

Genistein, a Natural Phytoestrogen From Soy,
Relieves Neuropathic Pain Following Chronic
Constriction Sciatic Nerve Injury in Mice:
Anti-Inflammatory and Antioxidant ActivityThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Neurochem. 2008 (Oct); 107 (1): 230–240 ~ FULL TEXT
Anna Elisa Valsecchi, Silvia Franchi, Alberto Emilio Panerai,
Paola Sacerdote, Anna Elisa Trovato, Mariapia Colleoni
Department of Pharmacology,
Chemotherapy and Medical Toxicology,
University of Milan,
Milan, Italy.There is great interest in soy isoflavones as alternatives to endogenous estrogens not only in hormonal pathologies, but also in inflammatory, neurodegenerative diseases, and pain. We investigated the effect of the isoflavone genistein on neuropathic pain. Genistein binds estrogen receptors (ER) with higher affinity for the ERbeta particularly expressed in neuronal and immune cells.
Neuropathy was induced in mice by means of chronic sciatic nerve constriction, and the subcutaneous administration of genistein from the third day after the lesion reversed pain hypersensitivity in a time- and dose-dependent manner. This effect may have been due to the activation of classical nuclear receptor and/or anti-oxidant, anti-inflammatory, and immunomodulating properties of genistein. The fact that a specific ERbeta antagonist prevented both its anti-allodynic and anti-hyperalgesic action, whereas a specific ERalpha antagonist was ineffective and a non-selective ER antagonist only reversed the anti-allodynic effect, suggests the involvement of ERbeta.
Antioxidant effects are also involved as the anti-nociceptive dose reversed the increase in reactive oxygen species and malondialdehyde in injured paw tissues, and increased the activity of anti-oxidant enzymes. The phytoestrogen had immunomodulatory and anti-inflammatory activities as it reduced peripheral and central nuclear factor-kappaB, nitric oxide system and pro-inflammatory cytokine over-activation.
Taken together, our results suggest that genistein could ameliorate painful neuropathy by multiple mechanisms.
From the FULL TEXT Article:
Introduction
Genistein, the major natural phytoestrogen isoflavone in soybean, has a weak estrogenic effect and well-known non-specific tyrosine kinase inhibitory activity at pharmacological doses (McClain et al. 2007). It has 7–8 times less binding affinity for estrogen receptor (ER) α than ERβ (Kuiper et al. 1997; An et al. 2001), and is therefore devoid of unwanted severe ERα agonist side effects, such as cancer promotion. It also has effects that are independent of its estrogenic activity, including protein tyrosine kinase inhibition or down-regulation, immune system modulation, and anti-oxidant activity (Duffy et al. 2007). Finally, over the last few years, public and scientific interest in phytoestrogens has increased because of their proposed neuroprotective effects against neurodegenerative diseases, neuronal damage, cerebral stroke, and ischemia (Sawada and Shimohama, 2003; Currie et al. 2004).
Neuropathic pain occurs secondarily to CNS injury or, more commonly, in association with injury to the PNS. These injuries can be caused by tumors compressing peripheral nerves, the toxins used in chemotherapy, metabolic disease (diabetes), viral disease (Herpes Zoster), severe ischemic insults, trauma, and disc herniations that stretch, compress, or inflame a nerve root but, despite more than 40 years of research, there are still no satisfactorily effective treatments.
The typical treatments for neuropathic pain of any origin are anti-depressant, anti-convulsant, or topical agents; opiates are often ineffective. There is therefore a need for new therapeutic approaches and, interestingly, it has been shown that soy diets are effective in preventing the development of neuropathic pain in animal models, and that specific plasma phytoestrogen concentrations are associated with the relief of chronic neuropathic sensory disorders (Shir et al. 2002).
We investigated the effects of genistein on neuropathic pain induced by mouse sciatic nerve chronic constriction injury (CCI), a well-established animal model of human sciatic neuritis (Clatworthy et al. 1995). As various and not fully elucidated pathological mechanisms contribute to the painful sequences of peripheral neuropathies, we investigated a number of molecular mechanisms possibly involved in the genistein-induced suppression of neuropathic hypersensitivity. In particular, we studied its anti-inflammatory properties by dosing nuclear factor (NF)-κB activation, and the involvement of nitric oxide (NO) system and pro-inflammatory cytokines [interleukin (IL)-1β and IL-6]; anti-oxidant mechanisms by assaying anti-oxidant enzyme activity, and reactive oxygen species (ROS) and lipoperoxide content; and classic ER-mediated activity by administering ER antagonists.
Materials and methods
Animals and surgical procedure
All of the experiments were performed in accordance with the Italian and European regulations governing the care and treatment of laboratory animals (Permit No. 94/2000A), and conformed to the guidelines for the study of pain in awake animals established by the International Association for the Study of Pain (Zimmermann, 1983).
Painful neuropathy was induced in 20–25 g C57BL/6J male mice (Harlan, Italy) using the CCI model originally described for rats by Bennett and Xie (1988) and adapted for mice as described in our previous paper (Martucci et al. 2008). Briefly, the animals were anesthetised using intraperitoneal (i.p.) sodium pentobarbital 60 mg/kg (0.1 mL/10 g) and, under a dissecting microscope, the right common sciatic nerve was exposed at the level of the mid-thigh and, proximal to the nerve trifurcation (while taking care to preserve epineural circulation), three ligatures (4/0 chromic silk; Ethicon, Brussels, Belgium) were loosely tied around it at intervals of about 0.5 mm until they elicited a brief twitch in the related hind paw. Sham-operated animals (sciatic exposure without ligation) were used as controls. In the experiments aimed at evaluating cytokine expression, we also used a group of mice that did not undergo any surgical procedure (naïve mice).
Drug treatment
Genistein (Sigma-Aldrich, Milan, Italy) was dissolved in dimethyl sulfoxide (DMSO) 5% and 95% polyethylene glycol 400, and administered at doses of 1, 3, 7.5, 15, and 30 mg/kg (0.1 mL/10 g). The higher doses (15 and 30 mg/kg) were those used by Verdrengh et al. (2003) to suppress cell-mediated inflammatory responses following collagen-induced arthritis in mice.
The drug or its vehicle was administered subcutaneously (s.c.) to neuropathic mice once a day for 11 days, starting from the third day after surgery; the sham-operated mice received vehicle alone following the same treatment procedure. The effect of the acute administration of the lowest genistein dose capable of reversing nociceptive hypersensitivity (3 mg/kg) was evaluated on the third day after injury by means of behavioral assessments made 1.5, 3, 5, and 7 h after administration.
In order to investigate the involvement of ER in genistein-induced effects, we tested the ability of the non-selective ER antagonist ICI 182 780 (Tocris Cookson, Bristol, UK) (Wakeling and Bowler 1987), the selective ERβ antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl) pyrazolo [1,5-a]pyrimidin-3-yl]phenol (PHTPP) (Tocris Cookson) (Compton et al. 2004) and the selective ERα antagonist methyl-piperidino-pyrazole (MPP) (Tocris Cookson) (Sun et al. 2002) to reverse the anti-hyperalgesic and anti-allodynic effect of genistein. ICI 182 780 (25 mg/kg, s.c.) or its vehicle (20% DMSO and 80% corn oil) was given twice daily for 3 days together with genistein (3 mg/kg) or its vehicle starting on day 9 of genistein administration (11 days after surgery) according to Leventhal et al. (2006) and Bjorling and Wang (2001). PHTPP (4.7 mg/kg, s.c.) or its vehicle (77% DMSO and 23% saline) was given once a day for 3 days together with genistein (3 mg/kg) or its vehicle starting on day 9 of genistein administration (11 days after surgery); this antagonist dose was chosen by us and was equimolar to genistein. MPP (2 mg/kg, i.p.) or its vehicle (distilled water) was given once a day for 2 days together with genistein or its vehicle starting on day 10 of genistein administration (12 days after surgery) according to Davis et al. (2006). The sham-operated mice received the vehicles of genistein and the antagonists.
Evaluation of thermal hyperalgesia and mechanical allodynia
The responses to thermal and mechanical stimuli were measured before, and 3, 7, 10, and 14 days after surgery (24 h after the last administration of genistein, antagonists, or vehicles), on the ipsilateral and contralateral hind paws of all mice by researchers blind to the given treatments.
Thermal hyperalgesia was tested using Hargreaves’ procedure, slightly modified by us for mouse, and a Plantar Test Apparatus (Ugo Basile, Comerio, Italy) (Martucci et al. 2008). Briefly, the mice were placed in smaller clear plexiglass cubicles and allowed to acclimatise before a constant-intensity radiant heat source (beam diameter 0.5 cm; intensity 20 IR) was aimed at the mid-plantar area of the hind paw, and the time in seconds (s) between the activation of the heat source and paw withdrawal was recorded.
Mechanical allodynia was assessed using the Dynamic Plantar Aesthesiomether (Ugo Basile). The animals were placed in a test cage with a wire mesh floor, and the rigid tip of a von Frey filament (punctate stimulus) was applied to the skin of the mid-plantar area of the hind paw with increasing force (ranging up to 5 g in 20 s), starting below the threshold of detection and increasing until the animal removed its paw. The withdrawal threshold was expressed in grams. The withdrawal threshold of the ipsilateral and contralateral paws was measured four times, and then averaged.
Biochemical evaluations
The biochemical evaluations were made on animals receiving genistein (3 mg/kg) for 11 days by researchers who were blind to the treatments. Immediately after the behavioral assessments, the mice were anesthetised with sodium pentobarbital 60 mg/kg, i.p. (0.1 mL/10 g) and, under a dissecting microscope, the ipsilateral sciatic nerve proximal to the trifurcation (about 1 cm before the three ligatures in the CCI animals), the ipsilateral L4, L5, and L6 dorsal root ganglia (DRG), the lumbar dorsal spinal cord (at the same L4–L6 level), and the ipsilateral and contralateral thalamus were removed, immediately frozen in liquid nitrogen and stored at –80°C until they were assayed for their NO synthase (NOS) content, and cytokine expression and content.
In some experiments, a small portion of the ipsilateral sciatic nerve proximal to the trifurcation (before the three ligatures in the CCI animals) and the spinal cord L4–L6 dorsal metamers were used to prepare nuclear extracts, which were stored at –80°C until NF-κB was assayed.
Given the technical difficulty of measuring NO, which requires accuracy in the time of sampling and prompt measurement immediately after sampling because of the instability of NO and nitrite, we assayed inducible (iNOS) and constitutive neuronal NOS (nNOS) in central and peripheral nervous tissues as a means of representing NO changes, as previously described by us (Martucci et al. 2008).
In one group of anesthetised mice, the paws were cut at the level of the calcaneus bone, weighed, crushed at 4°C, and used to determine the levels of malondialdehyde (MDA) and ROS, and the activity of glutathione peroxidase (GPX) and catalase (CT).
Sciatic nerve IL-1β content
The levels of IL-1β protein in the sciatic nerve of sham-operated, CCI and genistein-treated CCI mice were quantitatively determined using an ELISA. The sciatic nerves were removed, flash-frozen and stored at –80°C after surgery. The nerve samples pooled from two mice were homogenised in 0.25 mL of ice-cold phosphate buffered saline containing a protease inhibitor cocktail (Sigma-Aldrich) and centrifuged. The supernatant was used to measure IL-1β level and total protein content (Lowry’s method).
Interleukin-1β protein level was determined using a custom ELISA development kit (Endogen, Woburn, MA, USA). The concentrations of the capture and of the secondary biotinylated antibodies were respectively 2 and 1 µg/mL. The standard curves generated from recombinant protein ranged from 7.8 to 1000 pg/mL. Streptavidin-peroxidase and tetramethylbenzidine were used for color development. The color reaction was stopped with 2 N H2SO4 and read as optical density at 450 nm.
Real-time RT-PCR
RNA isolation Sciatic nerves, DRG and the lumbar dorsal spinal cord (L4–L6) were collected as described above and pooled from two mice (six samples; 12 mice per experimental group). Total RNA was purified using TRIzol reagent (Invitrogen, Life Technologies, San Giuliano Milanese, Italy) according to the manufacturer’s instructions, and resuspended in 6 µL of DEPC-treated water (Sigma-Aldrich). After purification, total RNA concentrations were determined from the sample absorbance value at 260 nm. Two thousand nanograms of total RNA were treated with Dnase (DNA-free Ambion, Austin, TX, USA) to avoid false-positive results because of the amplification of contaminating genomic DNA. First-strand cDNA was synthesised from 1000 ng of total RNA in a final volume of 20 µL using M-MLV RT (Moloney Murine Leukemia Virus Reverse Transcriptase; Invitrogen) (EC 2.7.7.49).
Real-time RT-PCR cDNA (2 µL) underwent real-time quantitative PCR using an ABI PRISM 7000 (Applied Biosystems, Forster City, CA, USA). The TaqMan PCR was performed in 25 µL volumes using TaqMan Universal PCR Real Master Mix Probe Rox (Eppendorf, Milan, Italy). The TaqMan probe/primers specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (code number Hs99999915_g1), murine IL-1β (code number Mm00434228_m1), and murine IL-6 (code number Mm00446190_m1) were purchased from TaqMan Assays-on-Demand Gene Expression Products (Applied Biosystems). All of the PCR assays were performed in triplicate. Before using the ΔΔCT method for relative quantification, a validation experiment was performed to demonstrate that the efficiencies of the target and reference were equal. The reaction conditions were as follows: 95°C for 10 min, followed by 40 cycles at 95°C for 15 s (denaturation) and 60°C for 1 min (annealing and elongation); the reaction mixture without the cDNA was used as a control. Threshold cycle numbers (CT) were determined using an ABI PRISM 7000 Sequence Detection System (version 1.2.3. software) and transformed using the ΔCT (2–ΔΔCT) comparative method. The gene-specific expression values were normalised to the expression values of GAPDH (endogenous control) within each sample. The levels of IL-1β and IL-6 were expressed in relation to their calibrated values in the naïve group, and relatively quantified by means of the comparative method: the amount of target, normalised to an endogenous reference and relative to a calibrator, is given by 2–ΔΔCT. Briefly, the ΔCT value is determined by subtracting the average GAPDH CT value from the average cytokine CT in the same sample. The calculation of ΔΔCT involves subtracting the ΔCT calibrator value.
Determination of NOS by western blot analysis
On the day of NOS determination, peripheral and central nervous samples were defrosted at room temperature (22 ± 2°C), weighed, diluted 1 : 20 w/v (thalamus 1 : 30 w/v) in lysis buffer (50 mmol/L Tris–HCl, pH 7.4, 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate, 1% Igepal CA-630, 0.5% sodium deoxycholate, 0.02% sodium azide, 1 mmol/L phenylmethylsulphonyl fluoride, 10 mmol/L leupeptin), homogenised using an ultrasonic processor homogeniser UP 50H (Dr Hielscher Gmbh, Berlin, Brandenburg, Germany), and centrifuged at 1500 g for 10 min at 4°C. After protein assay, the supernatant was diluted in Laemli buffer (0.3 mol/L Tris–HCl, pH 6.8, containing 10% sodium dodecyl sulfate, 50% glycerol, 5% dithiothreitol, and 0.05% bromophenol blue) in order to obtain 40 µg of proteins. nNOS and iNOS were assayed as described in Martucci et al. (2008) using a primary polyclonal antibody directed against mouse nNOS or iNOS (Cayman Chemical, Ann Arbor, MI, USA). The immunoreactive bands were analyzed by means of a computer-based densitometry National Institute of Health image program. The gray levels obtained by densitometrically analyzing the immunoreactive bands were normalised to β-actin using the actin polyclonal antibody (Cytoskeleton Inc., Denver, CO, USA), and expressed as percentages of sham-operated mouse levels in the case of sciatic nerve, DRG and spinal cord; in the case of thalamus, the gray levels of each treatment group were expressed as percentages of the contralateral versus ipsilateral portion.
Transcription factor NF-κB assay
The nuclear transcription factor NF-κB was measured by means of an ELISA kit (Active Motif, Rixensart, Belgium) that detected NF-κB activation by combining NF-κB-specific oligonucleotide binding with the subsequent detection of the p65 subunit of NF-κB using a specific antibody. Fourteen days after surgery, portions of the sciatic nerve and dorsal spinal cord (L4–L6) were homogenised in 100 µL ice-cold hypotonic lysis buffer (supplied with the nuclear extract kit) per milligrams of wet tissue. After centrifugation at 850 g for 10 min, 500 µL of the hypotonic buffer supplemented with 25 µL of Nonidet P-40 (Roche, Mannheim, Germany) were added to the pellet, and the mixture was centrifuged at 14 000 g for 2 min at 4°C, after which the pellets were suspended in 50 µL of hypertonic lysis buffer and incubated with shaking for 30 min at 4°C. The samples were then centrifuged at 14 000 g for 10 min at 4°C, and the supernatant containing the nuclear extracts was stored at –80°C until use.
The nuclear protein extracts (20 µg) were added to the oligonucleotide-coated ELISA plate and incubated for 1 h at room temperature (22 ± 2°C). A primary antibody recognising an epitope on p65 (which is only accessible when NF-κB is activated and bound to its target DNA) was added to the wells and incubated for 1 h. This was followed by the addition of a horseradish peroxidase (EC 1.11.1.7)-conjugated secondary antibody and, after 1 h, the horseradish peroxidase substrate. The reaction was stopped after 5–10 min, and absorbance was measured using a programmable microplate reader (DV990BV6, Gio’ de Vita, Rome, Italy) at 450 nm. Jurkat cell nuclear extracts were used as an activated NF-κB positive control. NF-κB wild-type and mutated consensus oligonucleotides were used in order to monitor the specificity of the assay: a wild-type oligonucleotide should compete with NF-κB for binding, whilst the mutated consensus oligonucleotide should have no effect on NF-κB binding.
Oxidative stress
Lipid peroxide Lipid peroxide levels were determined after surgery by measuring MDA in a hind paw tissue homogenate prepared using a T25, 18N Ultra-Turrax in a ratio 1 : 10 (w/v) potassium phosphate (50 mmol/L)-EDTA (0.1 mmol/L) buffer, pH 7.4. The lipid peroxide level was established spectrophotometrically at 532 nm by means of the thiobarbituric acid test with 0.156 µmol/L/cm being used as the extinction coefficient and expressed as nmol MDA/mg wet weight tissue as previously described (Costa et al. 2007).
Reactive oxygen species Reactive oxygen species levels were determined in hind paw tissue homogenate after surgery by means of fluorimetric analysis (Wallac Victor 2 1420 Multilabel Counter; Perkin Elmer, Shelton, CT, USA), which uses the non-fluorescent diacetate promoiety, 5 (and 6)-carboxy-2'-7'-dichlorofluorescein (CDF) diacetate (Sigma) that is converted into highly fluorescent CDF in the presence of ROS. The CDF procedure is a highly sensitive method that allows the detection of hydroperoxide pmols (excitation 485 nm, emission 525 nm) (Zamek-Gliszczynski et al. 2003). ROS levels are expressed as units of fluorescence.
GPX-specific activity Paw tissues were homogenised 1 : 4 (w/v) in Tris–HCl buffer (20 mmol/L, pH 7.6). The homogenate was centrifuged at 4°C for 10 min at 9000 g, and the supernatant was centrifuged at 4°C for 1 h at 100 000 g in order to obtain the cytosolic fraction, which was used to measure the activity of GPX. The specific activity of cytosolic GPX was assayed spectrophotometrically at 37°C using H2O2 as peroxide. The oxidation of NADPH was followed by monitoring the changes in absorbance at 366 nm as previously described (Costa et al. 2007). The reaction mixture contained GSH 1 mmol/L, glutathione reductase 0.3 U/mL and NADPH 0.2 mmol/L, buffered in a solution with 50 mmol/L K phosphate, 1 mmol/L EDTA, and 1 mmol/L NaN3, pH 7. Specific activity was expressed as NADPH µmol/min/µg protein.
Catalase-specific activity To assay CT-specific activity, paw tissues were homogenised in 1 : 10 (w/v) 0.25 M sucrose. The homogenate was centrifuged at 1000 g for 10 min, and the supernatant fraction was centrifuged at 10 000 g for 20 min; the resulting supernatant was used on the day that the mice were killed. CT activity was assayed using the procedure described by Carrillo et al. (1992), and slightly modified by us, in which the disappearance of peroxide is followed spectrophotometrically at 240 nm. The incubation mixture contained 0.05 mol/L K phosphate pH 7.00, 1.7 mol/L H2O2 and a sample of 50 µL of supernatant fluid in a final volume of 3 mL. The rate of decrease in absorbance was recorded for 2 min at 30°C, and the rate of decrease in absorbance per minute was calculated from the linear portion of the curve. The value of 0.0394 cm2/µmol was used as the extinction coefficient of H2O2. Specific enzymatic activity was defined as the amount of enzyme that decomposed 1 nmol of H2O2/min/µg protein.
Statistical analysis
The data are presented as mean values ± SEM, and were statistically analysed using one-way anova for parametric data followed by Tukey’s or Bonferroni’s test for multiple comparisons; in the case of non-parametric data, Kruskal–Wallis’anova was applied followed by Dunn’s test. The thalamus data were analysed using Mann–Whitney’s test. Differences were considered significant at p < 0.05. All of the statistical analyses were made using graphpad 4 Software (San Diego, CA, USA).
Results
The effect of genistein on thermal hyperalgesia and mechanical allodynia
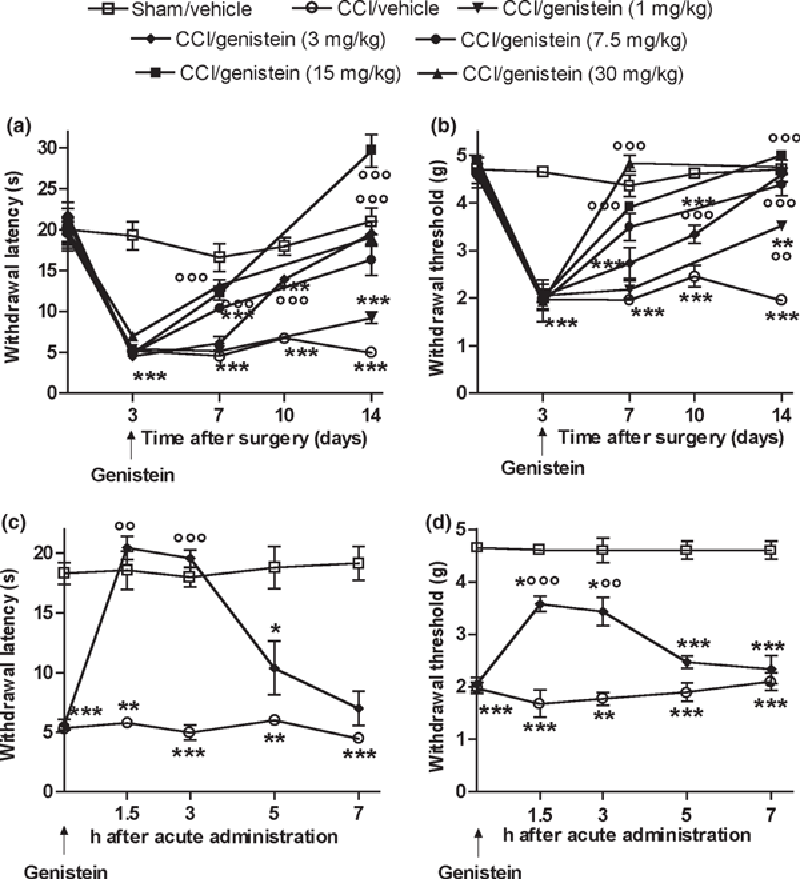
Figure 1 Nociceptive hypersensitivity was evaluated 24 h after the last genistein administration in order to avoid acute interference. The effects of genistein (1, 3, 7.5, 15, or 30 mg/kg s.c. once a day for 11 days, starting from the third day after nerve injury) on thermal hyperalgesia and mechanical allodynia are shown in Figure 1a and b. Three days after injury, thermal withdrawal latency significantly decreased in comparison with the sham-operated animals (p < 0.0001) only in the paw ipsilateral to the injury; the mice also developed mechanical allodynia to normally innocuous stimulation with the von Frey filament (p < 0.0001). Seven days after injury, thermal hyperalgesia and mechanical allodynia were still present in the neuropathic mice treated with vehicle, whereas genistein (30 and 15 mg/kg) reversed the nociceptive hypersensitivity, 7.5 mg/kg attenuated thermal hyperalgesia (p < 0.0001) and reversed allodynia (p < 0.0001), and 3 and 1 mg/kg were ineffective. After prolonged administration, the 3 and 7.5 mg/kg doses also abolished the nociceptive neuropathic hypersensitivity, whereas 1 mg/kg did not affect thermal hyperalgesia (p < 0.0001) but did relieve allodynia (p < 0.0001). In brief, genistein reduced the nociceptive hypersensitivity because of the neuropathic lesion in a time- and dose-dependent manner. The nociceptive thresholds of the paws contralateral to the nerve ligation were not affected by repeated treatment with 3 mg/kg (data not shown). The 3 mg/kg dose was used in all subsequent experiments as it was the lowest dose that reversed neuropathic pain after prolonged administration.
Figure 1c shows that a single administration of genistein (3 mg/kg) given 3 days after injury also reversed the hyperalgesia 1.5 h (p < 0.0001) and 3 h after treatment (p = 0.0002), but the hyperalgesia reappeared after 5 h (p = 0.0026); at the same time points, allodynia only partially decreased (Fig. 1d, 1.5 h, p < 0.0001; 3 h, p =0.0013) and reappeared after 5 h (p < 0.0001).
Effects of estrogen receptor antagonists on genistein-induced pain relief
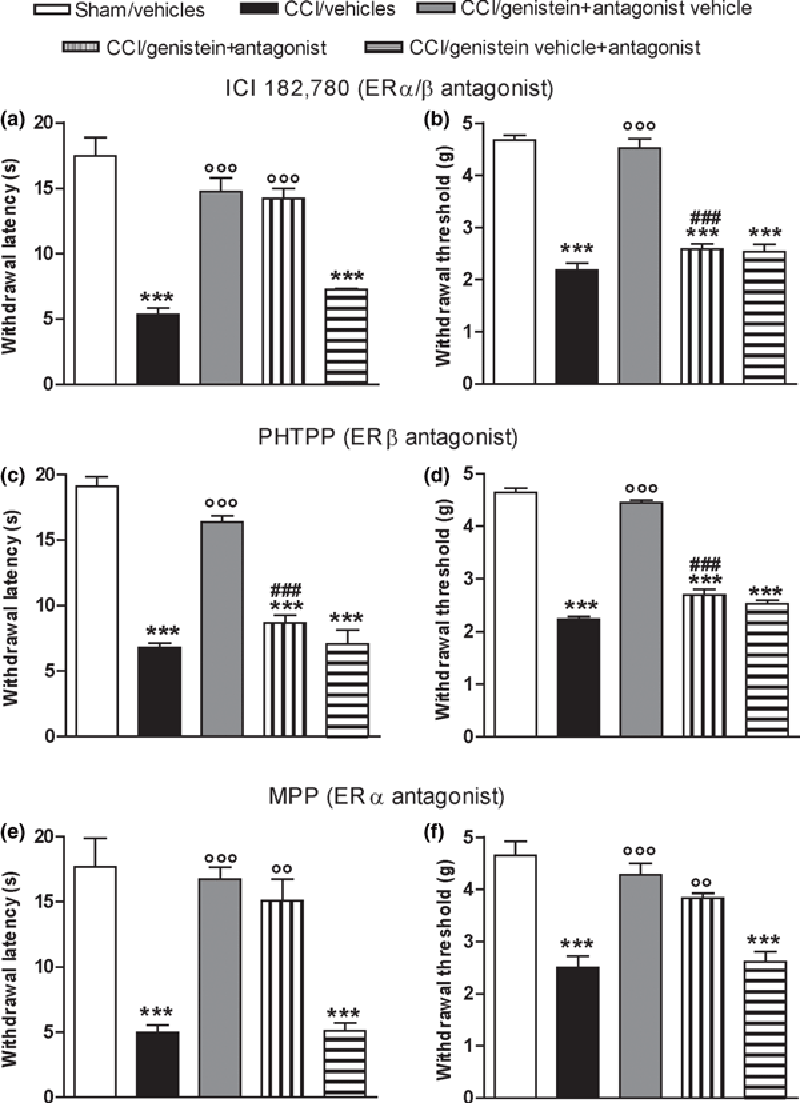
Figure 2
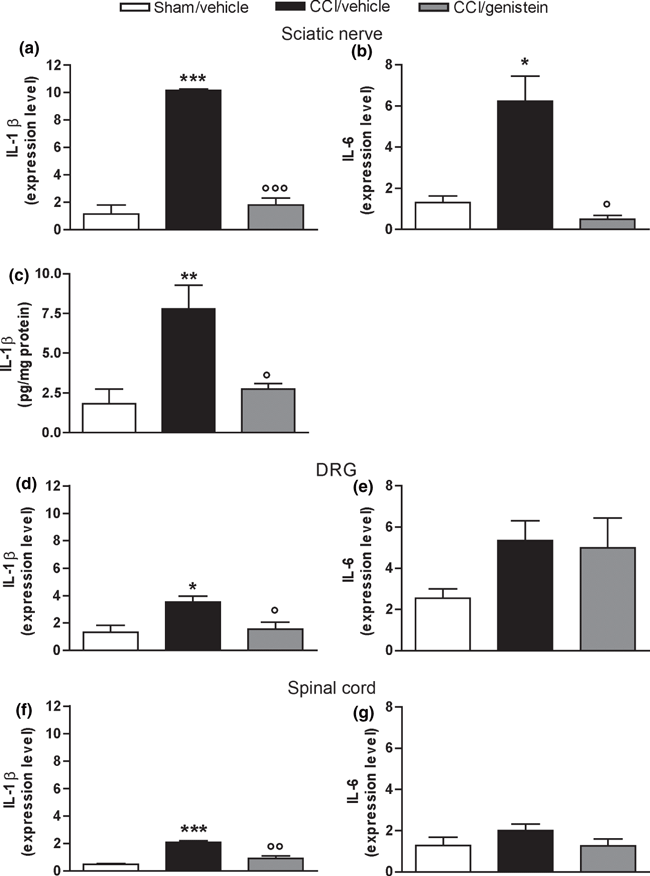
Figure 3
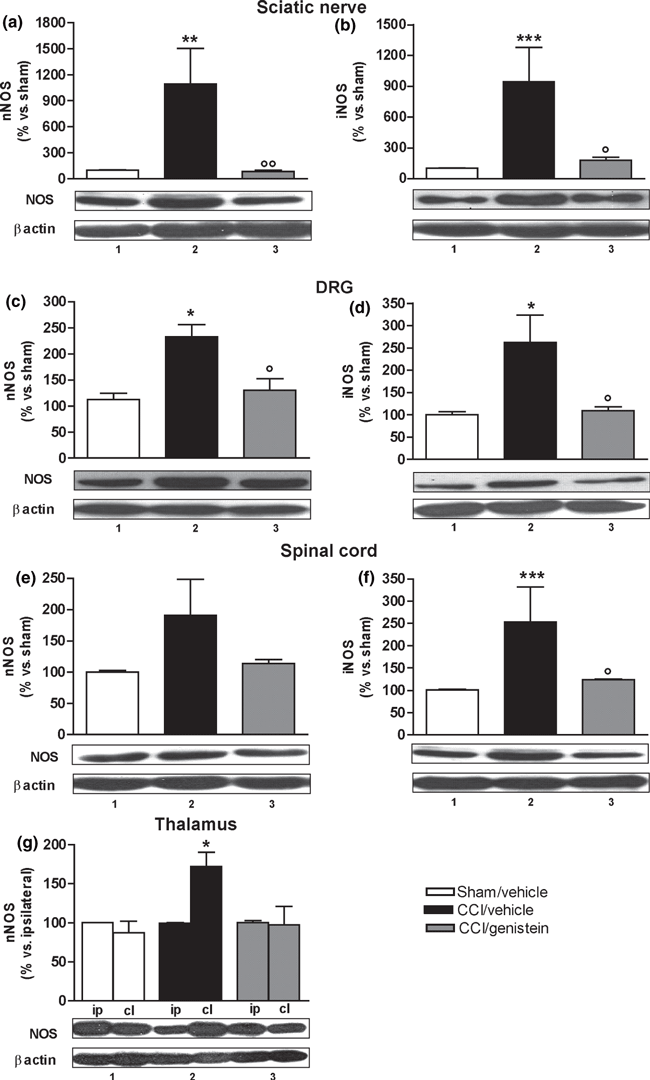
Figure 4 The ability of the non-selective ER antagonist ICI 182 780, the ERβ-specific antagonist PHTPP, and the ERα-specific antagonist MPP to reverse the anti-allodynic and anti-hyperalgesic effect of genistein 3 mg/kg was tested on day 14 after surgery, 24 h after the last coadministration. None of the antagonists alone modified withdrawal latency (Figure 2a, c and e) or the mechanical threshold of the ipsilateral (Fig. 2b, d and f) or contralateral paws (data not shown). ICI 182 780 reversed the anti-allodynic effect of genistein (p < 0.0001) (Fig. 2b), but not its anti-hyperalgesic effect (p < 0.0001) (Fig. 2a); PHTPP reversed both the anti-hyperalgesic (p < 0.0001) (Fig. 2c) and anti-allodynic effects (p <0.0001) (Fig. 2d); and MPP did not reverse either the anti-hyperalgesic (p < 0.0001) (Fig. 2e) or anti-allodynic effect (p < 0.0001) (Fig. 2f).
Effects of genistein on pro-inflammatory cytokine changes in sciatic nerve, DRG and spinal cord
Two weeks after nerve ligation, the sciatic nerve expression of both IL-1β and IL-6 mRNA was up-regulated in comparison with the sham-operated animals, and IL-1β protein level was also higher. Genistein reversed both the mRNA over-expression (IL-1β: p = 0.0005; IL-6: p = 0.0045) (Figure 3a and b) and the IL-1β increased protein content (p = 0.0056) (Fig. 3c). Only IL-1β was over-expressed in DRG and spinal cord, and this was reversed by genistein (DRG: p = 0.011; spinal cord: p = 0.0002) (Fig. 3d and f); IL-6 levels were not changed in DRG or spinal cord, and genistein did not change physiological IL-6 expression (Fig. 3e and g).
Effects of genistein on iNOS and nNOS changes in sciatic nerve, DRG, spinal cord, and thalamus
The sciatic nerve, DRG, and spinal cord results were shown as gray density percentages of sham-operated mouse levels (normalised on β-actin) and in the form of a representative western blot. Fourteen days after surgery, nNOS, and iNOS levels had increased in the injured nerve and were normalised by genistein 3 mg/kg (nNOS, p = 0.0074; iNOS, p =0.0121) (Figure 4a and b). In the ipsilateral DRG, both isoforms were over-expressed and normalised after genistein treatment (nNOS, p = 0.011; iNOS, p = 0.0128) (Fig. 4c and d). In the spinal cord dorsal horns (at the level of the L4–L6 metamers), only iNOS was significantly up-regulated and returned to sham-operated animal levels after genistein treatment (p = 0.0013) (Fig. 4e and f).
In the case of the thalamus, the mean gray density (normalised on β-actin) of the ipsilateral thalamus was set at 100 and the contralateral values were expressed as percentages. The results are shown in both gray density percentages and in the form of a representative western blot. The neuropathic mice showed an increase in nNOS (Fig. 4g) but not iNOS (data not shown) in the contralateral thalamus receiving spinothalamic sensitive fibers from the ligated nerve (p = 0.0286). This was normalised by genistein as no differences between the two sides were observed in the vehicle-treated sham-operated animals or the genistein-treated neuropathic mice (Fig. 4g).
Genistein effects on NF-κB in sciatic nerve and spinal cord
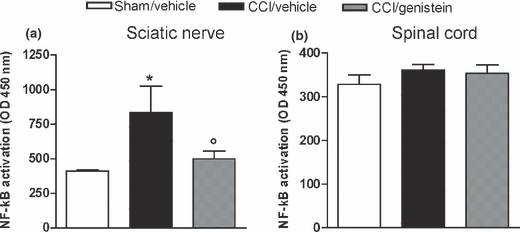
Figure 5
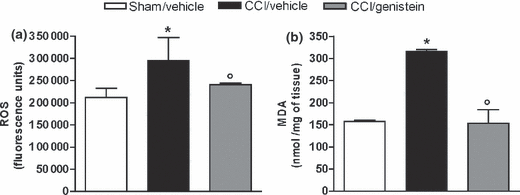
Figure 6 Two weeks after injury, NF-κB was over-activated in the sciatic nerve but not in the lumbar spinal cord. Genistein abolished the NF-κB activation (p < 0.05) (Figure 5a), but did not modify NF transcription in the spinal cord (Fig. 5b).
Effects of genistein on lipid peroxide and ROS levels
Two weeks after sciatic nerve ligation, neuropathic pain was associated with one-and-a-half times greater production of ROS in ipsilateral paw tissues (Figure 6a) and more than double the production of MDA (Fig. 6b). Genistein 3 mg/kg for 11 days reduced ROS and MDA levels to those observed in the sham-operated mice (ROS: p = 0.0236; MDA: p = 0.008) (Fig. 6a and b).
Genistein effects on GPX- and CT-specific activities
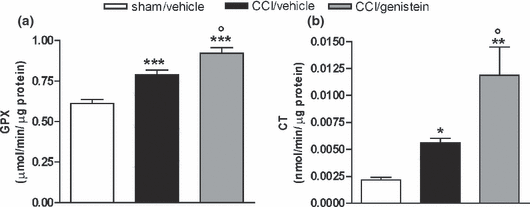
Figure 7 In order to compensate for oxidative damage, GPX and CT were activated in the paw tissues of the neuropathic mice. GPX- and CT-specific activity significantly increased in the neuropathic mice versus the sham-operated mice; repeated treatment with genistein 3 mg/kg for 11 days further increased the difference in enzymatic activities between the CCI and sham-operated mouse paw tissues (GPX: p < 0.0001; CT: p = 0.0016) (Figure 7a and b).
Discussion
The main finding of our study is that the soy phytoestrogen isoflavone genistein simultaneously diminishes the painful and inflammatory biochemical consequences of mouse sciatic nerve CCI, a neuropathic model. Furthermore, these effects also occur after the appearance of nociceptive hypersensitivity, thus suggesting the possible therapeutic efficacy of genistein.
It has previously been shown (Shir et al. 2002) that a soy diet decreases allodynia and hyperalgesia after partial sciatic nerve ligation in rat, but only if soy consumption precedes the injury; our findings suggest that genistein may be the soy phytoestrogen responsible for this effect. In line with published clinical and experimental evidence (Sawada and Shimohama, 2003; Currie et al. 2004) indicating that estrogens and phytoestrogens protect against progressive neurodegenerative diseases, our data suggest that genistein has a neuroprotective effect on the neuroinflammatory conditions caused by CCI in mouse.
Genistein has greater affinity for ERβ than ERα (Kuiper et al. 1997), and we found that a selective ERβ antagonist prevented the beneficial effect of phytoestrogen administration on nociceptive neuropathic hypersensitivity, whereas the selective ERα antagonist did not. The non-selective ERα and β antagonist only reversed the anti-allodynic effect of genistein, perhaps because ICI 182 780 does not cross the blood–brain barrier (Howell et al. 2000). ERβ may therefore be principally involved in the antinociceptive effect of genistein: a co-transfection assay in human endometrial cancer cells showed that PHTPP is 36 times more selective of this receptor subtype and fully antagonises ERβ, with minimal effects on ERα (Compton et al. 2004). Microglia (the primary immune cells in the CNS) are precociously activated in the dorsal horn in the case of nerve injury, whereas astrocytes are later also involved in the pathogenesis and modulation of pain hypersensitivity (Scholz and Woolf 2007), and it is interesting to note that both cell types express ERβ (McCarty 2006; Lewis et al. 2008). Furthermore, it is known that selective ERβ agonists have anti-inflammatory effects on microglia and astrocytes (Lewis et al. 2008), and that blocking microglia and astrocyte activation inhibits the development and maintenance of hyperalgesia and allodynia in neuropathic pain models (Myers et al. 2006).
However, we do not think that ERβ-mediated mechanisms alone explain the efficacy of genistein in reversing neuropathic allodynia and hyperalgesia, but believe that it is also necessary to consider its anti-inflammatory and immunomodulatory effects. Neuropathic pain is associated with local inflammation, the over-expression of NOS, and the increased presence of inflammatory cytokines in locally recruited macrophages, Schwann cells and glial cells (Martucci et al. 2008; Campbell and Meyer 2006) and our study shows that genistein reverses nociceptive hypersensitivity and simultaneously inhibits NOS and pro-inflammatory cytokine over-activity in both the peripheral and CNS. This effect might also be related to genistein’s ERβ agonist activity as it is well known that estrogens are involved in immunomodulation by interfering with cellular and humoral immune responses, and modulating the expression of cytokines in microglia, astrocytes, and immune cells (Mor et al. 1999).
Moreover, the binding of estrogens and isoflavones to ER is associated with the inhibition of NF-κB activation by pro-inflammatory cytokines, and may contribute to their neuroprotective effects (Siow et al. 2007). NF-κB plays an important role in the cascade of events following nerve injury, and we have previously demonstrated that blocking NF-κB activation considerably improves neuropathic pain (Martucci et al. 2008). Although it is not possible to identify the sequence of genistein-induced modifications, its ability to decrease painful behaviour may be related to its effects on NF-κB activation, IL-1β and IL-6 over-expression, and NOS over-production.
After s.c. injection, genistein can cross the blood/brain barrier and reach the CNS (Liu et al. 2008). On the basis of our results, it is difficult to establish whether genistein primarily acts peripherally or centrally, and it is feasible that both peripheral and central sites of action are involved. In this regard, it is worth bearing in mind that neurons, microglia, astrocytes (Liu et al. 2005), Schwann cells (Groyer et al. 2006), and immune cells (Straub 2007) all have ERβ receptors.
Our findings also show the anti-oxidant effects of genistein, which may be indirectly involved in its effect on neuropathic pain. It is well known that oxidative stress is involved in the pathogenesis of a number of neurodegenerative diseases, as well as in neural tissue degeneration. We found increased ROS and lipoperoxide production in the paw tissues of our mice that was reversed by genistein, and also observed that genistein increased the activity of the enzymes particularly involved in anti-oxidant defence such as GPX and CT (Naik et al. 2006; Costa et al. 2007). These effects may be directly because of the molecule’s phenolic ring (Dugas et al. 2000), which protects against lipid peroxidation, or to its ability to up-regulate anti-oxidant genes (Vina et al. 2007b; Siow et al. 2007). In line with our results, other recent studies have established that the induction of detoxification genes is mediated by the direct binding of phytoestrogens to ERβ, with the subsequent gene transcription being mediated by binding to anti-oxidant or electrophilic response elements (Bianco et al. 2005). This anti-oxidant activity of genistein could slow neural tissue damage or even help its repair, thus attenuating one of the putative causes of neuropathic pain-generating stimuli. It is well known that natural and synthetic ROS scavengers reduce hyperalgesia and allodynia in a number of neuropathic pain models (Kim et al. 2004; Sharma et al. 2007).
Although we found the presence of biochemical modifications 14 days after CCI in this study, we have recently reported that IL-1β is also over-expressed in peripheral and central nervous tissues earlier (Martucci et al. 2008). Similarly, we have observed an early increase in ROS production, with a peak 7 days after sciatic nerve lesion (unpublished results). As nociceptive hypersensitivity was not completely reversed until after 11 days of treatment, we can hypothesise that repeated treatment is needed to reduce this permanent inflammatory and oxidative condition of neuropathic tissues.
Interestingly, genistein could be rapidly transferred to clinical use, as it does not have the disadvantages of endogenous estrogens (Vina et al. 2007a), because isoflavones are not feminising in males as they bind quite specifically to ERβ, whereas these effects are associated with ERα activation. Furthermore, no carcinogenic effects of phytoestrogens have been reported.
In conclusion, a wide range of chemical, biochemical and genomic mechanisms may be involved in genistein-induced neuropathic pain relief, but it will require further studies before their roles can be clarified.
References:
An J., Tzagarakis-Foster C., Scharschmidt T. C., Lomri N. and Leitman D. C. (2001) Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J. Biol. Chem. 276, 17808–17814.
Bennett G. J. and Xie Y. K. (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33, 87–107.
Bianco N. R., Chaplin L. J. and Montano M. M. (2005) Differential induction of quinone reductase by phytoestrogens and protection against oestrogen-induced DNA damage. Biochem. J. 385, 279–287.
Bjorling D. E. and Wang Z-Y. (2001) Estrogen and neuroinflammation. Urology 57 (Suppl. 6A), 40–46.
Campbell J. N. and Meyer R. A. (2006) Mechanisms of neuropathic pain. Neuron 52, 77–92.
Carrillo M. C., Kanai S., Ivy G. O. and Kitani K. (1992) Sequential changes in activities of superoxide dismutase and catalase in brain regions and liver during (-)deprenyl infusion in male rats. Biochem. Pharmacol. 44, 2185–2189.
Clatworthy A. L., Illich P. A., Castro G. A. and Walters E. T. (1995) Role of periaxonal inflammation in the development of thermal hyperalgesia and guarding behavior in a rat model of neuropathic pain. Neurosci. Lett. 184, 5–8.
Compton D. R., Sheng S., Carlson K. E., Rebacz N. A., Lee I. Y., Katzenellenbogen B. S. and Katzenellenbogen J. A. (2004) Pyrazolo[1,5-a]pyrimidines: estrogen receptor ligands possessing estrogen beta antagonist activity. J. Med. Chem. 47, 5872–5893.
Costa B., Trovato A. E., Comelli F., Giagnoni G. and Colleoni M. (2007) The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 556, 75–83.
Currie L. J., Harrison M. B., Trugman J. M., Bennett J. P. and Wooten J. F. (2004) Postmenopausal estrogen use affects risk for Parkinson disease. Arch. Neurol. 61, 886–888.
Davis A. M., Ellersieck M. R., Grimm K. M. and Rosenfeld C. S. (2006) The effects of the selective estrogen receptor modulators, methyl-piperidino-pyrazole (MPP), and raloxifene in normal and cancerous endometrial cell lines and in the murine uterus. Mol. Reprod. Dev. 73, 1034–1044.
Duffy C., Perez K. and Partridge A. (2007) Implications of phytoestrogen intake for breast cancer. CA Cancer J. Clin. 57, 260–277.
Dugas A. J., Castaneda-Acosta J., Bonin G. C., Price K. L., Fisher N. H. and Winston G. W. (2000) Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: structure-activity relationships. J. Nat. Prod. 63, 327–331.
Groyer G., Eychenne B., Girard C., Rajkowski K., Schumacher M. and Cadepond F. (2006) Expression and functional state of the corticosteroid receptors and 11β-hydroxysteroid dehydrogenase type 2 in Schwann cells. Endocrinology 147, 4339–4350.
Howell A., Osborn C. K., Morris C. and Wakeling A. E. (2000) ICI 182,780 (Faslodex™). Development of a novel, “pure” antiestrogen. Cancer 89, 817–825.
Kim H. K., Park S. K., Zhou J. L., Taglialatela G., Chung K., Coggeshall R. E. and Chung J. M. (2004) Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 111, 116–124.
Kuiper G. G. J. M., Carlsson B., Grandien K., Enmark E., Haggblad J., Nilsson S. and Gustafsson J-A. (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138, 863–870.
Leventhal L., Brandt M. R., Cummons T. A., Piesla M. J., Rogers K. E. and Harris H. A. (2006) An estrogen receptor-β agonist is active in models of inflammatory and chemical-induced pain. Eur. J. Pharmacol. 553, 146–148.
Lewis D. K., Johnson A. B., Stohlgren S., Harms A. and Sohrabji F. (2008) Effects of estrogen receptor antagonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J. Neuroimmunol. 195, 47–59.
Liu X., Fan X. L., Zhao Y., Luo G. R., Li X. P., Li R. and Le W. D. (2005) Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-alpha and estrogen receptor-beta in microglia. J. Neurosci. Res. 81, 653–665.
Liu L. X., Chen W. F., Xie J. X. and Wong M. S. (2008) Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci. Res. 60, 156–161.
Martucci C., Trovato A. E., Costa B. et al. (2008) The purinergic antagonist PPADS reduces pain related behaviors and interleukin-1β, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain 137, 81–95.
McCarty M. F. (2006) Down-regulation of microglial activation may represent a practical strategy for combating neurodegenerative disorders. Med. Hypoth. 67, 251–269.
McClain R. M., Wolz E., Davidovich A., Edwards J. and Bausch J. (2007) Reproductive safety studies with genistein in rats. Food Chem. Toxicol. 45, 1319–1332.
Mor G., Nilsen J., Horvath T., Bechmann I., Brown S., Garcia-Segura L. M. and Naftolin F. (1999) Estrogen and microglia: a regulatory system that affects the brain. J. Neurobiol. 40, 484–496.
Myers R. R., Campana W. M. and Shubayev V. I. (2006) The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov. Today 11, 8–20.
Naik A. K., Tandan S. K., Dudhgaonkar S. P., Jadhav S. H., Kataria M., Prakash V. R. and Kumar D. (2006) Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur. J. Pain 10, 573–579.
Sawada H. and Shimohama S. (2003) Estrogens and Parkinson disease: novel approach for neuroprotection. Endocrine 21, 77–79.
Scholz J. and Woolf C. J. (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 10, 1361–1368.
Sharma S., Kulkarni S. K. and Chopra K. (2007) Effect of resveratrol, a polyphenolic phytoalexin, on thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Fundam. Clin. Pharmacol. 21, 89–94.
Shir Y., Campbell J. N., Raja S. N. and Seltzer Z. (2002) The correlation between dietary soy phytoestrogens and neuropathic pain behavior in rats after partial denervation. Anesth. Analg. 94, 421–426.
Siow R. C. M., Li F. Y. L., Rowlands D. J., De Winter P. and Mann G. E. (2007) Cardiovascular targets for estrogens and phytoestrogens: Transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radic. Biol. Med. 42, 909–925.
Straub R. H. (2007) The complex role of estrogens in inflammation. Endocr. Rev. 28, 521–574.
Sun J., Huang Y. R., Harrington W. R., Sheng S., Katzenellenbogen J. A. and Katzenellenbogen B. S. (2002) Antagonists selective for estrogen receptor α. Endocrinology 143, 941–947.
Verdrengh M., Jonsson I. M., Holmdahl R. and Tarkowski A. (2003) Genistein as an anti-inflammatory agent. Inflamm. Res. 52, 341–346.
Vina J., Gomez-Cabrera M. C. and Borràs C. (2007a) Fostering antioxidant defences: up-regulation of antioxidant genes or antioxidant supplementation. Br. J. Nutr. 98 (Suppl. 1), S36–S40.
Vina J., Lloret A., Vallés S. L., Borràs C., Badia M-C., Pallardò F. V., Sastre J. and Alonso M-D. (2007b) Effect of gender on mitochondrial toxicity of Alzheimer’s Aβ peptide. Antioxid. Redox Signal. 9, 1677–1690.
Wakeling A. E. and Bowler J. (1987) Steroidal pure antioestrogens. Endocrinology 112, R7–R10.
Zamek-Gliszczynski M. J., Xiong H., Patel N. J., Turncliff R. Z., Pollack G. M. and Brouwer K. L. R. (2003) Pharmacokinetics of 5(and 6)-carboxi-2',7'-dichlorofluorescein and its diacetate promoiety in the liver. J. Pharmacol. Exp. Ther. 304, 801–809.
Zimmermann M. (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16, 109–110.

Return to the SOY PROTEIN Section
Since 12-30-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |