

Weight Loss Without Losing Muscle Mass
in Pre-obese and Obese Subjects
Induced by a High-soy-protein Diet
in Pre-obese and Obese Subjects
Induced by a High-soy-protein Diet
Send all comments or additions to: Frankp@chiro.org




FROM: Int J Obes Relat Metab Disord. 2004 (Oct); 28 (10): 1349–1352 ~ FULL TEXT
Deibert P, König D, Schmidt-Trucksaess A, Zaenker KS, Frey I, Landmann U, Berg A.
Department of Rehabilitative and Preventive Sports Medicine,
University Hospital,
D-79106 Freiburg, Germany.
deibert@msm1.ukl.uni-freiburg.deOBJECTIVE: To determine change of weight, body composition, metabolic and hormonal parameters induced by different intervention protocols.
DESIGN: Randomized, controlled study including participants exhibiting a BMI between 27.5 and 35. Three different interventions containing lifestyle education (LE-G), or a substitutional diet containing a high-soy-protein low-fat diet with (SD/PA-G) or without (SD-G) a guided physical activity program.
SUBJECTS: A total of 90 subjects (mean weight 89.9 kg; mean BMI 31.5), randomly assigned to one of three treatment groups.
MEASUREMENTS: Change in body weight, fat mass and lean body mass measured with the Bod Pod device at baseline, 6 weeks and 6 months; change in metabolic and hormonal parameters.
RESULTS: In all, 83 subjects completed the 6-months study. BMI dropped highly significantly in all groups (LE-G: -2.2+/-1.43 kg/m(2); SD-G: -3.1+/-1.29 kg/m(2); SD/PA-G: -3.0+/-1.29 kg/m(2)). Subjects in the SD-G and in the SD/PA-G lost more weight during the 6-months study (-8.9+/-3.9; -8.9+/-3.9 kg) than did those in the LE-G (-6.2+/-4.2 kg), and had a greater decrease in fat mass (-8.8+/-4.27; -9.4+/-4.54 kg) than those in the LE-G (-6.6+/-4.59 kg). In contrast, no significant intraindividual or between-group changes in the fat-free mass were seen. In all groups, metabolic parameters showed an improvement in glycemic control and lipid profile.
CONCLUSIONS: Our data suggest that a high-soy-protein and low-fat diet can improve the body composition in overweight and obese people, losing fat but preserving muscle mass.
From the FULL TEXT Article:
Introduction
In high-income countries, people are eating more and exercising less, resulting in an increase of body weight. In many developed countries, as much as half of the adult population are overweight and more than 25% obese. [1] There are many conventional dietary approaches to weight management, recommended by the leading research and medical societies. However, only a few studies evaluated their long-term efficacy with respect to body composition, for example, changes in muscle vs fat mass. [2, 3] The balance of macronutrients in a diet to lose weight is still in debate. Diets with a high proportion of carbohydrates may reduce the oxidation of body fat, [4, 5] increase blood triglycerides [6, 7] and reduce satiety. [2, 8, 9] On the other hand, protein-rich diets may reduce energy efficiency and increase thermogenesis, [10, 11] reduce the resting energy expenditure in response to a diet to a lesser extent, [12, 13] spare muscle protein loss [3] and enhance glycemic control. [3, 14]
We conducted a 6-month randomized, controlled trial to estimate the efficacy of a staged high-soy-protein and low-fat diet. The primary objectives were weight loss and a reduction in BMI of at least 2.5 kg/m2 after 6 months; a secondary objective was loss of fat mass and preservation of muscle mass.
Methods
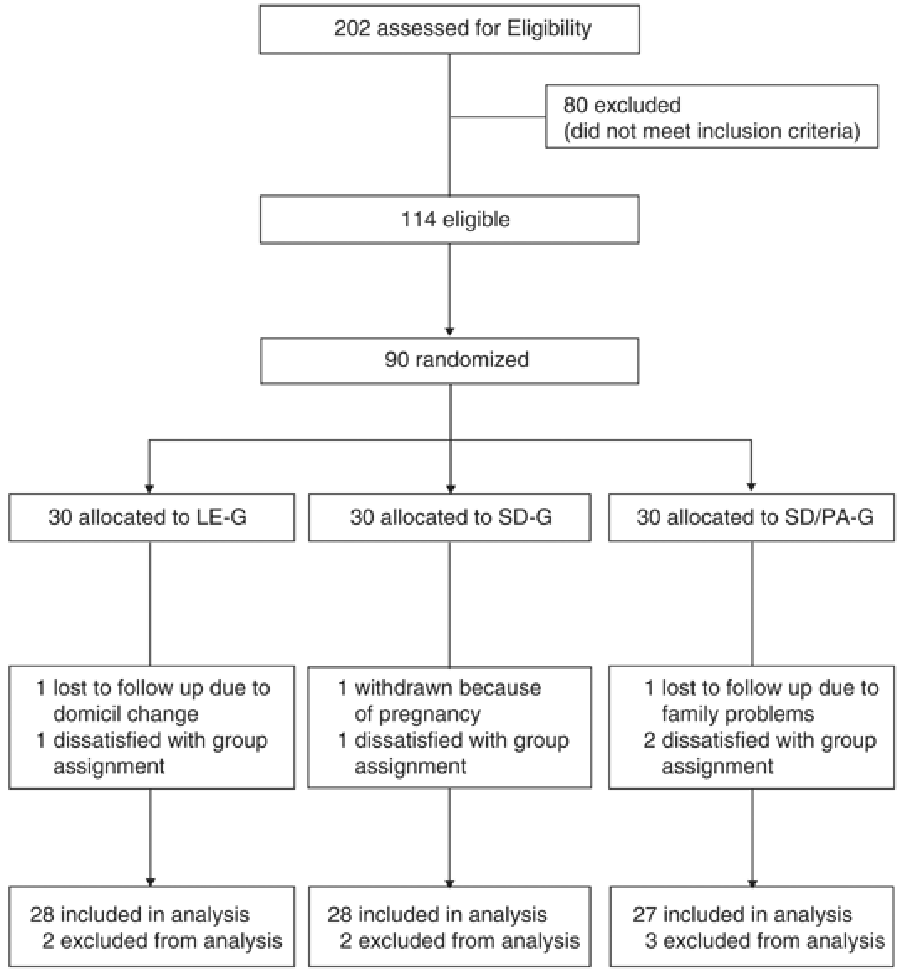
Figure 1 Out of a group of more than 500 interested people recruited by public advertisement, potential subjects were excluded if they had clinically significant illnesses, including type II diabetes, were taking lipid-lowering medication, or were taking medications that affect body weight. From 114 eligible people, 90 pre-obese and obese subjects [15] were randomized (see Figure 1) to participate in the study (mean weight 89.9±10.9 kg; mean BMI 31.5±2.26 kg/m2; mean fat mass 40.5±6.40 kg). All subjects completed a comprehensive medical examination and routine blood tests. Written informed consent was provided by all subjects, and the study protocol was approved by the local ethic review board.
The subjects were randomly assigned to three different treatment groups: the lifestyle education group (LE-G) attended three bi-monthly teaching sessions, and two individual visiting periods, 6 weeks and 6 months after enrolment; all sessions were led by experts in nutritional counselling. Subjects received a diet-overview handout, in accordance with the 'German Society of Nutrition' and the 'German Society of Sports Medicine and Prevention'. A moderate-fat, balanced nutrient reduction diet was prescribed (1200–1500 kcal/day for women and 1500–1800 kcal/day for men, with approximately 60% of calories from carbohydrate, 25% from fat, and 15% from protein). The subjects assigned to the substitutional diet group (SD-G) were instructed to replace two daily meals by a commercially available soy–yoghurt–honey preparation (Almased®) for the first 6 weeks, followed by the replacement of one daily meal for 18 weeks. For the latter time interval, the dietary intake of fat should not exceed 60 g/day. This diet contained about 1000 kcal/day for women and 1200 kcal/day for men in the first 6 weeks, and then was aimed not to exceed 1500 kcal/day for women and 1700 kcal/day for men in the following weeks. In addition, a third group of subjects was motivated to attend two times weekly a 60-min endurance physical activity program, which was delivered by a sport physician, otherwise, they followed the rules of the substitutional diet group (SD/PA-G).
Data collected at enrolment and thereafter monthly were body weight, waist and abdominal circumference, self-reported medical history, blood pressure, glucose, insulin, serum lipids and inflammatory markers (C-reactive protein, IL-6). For measurement of body composition, the technique of the air displacement plethysmography was used (Bod Pod®). [16] Dietary compliance was estimated by a 24-h recall of dietary consumption. However, the data derived from the self-reported 24-h recall showed a major under-reporting in all groups, so these data are not taken into account.
Testing for changes between examination at baseline and at examination after 24 weeks was carried out by paired sample t-test. For comparison of continuous variables between the groups, we calculated the change from baseline to 6 months in each subject and compared the mean changes in the three groups using analysis of variance with post hoc tests (Schiffé). Normality of all variables was tested before statistical testing. Leptin and insulin values were normalized by logarithmic transformation. All P-values were two-sided and a P-value of 0.05 or less was considered to indicate the statistical significance. Analysis was conducted using the SPSS software (version 11.5.2.1).
Results
A total of 83 subjects completed 6 months of the study (28 subjects in the LE-G, 28 subjects in the SD-G, 27 subjects in the SD/PA-G); the differences in characteristics of subjects who dropped out of the study were not statistically significant.
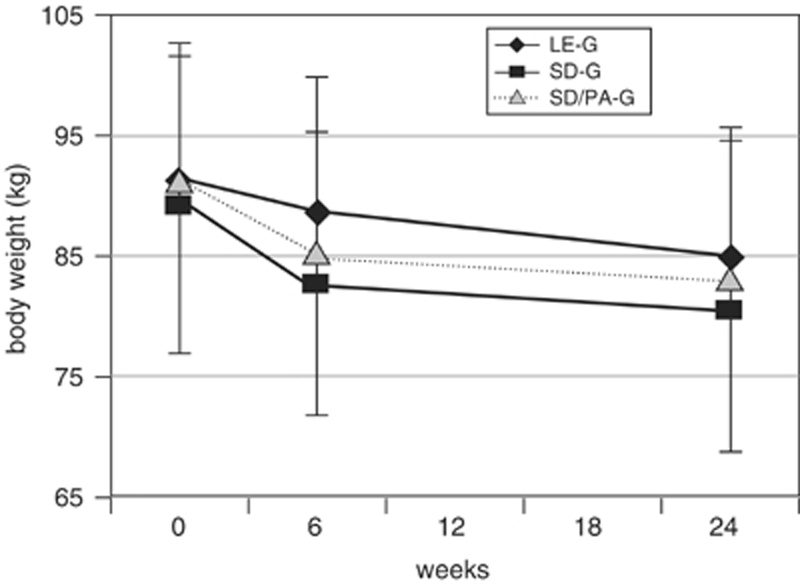
Figure 2

Table 1 With respect to weight changes during the 6-months study, there were differences between the three groups (F=4.292; P=0.048; ANOVA). Subjects in the SD-G and in the SD/PA-G lost more weight during the 6-months study (-8.9±3.9; -8.9±3.9 kg; NS) than did those in the LE-G (-6.2±4.2 kg) (P=0.048; post hoc analysis) (see Figure 2). The BMI, the second primary objective, dropped highly significantly in the LE-G (-2.2±1.43 kg/m2), in the SD-G (-3.1±1.29 kg/m2) and in the SD/PA-G (-3.0±1.29 kg/m2) (F=4.325; P=0.016; ANOVA). During the 6-months study, subjects had a greater decrease of fat mass in the SD-G and in the SD/PA-G (-8.8±4.27; -9.4±4.54 kg) than those in the LE-G (-6.6±4.59 kg) (F=3.049; P=0.053; ANOVA). In contrast, we did not observe significant intraindividual or between-group changes in the fat-free mass (LE-G: 0.4±1.76 kg; SD-G: -0.1±2.19 kg; SD/PA-G: 0.4±2.29 kg) (F=0.468; P=0.628; ANOVA). The biochemical changes are demonstrated in Table 1, exhibiting a significant improvement in glycemic control in all three groups; no adverse effects on serum levels of inflammatory markers were observed (data not shown).
Comment
The effects of any diet on weight loss with respect to body composition have been incompletely assessed in long-term trials. A high-soy-protein, low-fat diet without (28 subjects) or with an additional physical activity program (27 subjects) produced a greater weight loss and decrease in fat mass in the 55 pre-obese and obese subjects, than did a conventional diet supported by a teaching program for weight loss in 28 pre-obese and obese subjects for 6 months. However, we acknowledge the limitations of the present study over a short period of time. Further long-term evaluation is needed.
These data suggest that a caloric restriction with a soy-protein-enriched diet induces a larger reduction of fat mass without losing muscle mass as compared to a standard diet. Former studies revealed that up to 30% of weight loss may be due to reduction of muscle mass using different modalities of dieting. [17] Therefore, aside from a further increase in energy expenditure, dieting should be combined with physical exercise. However, diets with an elevated protein content seem to minimize the benefit of physical activity by preventing loss of muscle mass. Our results confirm the importance of a high-protein diet with respect to maintaining lean body mass, as a guided programme of aerobic exercise two times a week had no additional effect in participants using the soy-protein-enriched diet. It has been shown that an increased abundance of amino acids will increase muscle protein synthesis globally, [18] so the additional effect of physical activity may be masked.
A recent study assumed that energy expenditure was higher when the protein was derived from animal proteins compared to plant proteins. [19] As our results show, diets using plant-derived proteins also may lead to a considerable loss of weight. The used formula diet contains a high amount of essential and branched-chain amino acids. Essential amino acids have been shown to be responsible for the amino-acid stimulation of muscle protein anabolism in healthy elderly adults. Branched-chain amino acids promote muscle protein synthesis, too [20, 21] and may have regulatory roles on glycemic control. [22]
A major concern about using diets rich in animal products to maintain a higher protein content has been the association of cholesterol and saturated fatty acids with cardiovascular disease. This possible disadvantage is eliminated by using plant-derived proteins, where there may be an additional effect due to fibers and phytochemicals.
This is the first study that evaluates a soy-protein-enriched diet used in different weight reduction programmes with respect to changes in body composition. The simple measurement of body weight is not satisfactory any more, as muscle mass is determining the resting metabolic rate as well as motor competence and daily activity-induced energy expenditure. Therefore, muscle mass changes may be a parameter for long-term efficacy of a dietary programme. The described soy-protein-enriched diet limited in carbohydrates and fat is easy to follow, and apparently more effective in losing fat and preserving muscle mass than a conventional diet.
References:
Department of Health and Human Services. The Surgeon General's call to action to prevent and decrease overweight and obesity. Government Printing Office: Washington, DC; 2001.
Skov AR, Toubro S, Ronn B, Holm L, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord 1999; 23: 528–536.
Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr 2003; 133: 411–417.
McGarry JD. Glucose–fatty acid interactions in health and disease. Am J Clin Nutr 1998; 67: 500S–5504.
Wolfe RR. Metabolic interactions between glucose and fatty acids in humans. Am J Clin Nutr 1998; 67: 519S–5526.
Wolfe BM, Giovannetti PM. Short-term effects of substituting protein for carbohydrate in the diets of moderately hypercholesterolemic human subjects. Metabolism 1991; 40: 338–343.
Sidossis LS, Mittendorfer B, Walser E, Chinkes D, Wolfe RR. Hyperglycemia-induced inhibition of splanchnic fatty acid oxidation increases hepatic triacylglycerol secretion. Am J Physiol Endocrinol Metab 1998; 275: E798–E805.
Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003; 348: 2082–2090.
Anderson GH, Moore SE. Dietary proteins in the regulation of food intake and body weight in humans. J Nutr 2004; 134: 974S–9979.
Reeds PJ, Burrin DG, Davis TA, Stoll B. Amino acid metabolism and the energetics of growth. Arch Tierernahr 1998; 51: 187–197.
Parker B, Noakes M, Luscombe N, Clifton P. Effect of a high-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes. Diabetes Care 2002; 25: 425–430.
Baba NH, Sawaya S, Torbay N, Habbal Z, Azar S, Hashim SA. High protein vs high carbohydrate hypoenergetic diet for the treatment of obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord 1999; 23: 1202–1206.
Whitehead JM, McNeill G, Smith JS. The effect of protein intake on 24-h energy expenditure during energy restriction. Int J Obes Relat Metab Disord 1996; 20: 727–732.
Layman DK, Baum JI. Dietary protein impact on glycemic control during weight loss. J Nutr 2004; 134: 968S–9973.
WHO. Report of the World Health Organization Consultation of Obesity. Geneva; 1997.
McCrory MA, Gomez TD, Bernauer EM, Mole PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc 1995; 27: 1686–1691.
Ballor DL, Poehlman ET. Exercise-training enhances fat-free mass preservation during diet-induced weight loss: a meta-analytical finding. Int J Obes Relat Metab Disord 1994; 18: 35–40.
Brodsky IG, Suzara D, Hornberger TA, Goldspink P, Yarasheski KE, Smith S, Kukowski J, Esser K, Bedno S. Isoenergetic dietary protein restriction decreases myosin heavy chain IIx fraction and myosin heavy chain production in humans. J Nutr 2004; 134: 328–334.
Mikkelsen PB, Toubro S, Astrup A. Effect of fat-reduced diets on 24-h energy expenditure: comparisons between animal protein, vegetable protein, and carbohydrate. Am J Clin Nutr 2000; 72: 1135–1141.
Stein TP, Donaldson MR, Leskiw MJ, Schluter MD, Baggett DW, Boden G. Branched-chain amino acid supplementation during bed rest: effect on recovery. J Appl Physiol 2003; 94: 1345–1352.
Liu Z, Jahn LA, Long W, Fryburg DA, Wei L, Barrett EJ. Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab 2001; 86: 2136–2143.
Layman DK. The role of leucine in weight loss diets and glucose homeostasis. J Nutr 2003; 133: 261S–267S.

Return to LEUCINE
Since 1-21-2015


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |