibiotics, or no medications were compared using repeated-measures methods. [22] Cox proportional hazards regression was used to calculate the hazard ratio (HR) of developing an allergic condition based on exposure to PPIs, H2RAs, and antibiotics in the first 6 months of life. In the adjusted models, adjusted HRs (aHRs) were calculated after adjusting for prematurity, cesarean delivery, sex, the other drug classes, and any significant first-order interaction terms. The Cox proportional hazards regression assumption was evaluated by visual inspection for parallelism of the log-log survival curves for each variable in the models as well as evaluating correlation of the Schoenfeld residuals with time. When variables violated the Cox proportional hazards regression assumption with either log-log curves that were not parallel or the Schoenfeld residuals with time were significant, an interaction of the variable with time was included in the model. [23]
For evaluating whether there is a dose-related risk of developing allergy, patient groups receiving H2RAs or PPIs were divided into those receiving no medication, 1 to 60 days of medication, and more than 60 days of medication. For antibiotic use, low and high doses were defined as up to and including 10 days and over 10 days of antibiotics. Cutoff levels for low and high doses of acid-suppressive medications and antibiotics were based on median days prescribed. Cox proportional hazards regression was used to evaluate the HR for these medication groups. Estimated survival curves based on the Cox proportional hazards regression models were constructed for visualization purposes. [24] In addition, a subanalysis was performed to evaluate the effect of medications among those who had a diagnosis of gastroesophageal reflux disease (GERD). Data analysis was conducted from April 15, 2015, to January 4, 2018.
Two-tailed P values <.05 were considered statistically significant. Analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Results
Patient Characteristics and Use of Acid-Suppressive Medications and Antibiotics

Table 1 A total of 792,130 children had birth medical records in the database between October 1, 2001, and September 30, 2013, and met all other inclusion criteria (Figure 1). During the first 6 months of life, 60,209 children (7.6%) were prescribed a histamine-2 receptor antagonist (H2RAs), 13,687 (1.7%) were prescribed a proton pump inhibitor (PPI), and 131,708 (16.6%) were prescribed an antibiotic (Table 1). Several children were prescribed multiple medications. Of all children, 12,546 (1.6%) received H2RAs and antibiotics, 5,520 (0.7%) received H2RAs and PPIs, 1,590 (0.2%) received PPIs and antibiotics, and 2549 (0.3%) received all 3 medications. Data for each child were available for a median of 4.6 years (IQR, 2.5–7.9).
The most frequently used H2RA was ranitidine, accounting for 95.1% of all H2RA prescriptions. The next most frequently prescribed H2RAs were nizatidine and famotidine. Lansoprazole was the most commonly prescribed PPI, totaling 75.5% of all days supplied of PPI prescriptions. The median days supplied for both H2RAs and PPIs was 60 (IQR for H2RAs, 39–92; IQR for PPIs, 30-91).
Table 2 Median days of antibiotics prescribed was 10 (IQR, 10–20), and the total number of days was more than 3.4 million. The most common classes were penicillins, comprising 65.3% of the total days of prescribed antibiotics, cephalosporins comprising 14.2%, and macrolides comprising 10.1%. The top 3 individual antibiotics prescribed were amoxicillin (55.0%), amoxicillin clavulanate (9.0%), and trimethoprim with sulfamethoxazole (8.7%). Among those prescribed antibiotics in the first 6 months of life, the median age at which children were first prescribed antibiotics was 125 days (IQR, 88–155) and was greater than the age of first prescription for both H2RAs (61 days: IQR, 37–94) and PPIs (73 days: IQR, 47–117).
A greater percentage of boys was prescribed H2RAs (32,052 [8.1%]), PPIs (7,491 [1.9%]), and antibiotics (70,933 [17.9%]) than girls (28,157 [7.1%] for H2RAs; 6,196 [1.6%] for PPIs; and 60,775 [15.4%] for antibiotics; P < .001 for all comparisons). Premature infants were more likely to be prescribed H2RAs (4,177 [13.4%]) and PPIs (1,040 [3.3%]) than full-term infants (56,032 [7.4%] for H2RAs; 12,647 [1.7%] for PPIs; P < .001 for both comparisons). There was no significant difference in the percentage of children born prematurely given antibiotics compared with full-term infants (5212 [16.8%] vs 126,496 [16.1%]; P = .46).
Allergy Diagnoses
Of the 792,130 children in this cohort, 24,514 (58.7/10,000 person-years) were diagnosed with a food allergy.
Among the identified food allergies,peanut allergy was the most common (13.8/10,000 person-years), followed by
cow’s milk allergy (10.7/10,000 person-years) and
egg allergy (7.2/10,000 person-years) (Table 2).
With regard to other allergic diseases, the 2 most common diagnoses wereallergic rhinitis (771.5/10,000 person-years) and
contact dermatitis (703.2/10,000 person-years).
The next most common nonfood allergies wereatopic dermatitis (300.0/10,000 person-years),
asthma (297.7/10,000 person-years), and
urticaria (180.4/10,000 person-years) (Table 2).All covariates analyzed exhibited a statistically significant association with allergic disease (eTable 3 in the Supplement). A greater percentage of boys developed an allergic condition than girls (59.5% vs 56.6%; P < .001). Children born by cesarean delivery and those born prematurely also demonstrated an increased risk of allergic disease compared with children without those conditions (59.4% vs 57.5% and 59.0% vs 58.0%, respectively; P < .001 for both).
Association Between Acid-Suppressive Medications and Allergic Diseases
Table 3 Every allergic disease assessed exhibited a significantly increased risk in children who had received histamine-2 receptor antagonist (H2RAs) or proton pump inhibitor (PPIs) during infancy except for seafood allergy (Table 3), which had the lowest incidence of the conditions evaluated (Table 2). Adjusted HRs were greatest for food allergies as a category, with an aHR of 2.18 for H2RAs (95% CI, 2.04–2.33) and an aHR of 2.59 (95% CI, 2.25-3.00) for PPIs. Of the specific types of food allergies, use of acid-suppressive medications increased the risk of being diagnosed with cow’s milk allergy the most (aHR, 2.42; 95% CI, 2.22–2.64 for H2RAs and aHR, 4.43; 95% CI, 3.48–5.65 for PPIs). For children who received H2RAs, the aHRs for developing peanut, egg, or other food allergies were 1.21 (95% CI, 1.09–1.33), 1.74 (95% CI, 1.52–1.99), and 2.13 (95% CI, 1.98–2.30), respectively.
Similarly, prescription of PPIs during infancy was associated with an aHR for peanut, egg, or other food allergies of 1.27 (95% CI, 1.05–1.52), 1.35 (95% CI, 1.08–1.69), and 2.68 (95% CI, 2.23–3.20), respectively. Consistent with the entire study population, children diagnosed with GERD during infancy also demonstrated increased incidence densities and aHRs for food allergy after treatment with H2RAs and PPIs (eTables 4 and 5 in the Supplement).
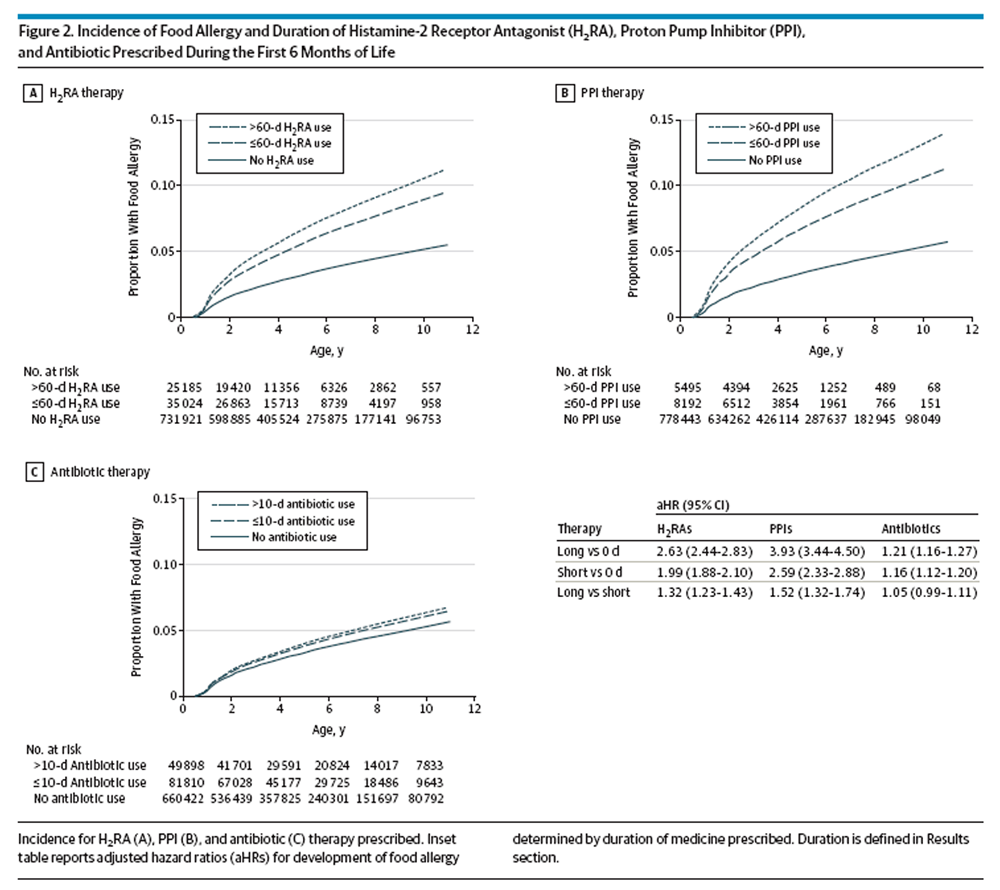
Figure 2 Risk of food allergy after treatment with acid-suppressive medications exhibited a dose-dependent effect with regard to days of medication prescribed (antibiotic short duration indicates 10 or fewer days; long duration, 11 or more days; H2RA and PPI short duration, 60 or fewer days; long duration, 61 or more days) (Figure 2). Children prescribed more than 60 days of PPIs had a 52% greater risk of being diagnosed with food allergy during childhood than those prescribed 1 to 60 days of PPIs (aHR of food allergy in children prescribed >60 days vs 1–60 days of PPIs: 1.52; 95% CI, 1.32–1.74). A dose-dependent effect was also observed with H2RAs (aHR of food allergy in children prescribed >60 days of H2RA vs 1–60 days: 1.32; 95% CI, 1.23–1.43).
All nonfood allergic diseases studies also were found to be significantly associated with the use of acid-suppressive medication therapy during infancy (Table 3). Of the nonfood allergy–related conditions, the allergic diseases exhibiting the greatest increased risk after acid-suppressive therapy were medication allergy (aHR, 1.70; 95% CI, 1.60–1.80 for H2RAs and 1.84; 95% CI, 1.56–2.17 for PPIs), allergic rhinitis (aHR, 1.50; 95% CI, 1.46–1.54 for H2RAs and 1.44; 95% CI, 1.36–1.52 for PPIs), anaphylaxis (aHR, 1.51; 95% CI, 1.38–1.66 for H2RAs and 1.45; 95% CI, 1.22–1.73 for PPIs), and other allergy (aHR, 1.63; 95% CI, 1.55–1.71 for H2RAs and 1.62; 95% CI, 1.45–1.80 for PPIs). Acid-suppressive medication therapy was also associated with a marked increase in asthma, allergic conjunctivitis, and urticaria during childhood.
Association Between Antibiotics and Allergic Diseases
As with acid-suppressive medications, prescriptions for antibiotics during infancy also were significantly associated with an increased risk of allergic diseases. The aHR for development of any food allergy was 1.14 (95% CI, 1.10–1.18) in children who had received antibiotics. While neither peanut allergy nor seafood allergy exhibited significantly increased risk, antibiotic use during infancy was associated with an increased risk of both cow’s milk allergy and egg allergy (aHR, 1.24 for both; 95% CI, 1.15–1.33 for cow’s milk allergy and 95% CI, 1.13–1.37 for egg allergy). Unlike acid-suppressive medications, antibiotics did not demonstrate a dose-dependent risk for development of food allergy (aHR, 1.05; 95% CI, 0.99–1.11 for developing any food allergy in children prescribed >10 days of antibiotics compared with children prescribed 1–10 days) (Figure 2C).
Several nonfood allergic diseases were significantly increased in children who had received antibiotics during infancy. Antibiotics prescribed during infancy were associated with a greater than 2–fold risk of asthma in childhood (aHR, 2.09; 95% CI, 2.05–2.13). Risks of anaphylaxis (aHR, 1.51; 95% CI, 1.38–1.66), allergic conjunctivitis (aHR, 1.42; 95% CI, 1.34–1.50), and medication allergy (aHR, 1.34; 95% CI, 1.29–1.40) also were significantly increased in children who had received antibiotics as infants (Table 3). In addition, the incidence of atopic dermatitis, allergic rhinitis, contact dermatitis, urticaria, and other allergies were significantly increased in children who were prescribed antibiotics during infancy (Table 3).
Discussion
In this retrospective cohort study of 792,130 children, we found significant associations between the use of acid-suppressive medications or antibiotics in infancy and the development of allergic diseases in childhood. Use of acid-suppressive medications was positively associated with increased risks for all major categories of allergic disease and most strongly associated with food allergy. Infants prescribed histamine-2 receptor antagonist (H2RAs) and proton pump inhibitor (PPIs) during the first 6 months of infancy exhibited respective aHRs for food allergy of 2.18 and 2.59. Antibiotics were also significantly associated with all major categories of allergic disease. The aHRs after antibiotic prescription in the first 6 months of life were 2.09 for asthma, 1.14 for food allergy, and 1.40 or greater for anaphylaxis, allergic rhinitis, and allergic conjunctivitis. This study adds to the mounting evidence that agents that disrupt the normal intestinal microbiome during infancy may increase the development of allergic diseases.
With respect to acid-suppressive medications, both adults and children have been shown to have a greater risk of food allergy after a course of acid-suppressive therapy. [18, 20] Acid-suppressive medications may enhance allergic responses not only to food allergens but also to nonfood allergens. In our study, all of the nonfood allergies examined were significantly increased in children who had received H2RAs or PPIs during infancy. Our results are consistent with those of a murine study that observed increased levels of allergen-specific IgE following intraperitoneal sensitization with cimetidine, [25] as well as with a human study in Spain that found that use of PPIs during hospitalization is associated with increased risks of allergy to injected and orally administered medications. [19]
Our results are also in line with those of prior investigations that suggest a link between administration of antibiotics early in life and subsequent development of allergic diseases. A systematic review published in 2011 found that children given antibiotics in the first year of life had a pooled odds ratio of 1.52 for risk of allergy. [26] Similarly, a recent retrospective study of 30,060 children in the United States found strong associations between antibiotic use in the first 3 months of life and subsequent development of food and nonfood allergic diseases. [27] In our study, food allergy, atopic dermatitis, urticaria, contact dermatitis, medication allergy, allergic conjunctivitis, and anaphylaxis were increased by 9% to 51% in children who had been prescribed antibiotics during the first 6 months of life. Antibiotics were associated with an even greater risk for development of allergic diseases triggered by aeroallergens. Asthma was increased by over 100% and allergic rhinitis by 75% in children exposed to antibiotics during infancy.
While not fully understood, potential mechanisms by which acid-suppressive medications and antibiotics can increase allergic sensitization include intestinal dysbiosis and, for acid-suppressive medications, decreased protein digestion in the stomach. [13] There is mounting evidence from human and animal studies that a diverse microbiome plays a central role in the development of a healthy immune system and that perturbations in the microbiome can increase the risk for allergic diseases. [11, 14, 15, 28, 29] Studies suggest that healthy normal flora modulate innate immune responses and augment regulatory T-cell populations, potentially by production of short-chain fatty acids. [28] The status of the microbiome early in life appears to be especially important, as animal studies have shown that antibiotic treatment of neonatal mice—but not adult mice—increases the risk of allergic disease. [30, 31]
Acid-suppressive medications were associated with a greater risk of food allergy than antibiotic use. In addition to causing intestinal dysbiosis, acid-suppressive medications may increase sensitization to ingested antigens by decreasing protein breakdown in the stomach. Animal studies have shown that acid suppression inhibits the breakdown of ingested proteins and facilitates IgE antibody production. [16, 17] In addition, H2RAs may increase the risk of allergic disease by direct effects on the immune system as histamine is increasingly recognized as having a role in modulating immune system function. [32]
While there has been increasing recognition of the potential risks of antibiotic use during infancy, H2RAs and PPIs are considered to be generally safe and are commonly prescribed for children younger than 1 year. [33, 34] In the present study, 7.6% of all infants were prescribed an H2RA and 1.7% were prescribed a PPI. These medications are frequently given to infants who regurgitate food and appear to be fussy. For most infants, however, regurgitation of gastric contents is not a disease, but rather is a developmentally normal process. [35] A systematic review found little evidence to support the efficacy of H2RAs in infants, [36] and trials have not found clinical benefit of PPI therapy in infants with symptoms attributed to gastroesophageal reflux. [37, 38] Accordingly, commentaries have advised against the overuse of acid-suppressive medications in infants. [35, 39]
Limitations
A limitation of this study is the potential bias from reverse causality because it is possible that acid-suppressive medications or antibiotics were given for allergic diseases that were misdiagnosed as GERD or infectious diseases. While this bias may have played a role, it is unlikely to explain the entirety of our findings. The increased rates of anaphylaxis, urticaria, and medication allergy observed in this study are not likely due to reverse causality because their clinical manifestations do not substantially overlap with GERD or infectious diseases. With regard to food allergy, which can be confused with GERD in infants, [40] the rates of this disease in children younger than 6 months may be too low to have been the main driver of acid-suppressive medication prescriptions in this study. A birth cohort study that confirmed cases by the use of a double-blind, placebo-controlled food challenge found an adjusted incidence of cow’s milk allergy of only 0.54% during the first 2 years of life. [41] In addition, in our subanalysis among those with a diagnosis of GERD, we found statistically significant increases in food allergy for infants prescribed H2RAs and PPIs. This finding suggests that the identified association for these medications is likely not driven exclusively by misclassified cases of food allergies manifesting with symptoms of GERD.
Conclusions
To our knowledge, this is the largest study demonstrating an association between H2RAs, PPIs, and antibiotics given during infancy and the subsequent development of allergic diseases. The results are consistent with those of prior studies and have biological plausibility. Thus, this study provides further impetus that antibiotics and acid-suppressive medications should be used during infancy only in situations of clear clinical benefit. Additional studies will be required to confirm causality and determine the mechanism of action.
Conflict of Interest Disclosures:
None reported.
Author Contributions:
Ms Susi and Dr Nylund had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mitre, Susi, Gorman, Nylund.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Mitre, Susi, Kropp, Schwartz.
Critical revision of the manuscript for important intellectual content: Mitre, Susi, Kropp, Gorman, Nylund.
Statistical analysis: Susi, Nylund.
Obtained funding: Gorman.
Administrative, technical, or material support: Mitre, Schwartz, Gorman, Nylund.
Study supervision: Mitre, Gorman, Nylund.Disclaimer:
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Air Force, Department of Defense, or the United States Government. Title 17 U.S.C. 105 provides that “copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a US government work as “a work prepared by a military service member or employee of the United States government as part of that person's official duties.” This work was prepared as part of our official duties.
Additional Contributions:
Courtney Judd, MD, Allison Malloy, MD, and Kathleen Madden, PhD (Uniformed Services University of the Health Sciences) provided suggestions for the manuscript; there was no financial compensation.
References:
Branum AM, Lukacs SL.
Food allergy among children in the United States.
Pediatrics. 2009;124(6):1549-1555Koplin JJ, Mills EN, Allen KJ.
Epidemiology of food allergy and food-induced anaphylaxis: is there really a Western world epidemic?
Curr Opin Allergy Clin Immunol. 2015;15(5):409-416Platts-Mills TA.
The allergy epidemics: 1870-2010.
J Allergy Clin Immunol. 2015;136(1):3-13Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA.
US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up.
J Allergy Clin Immunol. 2010;125(6):1322-1326Asher MI, Montefort S, Björkstén B, et al;
ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys.
Lancet. 2006;368(9537):733-743Eder W, Ege MJ, von Mutius E.
The asthma epidemic.
N Engl J Med. 2006;355(21):2226-2235Rönmark E, Bjerg A, Perzanowski M, Platts-Mills T, Lundbäck B.
Major increase in allergic sensitization in schoolchildren from 1996 to 2006 in northern Sweden.
J Allergy Clin Immunol. 2009;124(2):357-363, 63.e1-63.e15Mulla ZD, Lin RY, Simon MR.
Perspectives on anaphylaxis epidemiology in the United States with new data and analyses.
Curr Allergy Asthma Rep. 2011;11(1):37-44Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB.
Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005.
J Allergy Clin Immunol. 2007;120(4):878-884Pali-Schöll I, Jensen-Jarolim E.
Anti-acid medication as a risk factor for food allergy.
Allergy. 2011;66(4):469-477Prince BT, Mandel MJ, Nadeau K, Singh AM.
Gut microbiome and the development of food allergy and allergic disease.
Pediatr Clin North Am. 2015;62(6):1479-1492Reynolds LA, Finlay BB.
A case for antibiotic perturbation of the microbiota leading to allergy development.
Expert Rev Clin Immunol. 2013;9(11):1019-1030Untersmayr E, Jensen-Jarolim E.
The role of protein digestibility and antacids on food allergy outcomes.
J Allergy Clin Immunol. 2008;121(6):1301-1308Marsland BJ, Salami O.
Microbiome influences on allergy in mice and humans.
Curr Opin Immunol. 2015;36:94-100Arrieta MC, Stiemsma LT, Dimitriu PA, et al;
CHILD Study Investigators. Early infancy microbial and metabolic alterations affect risk of childhood asthma.
Sci Transl Med. 2015;7(307):307ra152Untersmayr E, Schöll I, Swoboda I, et al.
Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice.
J Allergy Clin Immunol. 2003;112(3):616-623Riemer AB, Gruber S, Pali-Schöll I, Kinaciyan T, Untersmayr E, Jensen-Jarolim E.
Suppression of gastric acid increases the risk of developing immunoglobulin E-mediated drug hypersensitivity: human diclofenac sensitization and a murine sensitization model.
Clin Exp Allergy. 2010;40(3):486-493Untersmayr E, Bakos N, Schöll I, et al.
Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients.
FASEB J. 2005;19(6):656-658Ramírez E, Cabañas R, Laserna LS, et al.
Proton pump inhibitors are associated with hypersensitivity reactions to drugs in hospitalized patients: a nested case-control in a retrospective cohort study.
Clin Exp Allergy. 2013;43(3):344-352Trikha A, Baillargeon JG, Kuo YF, et al.
Development of food allergies in patients with gastroesophageal reflux disease treated with gastric acid suppressive medications.
Pediatr Allergy Immunol. 2013;24(6):582-588American Society of Health-System Pharmacists.
AHFS pharmacologic-therapeutic classification.
In: McEvoy GK, ed.
AHFS Drug Information 2018.
Bethesda, MD: American Society of Health-System Pharmacists; 2018:viii-x.Guthrie D.
Analysis of dichotomous variables in repeated measures experiments.
Psychol Bull. 1981;90(1):189-195Allison PD.
In: SAS Institute, ed.
Interactions With Time as Time-Dependent Covariates—Survival Analysis Using the SAS System:
A Practical Guide. 2nd ed.
Cary, North Carolina: SAS Institute; 2010:177-178.Cantor AB.
SAS Survival Analysis Techniques for Medical Research. 2nd ed.
Cary, North Carolina: SAS Institute; 2003.Arae K, Oboki K, Ohno T, et al.
Cimetidine enhances antigen-specific IgE and TH2 cytokine production.
Allergol Int. 2011;60(3):339-344Murk W, Risnes KR, Bracken MB.
Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review.
Pediatrics. 2011;127(6):1125-1138Hirsch AG, Pollak J, Glass TA, et al.
Early-life antibiotic use and subsequent diagnosis of food allergy and allergic diseases.
Clin Exp Allergy. 2017;47(2):236-244Rachid R, Chatila TA.
The role of the gut microbiota in food allergy.
Curr Opin Pediatr. 2016;28(6):748-753Lynch SV, Boushey HA.
The microbiome and development of allergic disease.
Curr Opin Allergy Clin Immunol. 2016;16(2):165-171Stefka AT, Feehley T, Tripathi P, et al.
Commensal bacteria protect against food allergen sensitization.
Proc Natl Acad Sci U S A. 2014;111(36):13145-13150Russell SL, Gold MJ, Hartmann M, et al.
Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma.
EMBO Rep. 2012;13(5):440-447O’Mahony L, Akdis M, Akdis CA.
Regulation of the immune response and inflammation by histamine and histamine receptors.
J Allergy Clin Immunol. 2011;128(6):1153-1162Nelson SP, Kothari S, Wu EQ, Beaulieu N, McHale JM, Dabbous OH.
Pediatric gastroesophageal reflux disease and acid-related conditions: trends in incidence of diagnosis and acid suppression therapy.
J Med Econ. 2009;12(4):348-355Barron JJ, Tan H, Spalding J, Bakst AW, Singer J.
Proton pump inhibitor utilization patterns in infants.
J Pediatr Gastroenterol Nutr. 2007;45(4):421-427Hudson B, Alderton A, Doocey C, Nicholson D, Toop L, Day AS.
Crying and spilling—time to stop the overmedicalisation of normal infant behaviour.
N Z Med J. 2012;125(1367):119-126.PubMedGoogle Scholarvan der Pol R, Langendam M, Benninga M, van Wijk M, Tabbers M.
Efficacy and safety of histamine-2 receptor antagonists.
JAMA Pediatr. 2014;168(10):947-954Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M.
Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease.
J Pediatr. 2009;154(4):514-520.e4Moore DJ, Tao BS, Lines DR, Hirte C, Heddle ML, Davidson GP.
Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux.
J Pediatr. 2003;143(2):219-223Hassall E.
Over-prescription of acid-suppressing medications in infants: how it came about, why it’s wrong, and what to do about it.
J Pediatr. 2012;160(2):193-198Farahmand F, Najafi M, Ataee P, Modarresi V, Shahraki T, Rezaei N.
Cow’s milk allergy among children with gastroesophageal reflux disease.
Gut Liver. 2011;5(3):298-301Schoemaker AA, Sprikkelman AB, Grimshaw KE, et al.
Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort.
Allergy. 2015;70(8):963-972
Return to PEDIATRICS
Since 4-03-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |