Adverse Reactions of Medications in Children:
The Need for Vigilance, A Case StudyThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Clinical Chiropractic Pediatrics 2014 (Mar); 14 (2) ~ FULL TEXT
OPEN ACCESS Edward Holmes, DC and Joyce Miller, BSc, DC, DABCO, FCC
Joyce Miller, BSc, DC, DABCO, FCC,
Associate Professor,
Anglo-European College of Chiropractic,
Bournemouth, United Kingdom.Summary: This study demonstrates that adverse drug reactions do potentially pose a public health risk within the pediatric population and all healthcare providers need to be mindful of this risk. Adverse events to medication within this population are prevalent particularly in children under the age of two. Chiropractors must therefore be aware of adverse drug reactions and recognize symptoms within their patient population.
Key terms: off label use; non-prescription drugs, child, pediatrics, drug toxicity guidelines, adverse medication reactions in children
From the Full-Text Article:
Introduction
A 19–week-old premature infant presented to a chiropractic teaching clinic with a ‘poor feeding pattern, recent slowing in weight gain and increase in crying’ over a three week period. The mother stated that this had started following a respiratory infection which occurred two months ago, with an associated rash that spread from her abdomen to the back of her neck, face and head. At least six weeks previously her GP had prescribed a cold remedy for the day (Calcold®) and Calpol® Night for the evening for the respiratory infection, and cortisone cream when a rash developed two weeks later. The medications seemed to help the child sleep, in fact she seemed to sleep much more during the day and night, which was put down to illness. However this did not change in the ensuing weeks after the respiratory infection abated. The mother continued with the Calpol® Night at the recommendation of the GP along with a change to Calpol® (instead of Calcold®) in the day, since it seemed to have helped with sleep. We examined a lethargic infant with an erythematous rash covering the trunk, head and neck who had decreased almost two centiles on her growth chart in the previous several weeks, and although not losing weight, she was nevertheless not gaining weight. Without another obvious etiology, was there an association between the medication and the child’s signs and symptoms?
Upon further investigation, we discovered that advice from the Medicines and Healthcare products Regulatory Agency (MHRA) stated that cold and cough medications should not be given to children under six years of age. MHRA states, “There is no evidence that cough and cold medications work and can cause side effects such as allergic reactions, effects on sleep and hallucinations.” [1] It was noted on the Calpol® website that three of their products (Calcold®, Calcough® and Calpol® Night2) were in this category. They recommend discussing the use of these with the child’s doctor or pharmacist when children are under six years of age. [2] Calcold® contains paracetamol and diphenhydramine and Calpol® Night contains the exact same ingredients at the same concentrations. [2]
Paracetamol (aka acetaminophen) is an analgesic and an anti-pyretic drug, which has been associated with childhood asthma when taken in infancy. [3] Diphenhydramine is a sedative as well as an antihistamine used to treat allergic reactions involving the nasal passages. The website states that no paracetamol product is recommended for a child under three months of age. [2] It was realized that in this case, where the child was four weeks premature that these products had been supplied either at the actual age of 12 weeks or just at the cusp of that age. Side effects of paracetamol are listed as skin rash, blood disorders, swollen pancreas, liver damage and sudden death secondary to a severe overdose. [4] There are no side effects listed for children. It was noted that paracetamol has a narrow therapeutic index, with the therapeutic dose and the toxic dose being very close. In infants under three months the toxic dose is thought to be 10mg/kg of body weight. [5]
The World Health Organisation (WHO) defines an adverse drug reaction (ADR) as “a response to a drug that is noxious and unintended and occurs in doses normally used in adults for prophylaxis, diagnosis or therapy of disease or for modification of physiological function.” [6] ADRs are a major health issue and can range from short term mild effects to more chronic symptoms, and can even be life threatening. [7] Identification and evaluation of ADRs in the pediatric population is of particular importance since they may be more susceptible to toxicity at lower doses (Table 1). [8]
Table 1. Physiological factors which can increase risk for ADRs in Children [8]
Among neonates and children, decreased intestinal motility and delayed gastric emptying can result in a greater lag time between drug administration and plasma concentration compared to adults. There is therefore a potential for increased drug absorption.
The presence of increased gastric irritability in neonatal life such as reflux can result in loss of medication dose.
Children have higher levels of water and extracellular fluid; this will result in increased distribution and dilution of water-soluble drugs.
Reduced protein binding of drugs in neonates can result in higher concentrations of free drugs in the body.
The blood brain barrier is not fully formed in neonates; therefore some medications may have an enhanced effect.
Neonatal livers are not yet fully developed to be able to metabolise a large proportion of drug substrates.
Glomerular filtration and tubular function within the kidney are not as efficient in neonates; therefore drug excretion is decreased.
(Modified from Barnes: paediatrics a guide for nurse practitioners 2003)
The identification, reporting and monitoring of adverse drug reactions (ADRs) are vital in predicting drug safety. The yellow card reporting system used in United Kingdom (UK) hospitals is an essential means of identifying drug reactions. [9]
Reporting of ADRs is complicated by a number of factors. Many children are below speaking age, which provides diagnostic difficulties. [8] Information therefore relies heavily on observation from nurses, physicians and pharmacists. Clinicians have been found to under-estimate adverse reactions in patients. [10] Clinician communication has also been a factor; parent interviews in a recent study demonstrated that clinician's communication about ADRs was poor indicating improvements are needed. [11] Then there is mis-interpretation of correct dosage due to off-label prescribing. The definition of off-labeling differs between Europe and the United States (Table 2). [12] Essentially though, off-label prescribing refers to administration of a drug for a particular indication that has not yet received approval. [12] Many medicinal products currently used to treat the pediatric population have not been studied or authorized for such use. [13] A recent study in Italy showed that a number of respiratory drugs prescribed to children under two years of age were done in an off-label way. [14]
Table 2. Off-label definitions in the USA and Europe [12] Country Definition United States The use of an indication, dosage form, dose regime, population or other parameter not mentioned in the approved labelling.
- As defined by the Food and Drugs Administration (FDA)
- Unapproved use of a licensed drug
Europe The use of medication in children that have been authorized for adults.
- Defined according to directive 2001/83/EC
- Terms are included but ill defined
- Definitions only present for pediatric medication
- Off label medication in children is the use of medicines not authorized for children
(modified from Neubert et al 2009)
Additionally there is a high usage of over-the-counter medication (OTC) use in children. [15] A study conducted in Germany in August 2009 found that over the course of one week in a population of 17,450, 0 to 17 year olds, 17% used OTC medication. [15] A similar study carried out in the United States (US) in August 2009, stated that in a population of 2,857 infants, 56% had used more than one OTC drug in the seven days prior to interview. [16] OTC use is therefore very common (Table 3).
Table 3. Over-The-Counter medication use in children, Germany and USA [15, 16] Country Date Population Size Percentage of Use Age Range (years) United States March 2009 17,450 17 0–17 Germany August 2009 2,857 56 0–1 (modified from Yong et al 2009 and Vernacchio et al 2009)
One major issue is that there are considerable ethical restrictions to conducting drug trials in children. Current European guidelines as quoted by Sammons et al (2007) [17] state that “medical trials cannot be carried out unless the child may benefit directly from the intervention.” Consequently there are a limited number of clinical drug trials involving children. [17]
These restrictions are historically related to major incidents such as the use of sulphonamides in pregnancy causing Kernicterus in the infant, and notably Thalidomide which resulted in congenital defects after use of this medication during the first trimester. [18] Following these tragedies, medicine manufacturers have been required by drug agencies to carry out much more extensive research into the efficacy and safety of their products prior to marketing and distribution. [18] The drug licensing regulatory process was introduced by the Medicines Act 1968, and this was “established to ensure that drugs were safe, effective and of high quality.” [10]
This has been reflected in subsequent legislation. With respect to medicinal products for pediatric use, legislation came into force in January 2007. [18] This was aimed at enhancing the safety of medicine for children through the use of research and development, by authorizing safe medicines based on pediatric experience, without subjecting this population to clinical trials. [17]
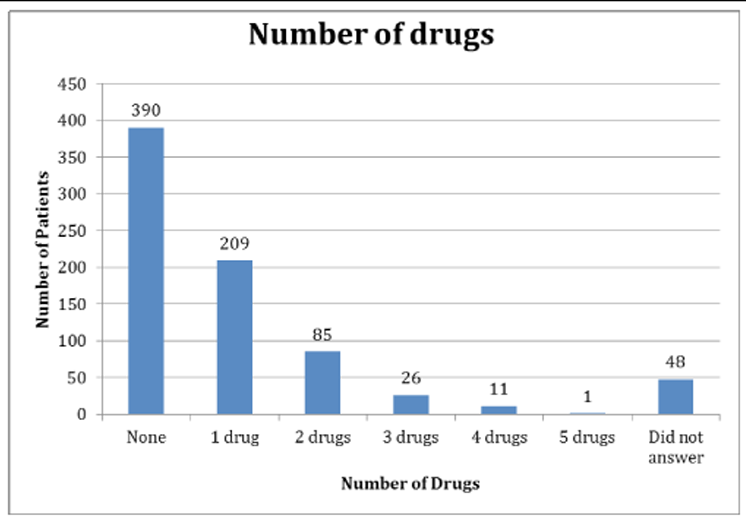
It was indicated to investigate further the use of drugs in children as information from a 2009 survey in a university-affiliated chiropractic teaching clinic (N=770) revealed 45% of crying babies had been treated with medication prior to their presentation to this clinic (Figure 1). [19] Health care providers must therefore be aware of the signs of ADRs and the misinterpretation of dosage via off-label prescribing. In order to determine the prevalence of ADRs in infants and children, a literature search was performed. Medline and PubMed were searched using the following search terms: off-label use, non-prescription drugs, child, pediatrics, prevalence, drug toxicity guidelines. Papers were limited to those published in the English language.
Figure 1. Number of drugs used in AECC patients under one year of age [19]
N=770 (reproduced from Miller et al 2009); AECC = Anglo-European College of Chiropractic
For the purposes of this investigation, research focused on adverse effects from OTC (over-the-counter) medication and off-label prescribing.
Over-the-counter medication effects
Paracetamol and non-steroidal anti-inflammatory drugs (NSAIDS) represent widely used forms of OTC medications within the pediatric population. [20] However a review was recommended in Australia following reports of an increasing number of ADRs related to NSAIDS over the previous five years. [20] Nineteen reports of ADRs to NSAIDS and six to Paracetamol were discovered with age groups ranging from four months of age to 22 years of age. [21] Patients presented with side effects including skin, gastrointestinal and respiratory symptoms. One patient died after acute exacerbation of asthma after taking Rofecoxib, (NSAID which was voluntarily withdrawn by Merck® in 2004, following trials that suggested use of this drug may lead to cardiovascular events, such as heart attack or stroke). [21] Titchen, Cranswick and Beggs (2005) showed that use of NSAIDS could be a significant cause of morbidity in children, and there was therefore an increased need for efficient drug surveillance. [20]
Much of the research regarding pediatric OTCs concerns cough and cold medications. A retrospective review of electronic records submitted to the New Jersey Poison Information and Education system was carried out between 2000 and 2007 by Vassilev. [22] Ninety-one cases demonstrated adverse drug reactions to OTCs in children with the majority of moderate to severe reactions occurring in children two to eleven years of age. This study highlighted the fact that there is no evidence to suggest this type of medication is effective in children under the age of two years. [22] Dart et al (2009) assessed all reported pediatric fatalities from 1983 to 2007 within the US, using a variety of databases. [23] They found that in children below the age of 12 years, a total of 118 deaths could be directly related to ingredients from cough and cold medications. Of these only 82 were due to OTCs in isolation. [23] They also discovered that these fatalities were more prevalent in children under the age of two years. The reason for this is, as highlighted by Fattahi et al (2009) that young children carry certain risk factors for ADRs. [7] These include differences in drug metabolism, which may increase their susceptibility to certain medications, and may mean some organs are more sensitive to side effects than others. [7]
In 2009, cough and cold medications were withdrawn for age groups under six years. [1] In a recent community-wide survey, 60% of a population of 179 parents had used OTC cough and cold medications for their pre-school child. [24] Many of the participants, when asked, indicated using an inappropriate dose. [24] In another 2009 study, all general pediatricians surveyed were aware of the withdrawal of these medications for children under the age of two years and the consideration of withdrawal for children under six years of age; however six per cent of physicians asked stated they would continue to prescribe these products. [24]
A significant problem identified with OTCs is mis-interpretation of use by the general public. [25] Lokker et al (2009) examined the perceptions of caregivers of children aged one year of age and below within three general pediatric outpatient clinics across the US. [25] All medication labels instructed consumers to seek medical advice before administering it to children under the age of two years. When shown these medications, however, 50% of the time child caregivers stated that they would give them to a 13–month-old child with flu-like symptoms. [25]
Mis-use of medications by caregivers was a common occurrence found in a study by Lokker et al (2009) who showed that the most common factors influencing parental decisions were packaging (if the bottle was brightly colored or had pictures of teddy bears for example) and labelling (if the product had infant or pediatric written on the label). [25] Their survey revealed that dosing directions on medication packets only influenced the dosing decisions of child caregivers 47% of the time. [25] This study showed that misunderstandings are common, and labelling and packaging can confuse parents.
Off-label medication events:
This case study related to prescribed medication, and there is a substantial amount of research related to off-label prescription of medication in children. Mcintyre et al (2000) conducted a retrospective study of all prescriptions over a one-year period within a single general practice. [26] They found that out of 3,347 prescriptions, 1,175 were for children. Of these, ten were used in an unlicensed manner and 351 in an off-label way. [26] This study highlighted that the use of off-label medications is widespread.
ADRs associated with off-label prescribing were found to be common in a one year cross sectional observational study carried out in Sweden. [27] From 112 patient reports, 158 ADRs were identified. Of these 158 ADRs, 30% (47.4) were considered serious. All reports concerned outpatients under the age of 16 years. The proportion of off-label drug prescribing amongst these 112 patients was 42%.27 The majority of these were related to inappropriate dosage. This suggests that off-label drugs frequently contribute towards ADRs in children.
In case control studies at a children’s hospital in the Netherlands it was found that out of a total of 138,449 prescriptions, clinicians had intervened on 1577 of them. Most of those interventions (81%) corrected prescriptions that may have resulted in ADRs. [28] This highlights that prescribing errors are a frequent occurrence. [28]
Further, this is a world-wide issue. In another children’s hospital setting, this time in Italy, 486 children were hospitalized for upper gastrointestinal complications; medication use within these cases were higher when compared with a control group. [29] A prospective survey into ADRs was conducted in 2005 by Jonville-Bera and Leca, which suggested a causal link between incorrect dosage and increased number of ADRs. [30] The study took information from the 'Regional Pharmacovigilance Centre (RPC) in Tours, France. [30] Drug use was assessed over a five-month period, and focused on off label medications and medications where inappropriate dosage was used. [30] Within the study, 642 medications were identified, and of those, 26% (167) were used incorrectly. Correctly used drugs appeared to be less likely to cause ADRs compared to incorrectly used drugs with a ratio of 59.45% to 75%, respectively. [30]
Clavenna and Bonati (2009) systematically reviewed 8 prospective studies published between 2001 and December 2007 in order to evaluate ADRs in the pediatric population. [31] They suggested greater regulation of medicinal warning labels was necessary to ensure paediatric safety. [31] The researchers showed that ADRs in children were more common in hospitalised patients compared to those admitted to hospital and this was statistically significant.
Discussion
A significant limitation of much of the research was the comparability of studies. Specifically when searching the literature, some information related to off-labelling and some to the use of OTC medication. It is therefore difficult to establish a causal link. Another limitation was the under-reporting of ADRs. Additionally, there was a significant lack of information relating to OTCs. Few studies were found relating to paracetamol and NSAIDS but much more information was related to cough and cold medications. [22–24] Studies did tend to suggest that there is significant potential for ADRs with OTC use and that increased drug surveillance is needed. [22–25] Research suggests that there is an increased prevalence of minor ADRs in patients under the age of two years, with ADRs of increasing severity in older children up to the age of 11 years. [22–25]
Despite the best efforts of clinicians and researchers, there is a deficit in reporting of ADRs in pediatric patients. Evidence does indicate, however, that off-label prescribing is widespread and the labelling of over-the-counter medications can sometimes be difficult to interpret. It is the lack of clinical trials conducted in the pediatric population, which is a significant obstacle.
Anderson and Holford (2013) highlighted that currently there are fewer pharmacodynamic (PD) studies when compared to pharmacokinetic (PK) studies in respect to ADRs in children. [32] This proves a huge problem for dosing, and whilst regulatory agencies are encouraging more studies to be done, these studies tend to be more PK based and most predict dose based on size difference between adults and children. [32] Anderson and Holford therefore state that these studies are insufficient without the corresponding infant specific PD data. [32]
The main issue, however, is that of ethical considerations. Consent to participate in a clinical trial must be obtained based on reliable and clear information and the individual or legal guardian must have capacity to give that consent. [33] Children over the age of 16 are considered to be legally competent to make such a decision, whereas those under 16 are not. [33] This was echoed by a recent article highlighting the difficulties of striking the balance between ethical demand to protect individual children and the importance of facilitating research. [33] Welzing et al (2007) found that pediatric trials were not included in the current legislation, and meeting requirements of the directive would prove difficult, expensive and unethical. [34] This has meant that the risk/benefit requirement hasn’t been applied to children. Current guidelines within the European Union were revised in 2007 and are based on the growing insight that it is unacceptable that drugs prescribed to children have not been proven to be safe and/or effective. [34] The guidelines state that medications must cause minimal risk, and the risk benefit ratio must be favorable when compared with alternative treatments. [34]
Conclusion
Adverse drug reactions do potentially pose a public health risk within the pediatric population and all healthcare providers need be mindful of this risk, whether they prescribe medications in their practice or not. Chiropractors must therefore be aware of ADRs and spot these symptoms within their patient population. Chiropractors should be aware that ADRs tend to be more prevalent in those under two years of age. Knowledge of ADRs is therefore extremely important in a clinical review of every patient regardless of age. In terms of the infant in the clinic, it was important to recognize the symptoms of ADRs and this aided the management of the case. Based on the information found in the literature and the symptoms of the infant, an ADR seemed very likely in this case. The parents were referred back to the GP regarding the suspicion of an ADR in this case. All medications were stopped and the patient recovered her energy levels and growth and the child was monitored for one month, without further adverse events.
Disclosure statement:
No competing financial interests exist.
REFERENCES:
Press Release: Better Medicines for children’s coughs and colds.
Medicines and Healthcare Products Regulatory Agency 2009 [internet].
Available from:
http://www.mhra.gov.uk/newscentre/pressreleases/CONO38902.New Recommendations on Children’s cough and cold treatments.
Calpol 2010 [internet]. Available from http://www.calpol.co.uk/Beasley S, Clayton T, Crane J, Von Mutius E,
Lai CKW, Montefort S, Stewart A.
Associations between paracetamol use in infancy and childhood, and risk of asthma,
rhinoconjunctivitis, and eczema in children aged 6–7 years:
analysis from phase three of the ISAAC programme.
Lancet 2008;372(9643):1039-1048British National Formulary.
London: British Medical Association 2009.Paracetamol Dosage. Paracetamol Information Centre. 2011 [internet].
Available from
http://www.pharmweb.net/pwmirror/pwy/paracetamol/pharmwebpicdosage.htmlMedicines: safety of medicines – adverse drug reactions.
World Health Organization 2008 [Internet].
Available from http://www.who.int/mediacentre/factsheets/fs293/en/.Fattahi F, Pourpak Z, Moin M, Kasemnejad A,
Khotaei GT, Mamishi S, Siadati A, Tabatabaei P.
Adverse Drug Reactions in Hospitalised Children in a Department of Infectious Diseases.
J Clin Pharmacol 2009;45:1313-1318Barnes K.
Paediatrics: a clinical guide for nurse practitioners.
London: Elseveir science limited 2003. 18-21.Yellow Card Scheme.
Medicines and Healthcare Products Regulatory Agency 2009 [Internet].
Available fromhttp://www.mhra.gov.uk/Safetyinformation/Howwemonitorthesafetyofproducts/
Medicines/TheYellowCardScheme/CON019685Medicine Act 1968.
National Archives. 2011 [internet].
Available from http://www.legislation.gov.uk/ukpga/1968/67.Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohead M, Smyth RL, Turner MA, Young B.
Enhancing communication about paediatric medicines: Lessons from a qualitative study
of parent’s experiences of their child’s suspected adverse drug reaction.
PLOS;7(10): 221-10.Neubert A, Felishi M, Bonifazi, Manfredi C, Wong I.C.K, Ceci A.
Off-Label and unlicensed use of medicines for children.
Pharmaceutical policy and law. 2009; 11:41-49Regulation (EC) n. 1901/2006 of the European Parliament and of the Council of 12
December 2006 on medicinal products for paediatric use and amending Regulation
(EEC) No. 1768/92, Directive 2001/20/EC and Regulation (EC) No 726/2004.
Official Journal of the European Union 27.12.2006, L378/1-L378/19.Baiardi P, Ceci a, Felisi M, Cantarutti L, Girotto S, Sturkenboom M, Baraldi E.
In label and off label use of respiratory drugs in the Italian paediatric population.
Acta Paediatr 2010; 99(4):544-9Yong DU, Knopf H.
Self-medication among children and adolescents in Germany: results of the national
health survey for children and adolescents.
Br J Clin Pharmacol. 2009; 68(4):599-608Vernacchio L, Kelly JP, Kaufman DW, Mitchell AA.
Medication use among children <12 years of age in the United States: Results from the slone survey.
Pediatrics 2009; 124(2):446-454.Sammons HM, Malhotra J, Choonara I, Sitar DS, Matsui D, Rieder MJ.
British and Canadian views on the ethics of paediatric clinical trials.
Eur J Clin Pharmacol 2007; 63:431-436Promoting safety of medicines for children.
World Health Organisation 2009 [Internet].
Available from: http://www.who.int/medicines/publications/essentialmedicines/
Promotion_safe_med_childrens.pdfMiller J, Newell D and Phelps S.
Identifying subgroups of infant patients with excessive crying disorders. WFC 2009.Titchen T, Cranswick N, Beggs S.
Adverse drug reactions to non-steroidal anti-inflammatory drugs, COX-2 inhibitors
and paracetamol in a paediatric hospital.
Br J Clin Pharmacol 2005; 59(6):718-723Press Release: Merck Announces Voluntary Worldwide Withdrawal of VIOXX® (rofecoxib) Sept 2004 [internet].
Available at: http://www.merck.com/newsroom/vioxx/pdf/vioxx_press_release_final.pdfVassilev ZP, Chu AF, Ruck B, Adams EH, Marcuss M.
Adverse drug reactions to over the counter cough and cold products among children:
the cases managed out of hospital.
J Clin Pharma Ther 2009; 34(3):313-318Dart RC, Paul IA, Bond GR, Winston DC, Manoguerra AS, Palmer RB, Kauffman RE, Banner W, Green JL, Rumack BH.
Pediatric fatalities associated with over –the- counter (non-prescription)
cough and cold medications.
Ann Emerg Med 2009; 53(4):411-417Yaghmal BF, Cordts C, Ahlers-schmidt CR, Issa BA, Warren RC.
One community’s perspective on the withdrawal of cough and cold medications
for infants and young children.
Clinical Paediatrics 2010; 49(4):310-315Lokker N, Sanders L, Perrin EM, Kumar D, Finkle J,
Franco V, Choi L, Johnston PE, Rothman RL.
Parental misinterpretations of over-the-counter paediatric cough and cold medication labels.
Pediatrics 2009; 123(6):1464-1471Mcintyre J, Conroy S, Avery A, Corns H, Choonara I.
Unlicensed and off label prescribing of drugs in general practise.
Arch Dis Child 2000;83:498-501Ufer M, Kimland E, Bergman U.
Adverse drug reactions and off-label prescribing for paediatric outpatients:
a one-year survey of spontaneous reports in Sweden.
Pharmaco-epidemilogy and drug safety. 2004;13:147-152Maat B , San YA, Bollen CW, Van Vaught AJ, Egberts TCG, Rademaker CMA.
Clinical pharmacy interventions in paediatric electronic prescriptions.
Arch Dis Child 2013;98:222-227Biancotto M, Chiappini E, Raffaldi I, Gabiano C,
Tovo PA, Sollai S, de Martino M, Manelli, Tipo V, Da Cas R, Traversa G, Menniti-Ippolito F.
Drug use and upper gastrointestinal complications in children: a case-control study.
Arch Dis Child 2013;98:218-221Jonville-Bera AP, Autret Leca E.
Are incorrectly used drug more frequently involved in adverse drug reactions?
A prospective study.
Eur J Clin Pharmacol 2005;61:231-236Clavenna A, Bonati M.
Adverse drug reactions in childhood: a review of prospective studies and safety alerts.
Arch Dis Child 2009;94:724-728Anderson BJ, Holford NHG.
Understanding dosing: children are small adults, neonates are immature children.
Arch Dis Child 2013. 2013;98:737-744Westra AE, Engberts DP, Sukhai RN, Wit JM, de Beaufort ID.
Drug development for children: how adequate is the current EU ethical guidance.
Arch Dis Child 2010;95:3-6Welzing L, Harnischmacher, Weyersberg, Roth B.
Consequences of directive 2001/20/EC for investigator initiated trials
in the paediatric population-a field report.
Eur J Pediatr 2007;166:1169-1176
Return to PEDIATRICS
Return to ADVERSE EVENTS
Since 1-22-2014


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |