Exploring the Neuromodulatory Effects
of the Vertebral Subluxation and Chiropractic CareThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Chiropractic Journal of Australia 2010 (Mar); 40 (1): 37–44 ~ FULL TEXT
OPEN ACCESS Thanks to the Chiropractic Journal of Australia, and the Editor Rolf E. Peters, DC, MCSc, FICC, FACC for permission to reproduce this full-text article, exclusively at Chiro.Org!
Heidi Haavik Taylor, BSc (Chiro), PhD [1], Kelly Holt, BSc (Chiro), PGDipHSc [2],
and Bernadette Murphy, DC, PhD [3]
1. Director of Research,
New Zealand College of Chiropractic,
Auckland, New Zealand
2. Researcher,
Research Department,
New Zealand College of Chiropractic,
Auckland, New Zealand.
3. Associate Professor,
Faculty of Health Sciences,
University of Ontario Institute of Technology, Ontario, Canada
This paper was voted best paper at the November 2009 Policy Forum and Scientific Symposium
The elusive vertebral subluxation is the central defining clinical principle of the chiropractic profession. After almost 115 years of discussion there is still little consensus regarding the nature of the vertebral subluxation or its potential associated neurological manifestations. Some authors even deny that the subluxation exists. In this paper a model is presented that assumes that the putative vertebral subluxation represents a state of altered afferent input which is responsible for ongoing maladaptive central plastic changes that over time can lead to dysfunction, pain and other symptoms. A growing body of research that investigates the neuromodulatory effects of chiropractic care supports this model. This paper explores this research and discusses it in light of the vitalistic principles upon which chiropractic was founded. The model outlined in this paper may go some way to explain some of the beneficial effects of chiropractic care on nonmusculoskeletal conditions previously reported in the literature.
INTRODUCTION
The elusive vertebral subluxation is the central defining clinical principle of the chiropractic profession. [1] A century ago the founder of chiropractic, D.D. Palmer, described vertebral subluxations as “slightly displaced vertebrae which press against nerves causing impingements, the result being too much or not enough functionating.” [2] Palmer argued that vertebral subluxations cause inflammation which is stressful to the body and viscera and results in “lowered tissue resistance.” [2] He incorporated these concepts into a metaphysical philosophy of chiropractic which emphasised the supremacy of vital forces and the concept of the body possessing an Innate Intelligence which is compromised in its ability to care for and direct the vital functions of the body by nerve interference caused by vertebral subluxations. [2] The use of this vitalistic paradigm and metaphysical constructs in his philosophy of chiropractic led detractors to malign his early theories and accuse him of being unscientific. [3] However, others have recognized that metaphysics plays a very important role in science by being the science of first principles, i.e. the fundamental a priori assumptions that lay the foundation for any research programme. [4]
Over the past century various authors have described the vertebral subluxation using a multitude of models that range from theories of pinched nerves within the intervertebral foramen (that even D.D. Palmer had discounted by 1910), [2,3] through to more modern developments of Palmer’s early theories that postulate a neurodystrophic hypothesis where the vertebral subluxation leads to lowered tissue resistance by modifying hypothalamic function and ultimately immune responses. [3] It is interesting to note that after almost 115 years of discussion there is still little consensus regarding the nature of the vertebral subluxation or its associated neurological manifestations. Some authors even deny that they exist. [1] It is clear therefore that the chiropractic profession needs to continue to invest time and money into subluxation-focussed-research in order to better understand the clinical entity that defines the profession.
Over the past 15 years our research group has been conducting a variety of experiments aimed at testing out the theory that adjusting subluxations improves central nervous system functioning and overall expression of health and well being. To do this the theory was first formulated into a model (Figure 2) that could be scientifically tested with a programme of research studies. This model became the basis for the lead author’s PhD research, [5] and continues to be a foundational premise that our research group is attempting to elucidate with our work. The model was constructed using early chiropractic research data and a thorough review of the neurophysiology scientific literature. The model assumes that the putative vertebral subluxation represents a state of altered afferent input which is responsible for ongoing maladaptive central plastic changes that over time can lead to dysfunction, pain and other symptoms. Thus a potential mechanism which could explain how chiropractic adjustments improve function is that altered afferent feedback from a vertebral subluxation alters the afferent “milieu” into which subsequent afferent feedback from the spine and limbs is received and processed, thus leading to altered sensorimotor integration of the afferent input, which is then normalised by high-velocity, low-amplitude adjustments of the vertebral subluxation. This theory is plausible considering that it is now well established that the human central nervous system (CNS) retains its ability to adapt to its ever-changing environment, and that both increased (hyperafferentation) and decreased (deafferentation) afferent input leads to changes in CNS functioning. [6–8]
Figure 1: Vertebral Subluxation may lead to
altered sensorimotor integration
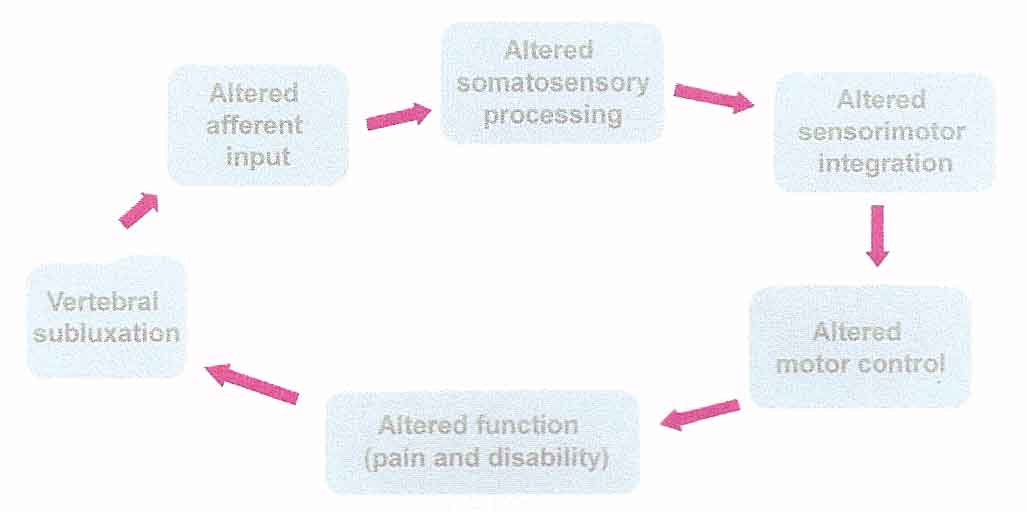
Figure 2: Chiropractic adjustments may normalise
afferent input and therefore promote appropriate
sensorimotor integration
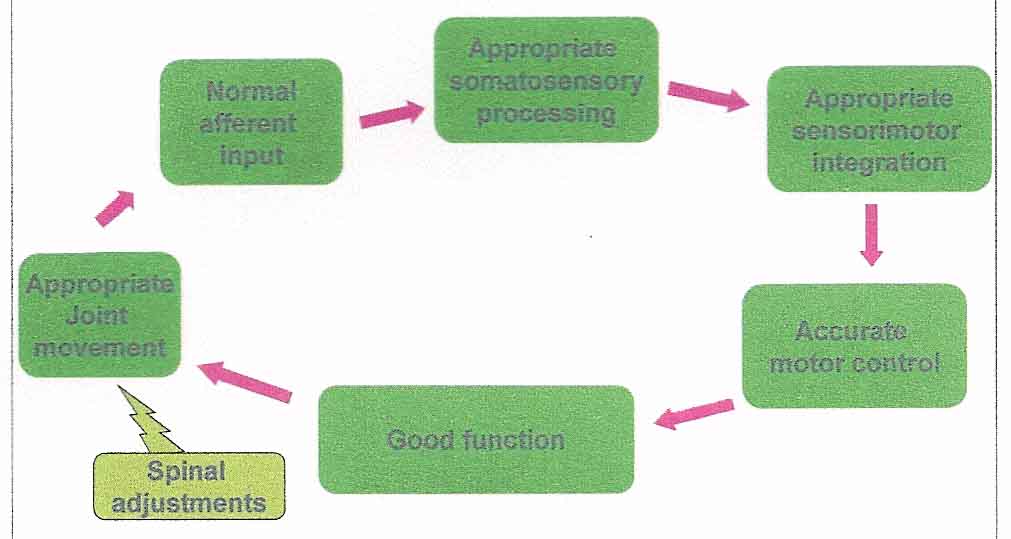
It is the aim of this paper to discuss some of the studies conducted by our group over the past 15 years and to consider how this work relates to our model and its a priori vitalistic assumptions. By developing a greater understanding of the effects of the vertebral subluxation on sensorimotor integration researchers may be able to help elucidate some of the many beneficial clinical effects reported by chiropractors in day to day practice.
DISCUSSION
To attempt to provide any clarification regarding the neurophysiological effects, if any, of adjusting subluxated spinal segments, it was first necessary to explore whether adjusting the spine had any lasting central neural effects at all. Very limited evidence for this existed 15 years ago. [9, 10] According to our model above, adjusting subluxated spinal segments should alter sensorimotor processing, sensorimotor integration and motor control.
Sensorimotor Integration
Numerous activities of daily living are dependent on appropriate sensorimotor integration. Interactions between sensory and motor systems allow us to engage with our environment, they allow us to reach for and grasp an object, turn towards an auditory stimulus or respond to perturbations of the environment in order to maintain postural stability and balance. [11]
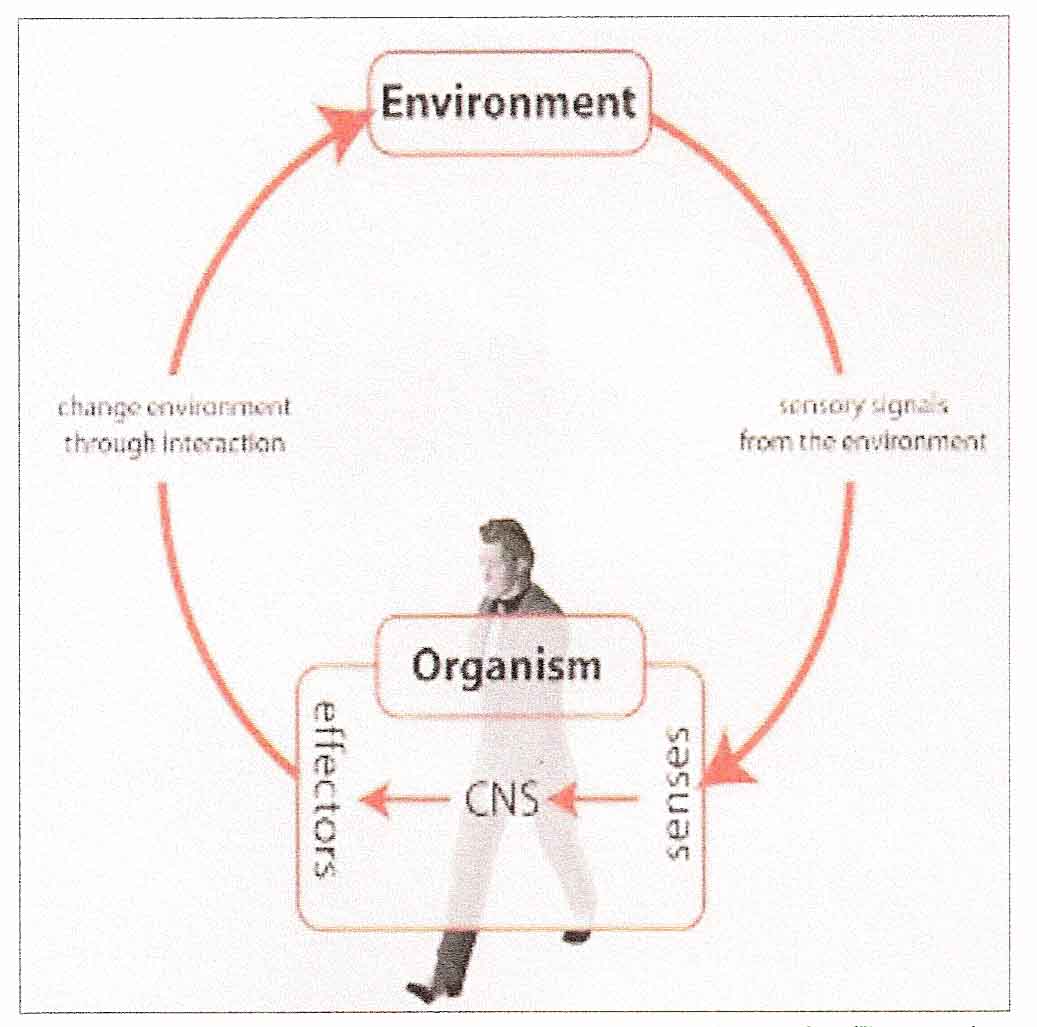
Figure 3: Sensorimotor integration illustrated by the Action/Perception Cycle
Sensorimotor integration involves strong feedback connections between different brain structures that are associated with numerous, and perhaps all, neuroanatomical subsystems. [4] These subsystems interconnect to form a dynamic, multimodal, sensorimotor integrative system. This system is dependent on motoric responses to reafferent proprioceptive signals in order to complement and define further sensory input. This adds organisational complexity which results in a higher order sensorimotor integrative system that may be said to have emergent properties. [11] This is similar to the emergent properties of consciousness, where a ‘thought’ is not made up of a single synapse or active neuron but involves a much higher level of processing and integration and cannot necessarily be explained by its constituent parts. [5] A breakdown anywhere in these multimodal sensorimotor feedback loops has the potential to influence other interconnected neuroanatomical subsystems, or, perhaps more importantly, the emergent properties of the higher order system. [11] Therefore, if a vertebral subluxation creates neuroplastic changes (i.e. lasting functional neurophysiological changes) in the CNS due to altered afferent input, its impact on the higher order sensorimotor integrative system may have neurological manifestations that extend well beyond the mechanical site of the lesion. There is, for example, a growing body of evidence that suggests that sensorimotor integration involves highly complex emergent properties that are linked to adaptation and homeostasis [12] and that chiropractic adjustments influence many of these integrative neural processes such as proprioception, somatosensory processing and feed forward activation. [13–15]
Proprioception
Proprioception is an important component of sensorimotor integration in the CNS. Proprioception includes joint position sense (JPS) and kinaesthesia (the sense of limb movement in the absence of visual cues). [for review see 16] The main source of afferent information for JPS arises from muscle spindles. [for review see 16] However, both mechanoreceptors in joint capsules and cutaneous tactile receptors may also contribute. [for review see 16] Joint position sense has been extensively studied in the ankle, knee and hip joints, [17–29] particularly to investigate the effects of reconstructive surgery, [19, 24, 25] osteoarthritis, [28, 30, 31] joint bracing, [for review see 26] and various exercise or re-training programmes. [17, 18, 21, 32] Recently there has also been an increased focus in the literature on spinal JPS, [33–37] however, much less research has looked at the effect of the spine on limb JPS. [38–40]
Accurate JPS is very important for balance and for the regulation of locomotion. [41] Impaired balance and locomotion control are known to impact the falls-risk in the elderly, [42, 43] which is a major health concern for this population group. [44–49] Impaired ankle and knee proprioception has been demonstrated in elderly populations compared to younger groups. [50, 51] This is thought to negatively impact balance and locomotor control, leading to more falls. [50, 51] In addition to the sensorimotor system, the vestibular system is vital for good balance and reduced risk of falls. However, the function of the vestibular system is known to decline with age, thus the elderly must rely more heavily on their proprioceptive system to maintain good balance and normal locomotor control. [52] There are numerous studies that implicate cervical spine impairment in reduced postural control, for example, due to chronic neck pain, [53, 54] neck muscle fatigue, [55] cervicobrachial pain syndrome, [56] cervical root compression, [57, 58] head injury or whiplash injury. [55, 59] Therefore, there appears to be a considerable link between cervical function and accurate proprioceptive processing and thus postural control. Although most of these previous studies related to significant cervical problems, one recent study has demonstrated that changes in head and neck position in a group of subjects without any history of neck pain or injury led to reduced accuracy of elbow joint position sense (JPS). [40] The authors of this study discussed how accurate execution of movement depends on the ability of the CNS to integrate somatosensory, vestibular, and visual information regarding the position of the body. [40] They argued that placing their subjects’ heads in full flexion and rotation could have led to an overload of the computational capacity of the CNS, thus resulting in increased JPS error. [40] The same group of researchers also demonstrated that people with whiplash associated disorder (WAD) are affected by smaller angles of neck rotation than individuals who had no history of WAD, [60] further suggesting that cervical spine dysfunction leads to reduced accuracy of JPS. Recent research, utilising somatosensory evoked potentials [61] and transcranial magnetic stimulation, [62, 63] has shown that adjusting vertebral subluxations in the cervical spine of patients without frank neck pain, but with a history of some form of subclinical neck pain syndrome (SCNPS) (i.e. individuals with reoccurring neck dysfunction such as minor neck pain, ache and/or stiffness, but asymptomatic between episodes, and who have not considered their symptoms severe enough to seek treatment) [64, 65] can alter cortical somatosensory processing, sensorimotor integration of input from the upper limb, and motor control of upper limb muscles. It is therefore possible that such changes in CNS processing following cervical adjustments could include alterations in proprioceptive processing.
Somatosensory Gating
Another important property of the CNS is its ability to gate sensory information. It is thought that this is necessary for the CNS to maintain the internal representation of its current posture and to avoid undesirable reactions to external or internal perturbations. [65, 66] Tinazzi et al have shown that gating of sensory information is distorted in patients with focal hand dystonia. [67] The authors argued that there was a lack of surround-like inhibition (a neural mechanism that focuses neuronal activity and is considered a fundamental property of retinal ganglion cells and the circuitry of the visual system) [68] of mainly proprioceptive afferent input in these patients, and that this inefficient integration could give rise to the abnormal motor output and might therefore contribute to the motor impairment present in dystonia patients. [67] Other groups have also demonstrated that there is a shift in the gain of the sensory signals, i.e. a central re-weighting of proprioceptive input, in patients with spasmodic torticollis [69] and low back pain patients. [70] A recent study demonstrated that adjusting dysfunctional cervical segments in SCNPS patients can increase the surround-like inhibition of proprioceptive afferent input, [71] again suggesting the possibility of central mechanisms of action for high-velocity, low-amplitude spinal adjustments.
Sensorimotor Processing
Recent research utilising somatosensory evoked potentials (SEPs) [61] and transcranial magnetic stimulation (TMS) [62, 63] has shown that adjusting dysfunctional segments in the cervical spine can alter somatosensory processing, sensorimotor integration of input from the upper limb, and motor control of upper limb muscles. These studies have shown alterations in the processing of the cortical SEP peaks N20 and N30 following high-velocity, low-amplitude cervical adjustments. [61] The N20 SEP peak represents processing at the primary somatosensory cortex [72–74] and thus reflects cortical perception. The neural generator(s) of the N30 SEP component remains more controversial. Although some authors suggest this peak is generated in the post-central cortical regions (i.e. S1), [75–77] most evidence suggests that this peak is related to a complex cortical and subcortical loop linking the basal ganglia, thalamus, pre-motor areas, and primary motor cortex. [78–86] The N30 peak is therefore thought to reflect sensorimotor integration. [87] This means that adjusting cervical vertebral subluxations can alter cortical perception and sensorimotor integration of information from the upper limb. It has also recently been demonstrated that adjusting cervical vertebral subluxations alters cortical upper limb muscular control in a muscle specific manner. [63,88] The TMS experimental measures utilised in these studies, such as short-interval-intracortical-inhibition (SICI), short-interval-intracortical-facilitation (SICF) and the cortical silent period (CSP), all reflect sensorimotor integration and are believed to reflect processing at the level of the cortex. [89–100]
Muscle perception impairments are also present in chronic neck pain patients. Impairment of deep cervical neck flexors and significant postural disturbances during walking and standing have been demonstrated in both insidious-onset and trauma-induced chronic neck pain conditions. [56, 101–106] Altered sensitivity of proprioceptors within the neck muscles has been suggested to be related to the postural (i.e. motor control) disturbances seen in these patients. [102, 106] It has also been argued that the degree to which proprioceptive input to the central nervous system is disturbed and possibly even more importantly how the CNS processes, interprets and transforms this afferent information into motor commands determines the degree to which subjects can successfully execute more challenging balance tasks. [65, 106] It is therefore possible that adjusting vertebral subluxations in patients with sub-clinical or chronic neck pain actually alters the central processing of proprioceptive information, and that this in part is the mechanism by which high-velocity, low-amplitude spinal adjustments reduce pain and improve function in these patient populations. It is possible that the changes in cortical somatosensory processing, [61, 107, 108] sensorimotor integration [61–63] and motor control [62, 63, 109–111] that have been previously documented following high-velocity, low-amplitude spinal adjustments reflect changes in central processing of proprioceptive afferent input.
Centrally Modulated Pain
The CNS utilises peripheral signals continuously to build and maintain an internal reference frame. [112, 113] Motor commands or motor intention (also known as “efference copies”) are also known to interact with afferent signals to generate sensation, and are known to contribute to joint position sense. [114] Under normal circumstances there is an integration of intention, action and sensory feedback. Furthermore, in a healthy state there is congruence between motor intention and sensory experience (both proprioceptive and visual) when we for example move a limb through space. Thus goal-directed action requires ongoing monitoring of sensorimotor inputs to ensure that motor outputs are congruent with current intentions. This monitoring is automatic but can become conscious if there is a mismatch between expected and realised sensorimotor states. A recent study has demonstrated that providing a sensory–motor conflict, i.e. providing unexpected visual feedback when moving a limb (via hiding a moving limb and/or distorting visual feedback of the movement of that limb) is sufficient to produce additional somaesthetic disturbances, and exacerbation of pre-existing symptoms in a group of fibromyalgia patients. [115] This suggests that a conflict between our expected and realised sensorimotor states can in some individuals produce or worsen pain sensations. It is therefore possible that a mechanism by which spinal adjustments relieve pain in patients is due to a central effect by improving somatosensory integration processes and removing the conflict between the expected and actual sensorimotor state.
Feed Forward Activation
When performing bodily movements, like throwing a ball for example, the central nervous system will activate a variety of postural muscles prior to any movement of the arm in order to maintain postural stability during the throwing action. This process is known as feed-forward activation (FFA). Delays in FFA are known to occur in individuals suffering from chronic low back pain. [116] Based on our model, such a delay in muscle activation would be an example of altered motor control.
We were interested to understand what the incidence of delayed feed-forward activation might be in an asymptomatic population and whether this might be related to underlying vertebral subluxations. In order to do this, we selected a uniform population of 90 healthy young males who were evaluated for delays in FFA of the transversus abdominis muscle and internal obliques when undertaking rapid movements of the upper limb. Seventeen subjects had a delay in FFA which was reproducible when retested six months later. These subjects were examined by a chiropractor and were all found to have a sacroiliac joint subluxation on the side of delayed FFA. Following a single chiropractic adjustment of the subluxated sacroiliac joint the FFA activation time improved by an average of 38%. [15] This study demonstrated an improvement in central nervous system control of muscles associated with the stability of a specific joint due to a chiropractic adjustment. Only one prospective study has investigated the potential role of delayed trunk muscle activation in actually predicting low back pain over a two year period. [117] The authors found that the odds of sustaining a low back injury increased 2.8–fold when a history of low back pain was present and increased by 3% with each millisecond of abdominal muscle shut-off latency. They found that the latency was an average of 14 milliseconds longer for athletes who sustained low back pain as compared to those who didn’t. Considerably more work needs to be done in this area to determine whether delayed trunk muscle latencies may be a marker of disordered sensorimotor integration, and whether the improvement in activation is sustained following chiropractic care.
Relationship to Observations in Practice
We are currently developing a questionnaire to measure self-reported “body awareness” which might be linked to impaired sensorimotor integration. This questionnaire could be used to assess the incidence of disordered sensorimotor integration in a chiropractic patient group. Some sample questions which reflect the sort of things that a patient with disordered sensorimotor integration may experience include:
- Have you noticed that you have been hitting your head getting out of the car since your neck has been sore?
- Have you been bumping your wrists or elbows more frequently?
- Have you had trouble seeing clearly or focusing on objects since your neck has been sore?
- Have you felt clumsy or uncoordinated since your neck has been sore?
CONCLUSION
The fact that chiropractic adjustments result in such a plethora of changes to sensorimotor integration is interesting when considering the possible mechanisms associated with the beneficial clinical effects of chiropractic care. Researchers from many diverse fields have recently suggested that sensorimotor integration involves multiple layers of processing that display emergent properties which cannot be explained by individual neurons and pathways. [11, 12, 118] Of interest when considering the vitalistic principles upon which chiropractic was founded is that some researchers are now proposing that emergent signals from optimal sensorimotor integration may underlie appropriate adaptation of respiratory patterns and homeostasis. [12] This may go some way to explain some of the beneficial effects of chiropractic care on nonmusculoskeletal conditions previously reported in the literature. [119] Many of the studies discussed in this paper show that chiropractic adjustments result in changes to sensorimotor integration within the central nervous system. What is not yet clear is whether these changes correlate with beneficial clinical outcomes or not. It is also not clear whether these changes are due to the correction of a vertebral subluxation, therefore normalising aberrant afferent input to the CNS, or are they merely due to an afferent barrage associated with the adjustive thrust. These questions remain to be answered and are the focus of our ongoing research efforts.
Acknowledgments
The authors would like to thank the following organisations for providing funding to help conduct their research over the past fifteen years: Australian Spinal Research Foundation, the Hamblin Chiropractic Research Fund Trust, the University of Auckland Vice Chancellors Fund, New Zealand Tertiary Education Commission Top Achievers Doctoral Scholarship and the Foundation for Chiropractic Education and Research.
REFERENCES
Nelson C.
The subluxation question.
J Chiropr Humanit. 1997; 7(1):46-55Palmer DD.
Text-book of the Science, Art and Philosophy of Chiropractic.
Portland, Oregon:
Portland Printing House Company; 1910. 107, 28, 60, 89, 294-5, 399, 491-5 p.Leach RA.
The chiropractic theories: a textbook of scientific research. 4th ed.
Baltimore: Lippincott Williams and Wilkins; 2004. 18-20, 137, 251, 359 p.Coulter ID.
Chiropractic: A Philosophy for Alternative Health Care 1st ed.
Oxford: Reed Educational and Professional Publishing Ltd; 1999.Haavik Taylor H.
The effect of altered peripheral input on sensorimotor integration.
Auckland: University of Auckland, 2007. 354 p.Tinazzi M, Zanette G, Polo A, et al.
Transient deafferentation in humans induces rapid modulation of primary sensory cortex not associated with subcortical changes: a somatosensory evoked potential study.
Neurosci Lett. 1997;223(1):21-4.Bertolasi L, Priori A, Tinazzi M, Bertasi V, Rothwell JC.
Inhibitory action of forearm flexor muscle afferents on corticospinal outputs to antagonist muscles in humans.
J Physiol. 1998;511(Pt 3):947-56.Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, et al.
Rapid modulation of human cortical motor outputs following ischaemic nerve block.
Brain. 1993;116(Pt 3):511-25.Pickar JG.
Neurophysiological Effects of Spinal Manipulation
Spine J (N American Spine Society) 2002 (Sep); 2 (5): 357–371Haldeman S.
Neurologic Effects of the Adjustment
J Manipulative Physiol Ther. 2000 (Feb); 23 (2): 112–114Chen JL, Penhune VB, Zatorre RJ.
The role of auditory and premotor cortex in sensorimotor transformations.
Ann N Y Acad Sci. 2009;1169:15-34.Poon C-S, Tin C, Yu Y.
Homeostasis of exercise hyperpnea and optimal sensorimotor integration: The internal model paradigm.
Respir Physiol Neurobiol. 2007;159(1):1-13.Haavik-Taylor H, Murphy B.
Transient modulation of intracortical inhibition following spinal manipulation.
Chiropr J Aust. 2007;37(3):106-16.Haavik-Taylor H, Murphy B.
Cervical Spine Manipulation Alters Sensorimotor Integration:
A Somatosensory Evoked Potential Study
Clin Neurophysiol. 2007 (Feb); 118 (2): 391–402Marshall P, Murphy B.
The effect of sacroiliac joint manipulation on feed-forward activation times of the deep abdominal musculature.
J Manipulative Physiol Ther. 2006;29(3 ):196-202.Gilman S.
Joint position sense and vibration sense: anatomical organisation and assessment.
J Neurol Neurosurg Psychiatry. 2002;73(5):473-7.Tsauo J-Y, Cheng P-F.
The effect of sensorimotor training on knee proprioceptive and function for patients with knee osteoarthritis: a preliminary report.
Clin Rehabil. 2008;22:448-57.Ribeiro F, Mota J, Oliveira J.
Effect of exercise-induced fatigue on position sense of the knee in the elderly.
Eur J Appl Physiol. 2007;99(4):379-85.Adachi N, Ochi M, Uchio Y, Iwasa J, Ishikawa M, Shinomiya R.
Temporal change of joint position sense after posterior cruciate ligament reconstruction using multi-stranded hamstring tendons.
Knee Surg Sports Traumatol Arthrosc. 2007;15(1):2-8.Okuda T, Ochi M, Tanaka N, Nakanishi K, Adachi N, Kobayashi R.
Knee joint position sense in compressive myelopathy.
Spine. 2006;31(4):459-62.Hazneci B, Yildiz Y, Sekir U, Aydin T, Kalyon TA.
Efficacy of isokinetic exercise on joint position sense and muscle strength in patellofemoral pain syndrome.
Am J Phys Med Rehabil. 2005;84(7):521-7.Larsen R, Lund H, Christensen R, Rogind H, Danneskiold-Samsoe B, Bliddal H.
Effect of static stretching of quadriceps and hamstring muscles on knee joint position sense.
Br J Sports Med. 2005;39(1):43-6.Bennell K, Wee E, Crossley K, Stillman B, Hodges P.
Effects of experimentally-induced anterior knee pain on knee joint position sense in healthy individuals.
J Orthop Res. 2005;23(1):46-53.Hopper DM, Creagh MJ, Formby PA, Goh SC, Boyle JJ, Strauss GR.
Functional measurement of knee joint position sense after anterior cruciate ligament reconstruction.
Arch Phys Med Rehabil. 2003;84(6):868-72.Reider B, Arcand MA, Diehl LH, et al.
Proprioception of the knee before and after anterior cruciate ligament reconstruction.
Arthroscopy. 2003;19(1):2-12.Beynnon BD, Good L, Risberg MA.
The effect of bracing on proprioception of knees with anterior cruciate ligament injury.
J Orthop Sports Phys Ther. 2002;32(1):11-5.Ishii Y, Terajima K, Terashima S, Bechtold JE, Laskin RS.
Comparison of joint position sense after total knee arthroplasty.
J Arthroplasty. 1997;12(5):541-5.Marks R.
Further evidence of impaired position sense in knee osteoarthritis.
Physiother Res Int. 1996;1(2):127-36.Karanjia PN, Ferguson JH.
Passive joint position sense after total hip replacement surgery.
Ann Neurol. 1983;13(6):654-7.Bennell KL, Hinman RS, Metcalf BR, et al.
Relationship of knee joint proprioception to pain and disability in individuals with knee osteoarthritis.
J Orthop Res. 2003;21(5):792-7.Barrett DS, Cobb AG, Bentley G.
Joint proprioception in normal, osteoarthritic and replaced knees.
J Bone Joint Surg Br. 1991;73(1):53-6Friemert B, Bach C, Schwarz W, Gerngross H, Schmidt R.
Benefits of active motion for joint position sense.
Knee Surg Sports Traumatol Arthrosc. 2006;14(6):564-70.Learman KE, Myers JB, Lephart SM, Sell TC, Kerns GJ, Cook CE.
Effects of spinal manipulation on trunk proprioception in subjects with chronic low back pain during symptom remission.
J Manipulative Physiol Ther. 2009;32:118-26.Allison GT.
Trunk muscle onset detection technique for EMG signals with ECG artefact.
J Electromyogr Kinesiol. 2003;13:209-16.Swinkels A, Dolan P.
Regional assessment of joint position sense in the spine.
Spine. 1998;23(5):590-7.Jull G, Falla D, Treleaven J, Hodges P, Vicenzino B.
Retraining cervical joint position sense: the effect of two exercise regimes.
J Orthop Res. 2007;25(3):404-12.Strimpakos N, Sakellari V, Gioftsos G, Kapreli E, Oldham J.
Cervical joint position sense: an intra- and inter-examiner reliability study.
Gait Posture. 2006;23(1):22-31.Knox J, Cordo P, Skoss R, Durrant S, Hodges P.
Illusory changes in head position induced by neck muscle vibration can alter the perception of elbow position.
Behav Neurosci. 2006;120(6):1211-7.Knox JJ, Coppieters MW, Hodges PW.
Do you know where your arm is if you think your head has moved?
Exp Brain Res. 2006;173(1):94-101. Epub 2006 Mar 25.Knox JJ, Hodges PW.
Changes in head and neck position affect elbow joint position sense.
Exp Brain Res. 2005 2005/08//;165(1):107-13.Bove M, Courtine G, Schieppati M.
Neck Muscle Vibration and Spatial Orientation During Stepping in Place in Humans.
J Neurophysiol. 2002;88:2232-41.Gill T, Taylor AW, Pengelly A.
A population-based survey of factors relating to the prevalence of falls in older people.
Gerontology. 2005;51(5):340-5.Tinetti ME, Speechley M, Ginter SF.
Risk factors for falls among elderly persons living in the community.
N Engl J Med. 1988;319:1701-7.Walsh MJ, Polus BI, Webb MN.
The role of the cervical spine in balance and risk of falling in the elderly.
Chiropr J Aust. 2004;34(1):19-22.Kannus P, Khan KM, Lord SR.
Preventing falls among elderly people in the hospital environment.
Med J Aust. 2006;184(8):372-3.Stephenson S, Langley J, Trotter M.
Impact of injury in New Zealand. 2nd ed.
Dunedin: Injury Prevention Research Unit; 2005.American Academy of Orthopedic Surgeons.
Don’t let a fall be your last trip.
Rosemont, IL American Academy of Orthopedic Surgeons; 1997.Chang JT, Morton SC, Rubenstein LZ, et al.
Interventions for the prevention of falls in older adults: systematic review and meta-analysis
of randomised clinical trials.
Br Med J. 2004;328(7441):680.Hendrie D, Hall SE, Arena G, Legge M.
Health system costs of falls of older adults in Western Australia.
Aust Health Rev. 2004;28(3):363-73.You SH. Joint Position Sense in Elderly Fallers:
A preliminary investigation of the validity and reliability of the SENSERite measure.
Arch Phys Med Rehab. 2005;86:346-52.Hurley MV, Rees J, Newham DJ.
Quadriceps function, proprioceptive acuity and functional performance in healthy young, middle-aged and elderly subjects.
Age Ageing. 1998;27(1):55-62.Schweigart G, Chien RD, Mergner T.
Neck proprioception compensates for age-related deterioration of vestibular self-motion perception.
\Exp Brain Res. 2002;147(1):89-97.Falla D.
Unravelling the complexity of muscle impairment in chronic neck pain.
Man Ther. 2004;9(3):125-33.Michaelson P, Michaelson M, Jaric S, Latash ML, Sjolander P, Djupsjobacka M.
Vertical posture and head stability in patients with chronic neck pain.
J Rehabil Med. 2003;35(5):229-35.Stapley PJ, Beretta MV, Toffola ED, Schieppati M.
Neck muscle fatigue and postural control in patients with whiplash injury.
Clin Neurophysiol. 2006 2006/3;117(3):610-22.Karlberg M, Persson L, Magnusson M.
Reduced postural control in patients with chronic cervicobrachial pain syndrome.
Gait Posture. 1995;3:241-9.Takayama H, Muratsu H, Doita M, Harada T, Kurosaka M, Yoshiya S.
Proprioceptive recovery of patients with cervical myelopathy after surgical decompression.
Spine. 2005;30(9):1039-44.Takayama H, Muratsu H, Doita M, Harada T, Yoshiya S, Kurosaka M.
Impaired joint proprioception in patients with cervical myelopathy.
Spine. 2005;30(1):83-6.Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R.
Development of motor system dysfunction following whiplash injury.
Pain. 2003 2003/5;103(1-2):65-73.Knox JJ, Beilstein DJ, Charles SD, et al.
Changes in head and neck position have a greater effect on elbow joint position sense
in people with whiplash-associated disorders.
Clin J Pain. 2006;22(6):512-8.Haavik-Taylor, H and Murphy, B.
Cervical Spine Manipulation Alters Sensorimotor Integration:
A Somatosensory Evoked Potential Study
Clin Neurophysiol. 2007 (Feb); 118 (2): 391–402Haavik Taylor H, Murphy B.
World Federation of Chiropractic’s 9th Biennial Congress Award Winning Paper (3rd Prize):
Altered sensorimotor integration with cervical spine manipulation.
J Manip Physiol Ther. 2008;31(2):115-26.Haavik-Taylor H, Murphy B.
Transient modulation of intracortical inhibition following spinal manipulation.
Chiropr J Aust. 2007;37:106-16.Lee HM, Nicholson LLP, Adams RDP, Bae S-SP.
Proprioception and Rotation Range Sensitization Associated With Subclinical Neck Pain.
Spine. 2005;30(3):E60-E7.Paulus I, Brumagne S.
Altered interpretation of neck proprioceptive signals in persons with subclinical recurrent neck pain.
J Rehabil Med. 2008;40:426-32.Ivanenko YP, Solopova IA, Levik YS.
The direction of postural instability affects postural reactions to ankle muscle vibration in humans.
Neurosci Lett. 2000;292:103-6.Tinazzi M, Priori A, Bertolasi L, Frasson E, Mauguiere F, Fiaschi A.
Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow.
Brain. 2000;123(Pt 1):42-50.Beck S, Hallett M.
Surround inhibition is modulated by task difficulty.
Clin Neurophysiol. 2009 Nov 10.Anastasopoulos D, Nasios G, Mergner T, Maurer C.
Idiopathic spasmodic torticollis is not associated with abnormal kinesthetic perception
from neck proprioceptive and vestibular afferences.
J Neurol. 2003;250:546-55.Brumagne S, Cordo P, Verschueren S.
Proprioceptive weighting changes in persons with low back pain and elderly persons
during upright standing.
Neurosci Lett. 2004;366:63-6.Haavik-Taylor H, Murphy B.
Altered Central Integration of Dual Somatosensory Input After Cervical Spine Manipulation
J Manipulative Physiol Ther. 2010 (Mar); 33 (3): 178–188Desmedt JE, Cheron G.
Central somatosensory conduction in man: neural generators and interpeak latencies of the far-field
components recorded from neck and right or left scalp and earlobes.
Electroencephalogr Clin Neurophysiol. 1980;50(5-6):382-403.Mauguiere F.
Somatosensory evoked potentials: normal responses, abnormal waveforms and clinical applications
in neurological diseases.
In: Niedermeyer E (ed).
Electroencephalography: basic principles, clinical applications, and related fields.
Baltimore: Williams and Wilkins, 1999.Nuwer MR, Aminoff M, Desmedt J, et al.
IFCN recommended standards for short latency somatosensory evoked potentials.
Report of an IFCN committee.
International Federation of Clinical Neurophysiology.
Electroencephalogr Clin Neurophysiol. 1994;91(1):6-11.Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD.
Human cortical potentials evoked by stimulation of the median nerve. I.
Cytoarchitectonic areas generating short-latency activity.
J Neurophysiol. 1989;62(3):694-710.Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD.
Human cortical potentials evoked by stimulation of the median nerve. II.
Cytoarchitectonic areas generating long-latency activity.
J Neurophysiol. 1989;62(3):711-22.Allison T, McCarthy G, Wood CC, Jones SJ.
Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve.
A review of scalp and intracranial recordings.
Brain. 1991;114(Pt 6):2465-503.Cheron G, Borenstein S.
Gating of the early components of the frontal and parietal somatosensory evoked potentials
in different sensory-motor interference modalities.
Electroencephalogr Clin Neurophysiol. 1991;80(6):522-30.Cheron G, Borenstein S.
Mental movement simulation affects the N30 frontal component of the somatosensory evoked potential.
Electroencephalogr Clin Neurophysiol. 1992;84(3):288-92.Desmedt JE, Cheron G.
Non-cephalic reference recording of early somatosensory potentials to finger stimulation in adult or
aging normal man: differentiation of widespread N18 and contralateral N20 from the prerolandic P22 and N30 components.
Electroencephalogr Clin Neurophysiol. 1981;52(6):553-70.Desmedt JE, Huy NT, Bourguet M.
The cognitive P40, N60 and P100 components of somatosensory evoked potentials and the earliest
electrical signs of sensory processing in man.
Electroencephalogr Clin Neurophysiol. 1983;56(4):272-82.Kanovský P, Bare M, Rektor I.
The selective gating of the N30 cortical component of the somatosensory evoked potentials of
median nerve is different in the mesial and dorsolateral frontal cortex: evidence from intracerebral recordings.
Clin Neurophysiol. 2003;114(6):981-91.Mauguiere F, Desmedt JE, Courjon J.
Astereognosis and dissociated loss of frontal or parietal components of somatosensory evoked potentials
in hemispheric lesions. Detailed correlations with clinical signs and computerized tomographic scanning.
Brain. 1983;106(Pt 2):271-311.Rossini PM, Gigli GL, Marciani MG, Zarola F, Caramia M.
Non-invasive evaluation of input-output characteristics of sensorimotor cerebral areas in healthy humans.
Electroencephalogr Clin Neurophysiol. 1987;68(2):88-100.Rossini PM, Babiloni F, Bernardi G, et al.
Abnormalities of short-latency somatosensory evoked potentials in parkinsonian patients.
Electroencephalogr Clin Neurophysiol. 1989;74(4):277-89.Waberski TD, Buchner H, Perkuhn M, et al.
N30 and the effect of explorative finger movements: a model of the contribution of the motor cortex
to early somatosensory potentials.
Clin Neurophysiol. 1999;110(9):1589-600.Rossi S, della Volpe R, Ginanneschi F, et al.
Early somatosensory processing during tonic muscle pain in humans: relation to loss of proprioception and
motor ’defensive’ strategies.
Clin Neurophysiol. 2003;114(7):1351-8.Haavik Taylor H, Murphy B.
World Federation of Chiropractic’s 9th Biennial Congress Award Winning Paper (3rd Prize):
Altered sensorimotor integration with cervical spine manipulation.
J Manip Physiol Ther. 2008;31(2):115-26.Kujirai T, Caramia MD, Rothwell JC, et al.
Corticocortical inhibition in human motor cortex.
J Physiol. 1993;471:501-19.Chen R, Lozano AM, Ashby P.
Mechanism of the silent period following transcranial magnetic stimulation.
Evidence from epidural recordings.
Exp Brain Res. 1999;128(4):539-42.Cantello R, Gianelli M, Civardi C, Mutani R.
Magnetic brain stimulation: the silent period after the motor evoked potential.
Neurology. 1992;42(10):1951-9.Inghilleri M, Berardelli A, Cruccu G, Manfredi M.
Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction.
J Physiol. 1993;466:521-34.Kukowski B, Haug B.
Quantitative evaluation of the silent period, evoked by transcranial magnetic stimulation
during sustained muscle contraction, in normal man and in patients with stroke.
Electromyogr Clin Neurophysiol. 1992;32(7-8):373-8.Fisher RJ, Nakaura Y, Bestmann S, Rothwell JC, Bostock H.
Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking.
Exp Brain Res. 2002;143:240-8.Hanajima R, Ugawa Y, Terao Y, et al.
Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans.
J Physiol. 2002;538(Pt 1):253-61.Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W.
Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation.
J Physiol. 1998;511(Pt 1):181-90.Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC.
Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex.
Electroencephalogr Clin Neurophysiol. 1996;101(4):263-72.Di Lazzaro V, Restuccia D, Oliviero A, et al.
Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits.
Exp Brain Res. 1998;119(265-268).Di Lazzaro V, Rothwell JC, Oliviero A, et al.
Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli
applied to human motor cortex.
Exp Brain Res. 1999;129(4):494-9.Chen R, Tam A, Butefisch C, et al.
Intracortical inhibition and facilitation in different representations of the human motor cortex.
J Neurophysiol. 1998;80(6):2870-81.Alund M, Ledin T, Odkvist L, S.E. L.
Dynamic posturography among patients with common neck disorders.
J Vestib Res. 1993;3:383-9.Persson L, Karlberg M, Magnusson M.
Effects of different treatments on postural performance in patients with cervical root compression:
A randomized prospective study assessing the importance of the neck in postural control.
J Vestib Res. 1996;6(6):439-53.Jull G, Kristjansson E, Dall’Alba P.
Impairment in the cervical flexors: a comparison of whiplash and insidious onset neck pain patients.
Manual Therapy. 2004;9(2):89-94.Branstrom H, Malmgren-Olsson EB, Barnekow-Bergkvist M.
Balance performance in patients with whiplash associated disorders and patients with
prolonged musculoskeletal disorders.
Adv Physiother. 2001;3:120-7.Rubin AM, Woolley SM, Dailey VM, Goebel JA.
Postural stability following mild head or whiplash injuries.
Am J Otol. 1995;16(2):216-21.Michaelson P, Michaelson M, Jaric S, Latash ML, Sjolander P, Djupsjobacka M.
Vertical posture and head stability in patients with chronic neck pain.
J Rehabil Med. 2003;35(5):229-35.Zhu Y, Haldeman S, Hsieh CY, Wu P, Starr A.
Do cerebral potentials to magnetic stimulation of paraspinal muscles reflect changes in
palpable muscle spasm, low back pain, and activity scores?
J Manipulative Physiol Ther. 2000;23(7):458-64.Zhu Y, Haldeman S, Starr A, Seffinger MA, Su SH.
Paraspinal muscle evoked cerebral potentials in patients with unilateral low back pain.
Spine. 1993;18(8):1096-102.Marshall P, Murphy B.
The effect of sacroiliac joint manipulation on feed-forward activation times
of the deep abdominal musculature.
J Manipulative Physiol Ther 2006;29(3 ):196-202.Suter E, McMorland G.
Decrease in elbow flexor inhibition after cervical spine manipulation in patients with chronic neck pain.
Clin Biomech. 2002;17(7):541-4.Suter E, McMorland G, Herzog W, Bray R.
Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain.
J Manipulative Physiol Ther. 1999;22(3):149-53.Lackner J, DiZio P.
Vestibular, proprioceptive, and haptic contribution to spatial orientation.
Annu Rev Psychol. 2005;56:115-47.Sainsburg R, Ghez C, Kalakanis D.
Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms.
J Neurophysiol. 1999;81:1045-56.Smith JL, Crawford M, Proske U, Taylor JL, Gandevia SC.
Signals of motor command bias joint position sense in the presence of feedback from proprioceptors.
J Appl Physiol. 2009;106:950-8.McCabe CS, Cohen H, Blake DR.
Somaesthetic disturbances in fibromyalgia are exaggerated by sensory–motor conflict:
implications for chronicity of the disease?
Rheumatology. 2007;46:1587-92.Hodges PW, Richardson CA.
Inefficient muscular stabilization of the lumbar spine associated with low back pain.
A motor control evaluation of transversus abdominis.
Spine. 1996;21(22):2640-50.Cholewicki J, Silfies SP, Shah RA, et al.
Delayed trunk muscle reflex responses increase the risk of low back injuries.
Spine. 2005;30(23):2614-20.Blohm G, Keith GP, Crawford JD.
Decoding the Cortical Transformations for Visually Guided Reaching in 3D Space.
Cereb Cortex. 2009 June 1, 2009;19(6):1372-93.Hawk C, Knorsa R, Lisi A, Ferrance RJ, Evans MW.
Chiropractic Care for Nonmusculoskeletal Conditions: A Systematic Review
With Implications For Whole Systems Research
J Altern Complement Med. 2007 (Jun); 13 (5): 491–512
Return to SUBLUXATION
Since 6-02-2010


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |