

The Origin, and Application of Somatosensory
Evoked Potentials as a Neurophysiological
Technique to Investigate NeuroplasticityThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Can Chiropr Assoc. 2014 (Jun); 58 (2): 170–183 ~ FULL TEXT
Steven R. Passmore, DC, PhD, Bernadette Murphy, DC, PhD, and Timothy D. Lee, PhD
McMaster University
University of Ontario,
Institute of Technology
Somatosensory evoked potentionals (SEPs) can be used to elucidate differences in cortical activity associated with a spinal manipulation (SM) intervention. The purpose of this narrative review is to overview the origin and application of SEPs, a neurophysiological technique to investigate neuroplasticity. Summaries of:
1) parameters for SEP generation and waveform recording;
2) SEP peak nomenclature, interpretation and generators;
3) peaks pertaining to tactile information processing (relevant to both chiropractic and other manual therapies);
4) utilization and application of SEPs;
5) SEPs concurrent with an experimental task and at baseline/control/pretest;
6) SEPs pain studies; and
7) SEPs design (pre/post) and neural reorganization/neuroplasticity; and
8) SEPs and future chiropractic research are all reviewed.Understanding what SEPs are, and their application allows chiropractors, educators, and other manual therapists interested in SM to understand the context, and importance of research findings from SM studies that involve SEPs.
KEYWORDS: chiropractic; manipulation; neuroplasticity; somatosensory evoked potential
From the FULL TEXT Article:
Introduction
Evoking and recording somatosensory evoked potentials (SEPs) is appearing in scientific literature that pertains to spinal manipulation (SM). There is evidence to support that SEPs are a neurophysiological technique capable of elucidating differences in cortical activity associated with an SM intervention. [1, 2] Haavik and Murphy [3] hypothesized that appropriate spinal movement normalizes afferent input and restores sensorimotor function and integration by filtering and processing appropriate somatosensory input. The purpose of this manuscript is to provide an overview of the origin, and application of somatosensory evoked potentials as a neurophysiological technique to investigate neuroplasticity. Neuroplasticity is defined as how one’s central nervous system adapts to their ever-changing environment. Neuroplastic changes can be subjectively positive for the individual (adaptive) such as learning, or they can be subjectively negative (maladaptive) such as pain. [3] Understanding what the SEPs technique is, and how it has been applied will allow chiropractors, manual therapists and educators with an interest in SM to better understand the context, and importance of research findings from SM studies that involve SEPs as an outcome measure.
The most basic form of electrical communication between cells in the human body is the action potential. [4] A neuron, stimulated by other cells or other external stimuli, will reach a point at which an “all or none” burst of electricity is generated, and propagated. Depending on the type of neuron where this propagation is generated, the result will be either inhibitory, or excitatory in nature at the synapse where it terminates.
Excitatory post synaptic potentials facilitate action potential generation at the cells upon which they synapse. Such changes in electrical activity occur as a result of positive and negative ions crossing the cellular membrane. The ion flow results in changing regional polarity, and the resulting voltage changes in the area can be measured to demonstrate activity in the brain.
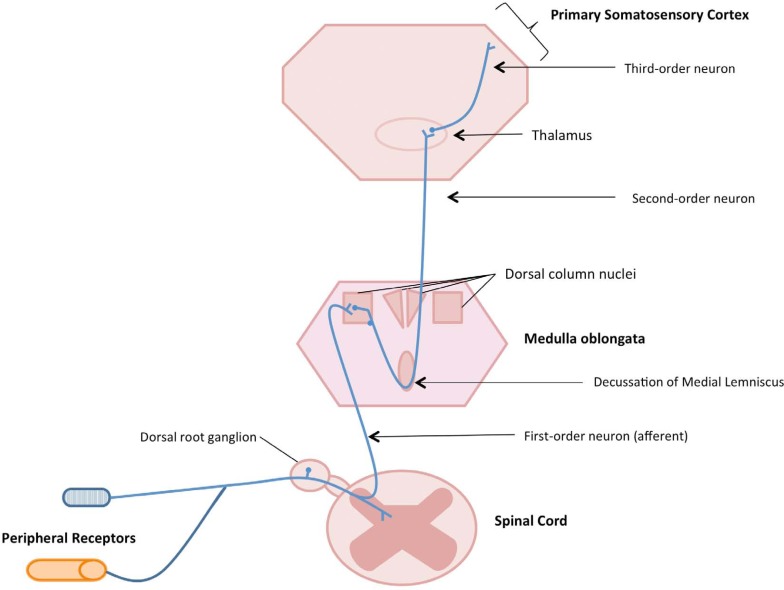
Figure 1 The brain is the site of integration, and perception of all external and internal stimuli, it is the keystone of the central nervous system. The somatosensory system is comprised of elements of the peripheral nervous system and central nervous system that serve the modalities of touch, vibration, temperature, pain and kinesthesia. [5] Neurologically this pathway consists of peripheral receptors and afferent neurons that enter the dorsal root ganglion prior to ascending the spinal cord to the medulla where they synapse with the ipsilateral dorsal column nuclei (Figure 1). Once in the medulla they cross to the contralateral side of the brain (decussate) and the pathway continues to the contralateral ventral posterior lateral nucleus of the thalamus prior arrival at the primary somatosensory cortex for processing. [6] This pathway consists of the dorsal column – medial lemniscal, and thalamo – cortical sensory systems. [7] Knowledge of the anatomical pathway of afferent and subsequently perceptual information can serve as a roadmap to the study of information acquisition and processing.
Origins of Somatosensory Evoked Potentials
An evoked potential occurs when the stimulation of sensory receptors or afferent nerve bundles past their resting threshold results in the generation of a compound action potential. While not mutually exclusive the evocative stimulation can consist of tactile, vibrational, painful or electrical elements. [8] The compound action potential transmitted can be recorded using electrodes to study the post-stimulus characteristics. [9] Potentials evoked by peripheral nerve stimulation can be recorded in the sensorimotor cortex and multiple other sites along the pathway. [10]
A somatosensory evoked potential (SEP) is the electrical activity response measured at the skin’s surface following controlled peripheral nerve stimulation. Electrical activity from peripheral stimulation measured over the scalp reflects cerebral action potentials and are best recorded contralateral to peripheral nerve stimulation. [11] The recorded electrical potential of this afferent volley bombardment generates a complex waveform. [12]
Waveform reproducibility is confirmed by taking the average of several controlled stimuli to waveform generation time-locked trials. The resulting average waveform can then be analysed in terms of the peaks and troughs present at different time points relative to the stimulation. To understand the significance of the waveforms, their components and their neurological interpretation, Giblin [13] observed SEPs in both healthy participants and patients with impairments including lesions of the peripheral nerves, spinal cord, and the brain. He described “early potentials” as those of brief duration that occur within the first 35 msec after stimulation of the median nerve at the wrist. Recorded latency will vary based on the distance from anatomical stimulation site. For example, lower extremity potentials have a slightly longer latency than upper extremity potentials as they have a longer distance to travel. Early potentials were accurately reproducible and Giblin [13] noted the positive and negative voltage changes at particular times in milliseconds.

Table 1 Early SEP studies had substantial variability in many facets of technique application. This variability included, but was not limited to: the stimulus intensity and inter-stimulus interval of the peripheral evoked potentials, the impedance and location of recording electrodes, the number of signals recorded to generate an average waveform, the filtering and amplification of recorded signals, and the measurement and recognition of specific peaks. Acknowledging this heterogeneity of method, but the usefulness of this approach to the study of the nervous system, the International Federation of Clinical Neurophysiology (IFCN) generated a report from a committee of recommended standards for short latency somatosensory evoked potentials. [14] The findings from the report have been used in part to generate suggested SEP stimulating and recording parameters as detailed in the following section of this manuscript. A brief overview comparisson of SEPs and other common neurological recording techniques can be found in Table 1.
Parameters for SEP generation & recording of waveforms
Different from electroencephalography (EEG) which reflects the brain’s spontaneous electrical activity over a short period of time, SEPs are not recorded continuously to spontaneous stimuli but are time locked to a stimulus with a pretrigger. [15] SEP peak amplitudes are traditionally in the under 10?V range (smaller then EEG [tens of ?V], EMG [mV], ECG [V]). [15] The stimulation most favoured is electrical stimulation as it has parameters that are easily manipulated and controlled. [16]
According the updated IFCN guidelines [17] the recommended electrical stimulus should consist of a 0.1–0.2 ms duration square wave pulse. These pulses can be delivered by constant current stimulators applied transcutaneously over the targeted nerve. When stimulating a mixed (motor and sensory fibre containing) nerve, stimulus intensity should exceed the motor threshold for eliciting a muscle twitch. But, the intensity should not be so high as to excite a-delta or c-fibres that are excited by nociceptive input. Gandevia and colleagues [18, 19] have demonstrated that muscle afferents most likely dominate the cerebral potentials produced by stimulation of the mixed median nerve at the wrist. IFCN guidelines recommend that the pulse delivery should repeat at a frequency between 3 and 5 Hz. Stimulation frequencies up to 8 Hz can be used for pulse delivery if the latency of a target peak to be measured occurs before 30 ms. After 30 ms peaks resulting from this higher (8hz) frequency of stimulation are subject to reduction or attenuation which is why a bandwidth of 3–5Hz is preferred. [20] Recently however, Haavik and Murphy [21], have demonstrated that stimulation rate may impact early peaks differentially. Specifically, a rate of 5 Hz enhanced the N24 SEP peak amplitude, while a rate of less than 3 Hz was needed to reliably record the N30 peak. Electrodes for stimulation should be placed over the course of the desired nerve, with the cathode placed 2 cm proximal to the anode. [17]
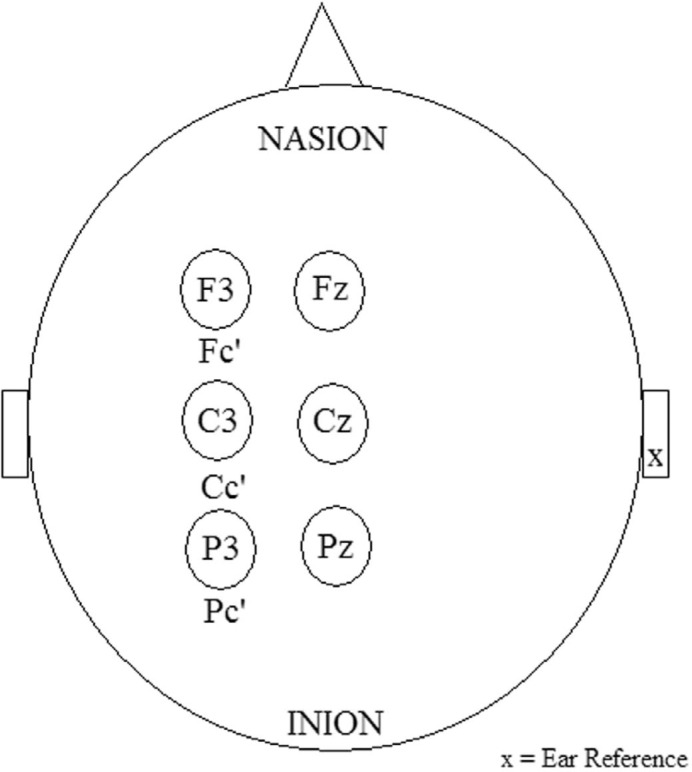
Figure 2 To most effectively, and efficiently record SEPs signals to measure the changing activity in the brain and central nervous system, it is recommended that one centimetre surface recording EEG electrodes should be placed as per the 10–20 international EEG system (Figure 2). [15] The cortical locations that should be used contralateral to the site of stimulation are the Fc’ (contralateral frontal) and Pc (contralateral parietal). [17] Skin at the scalp EEG electrodes should have less than 5 Kohms impedance. The number of waveforms that need to be averaged are from between 500 and 2000 stimuli presentations, in order to clearly differentiate the signal from noise. Updated IFCN guidelines [17] recommend a filtering bandwidth with a high pass of 3 Hz and low pass of over 2000 Hz to isolate reproducible waves from background noise. Scalp electrodes may utilize an earlobe reference [17] and a lip placement for the ground electrode [22]. Adherence to these recommendations will allow the optimal uniform technical recording environment to assess the neurophysiological changes associated with behavioural or perceptual experimental interventions and the resulting information processing.
SEP peak nomenclature, interpretation and generators
Waveform peaks are assigned a letter representing their polarity (positive or negative). By convention an upward wave deflection is a negative polarity (N), while a downward deflection is positive (P), and also assigned an integer based on the post stimulus latency (in ms) at which they appear in a healthy population. [17] Both the latency and the amplitude (uV) of these peaks can be used to interpret changes in neural activity. The amplitudes and latencies of the peaks are thought to represent a combination of the peripheral and central nervous system reception of the external stimulus, and the early processing by a given neural structure of that stimulus. Specifically, amplitude represents the magnitude of the incoming afferent volley. [15] Latency reflects the anatomical location along the somatosensory pathway impacted by the peripheral stimulus. [15]
The waveform is a post-stimulation cortical-electrical potential with predictable and reproducible peak and trough amplitudes and latencies based on recording site. The signals recorded are reflective of their neural generators. [17] The neural generator can be “near-field”, or anatomically close to the electrode (cortex surface), or “far-field”, relatively anatomically distant (subcortical). [6] This means that the near-field potentials represent the direct region of polarity change proximal to the electrode. Far-field potential responses reflect structures with a diffuse signal to a larger area of the surface, they are more likely to be detected at multiple electrode sites. [15]
Early SEP peaks also referred to as “short latency” SEPs are considered to be the most useful for the study of neurological activity as they are the least variable among participants with intact nervous systems free from pathology considered to represent the normal population. [15] Short latency refers to the peaks and troughs present within the first 40 msec following a single stimulation to the upper limb, and less than 50 msec for the lower limb. [23] Peaks of longer latency than 45 ms may be susceptible to cognitive factors, which may further increase their variability. [17]
Identification and meaning associated with specific temporal peaks have been derived from several different methodologies. One methodological example are the techniques used in laboratory obliteration studies which are traditionally performed with animal populations. Severe attenuation or abolishment of all SEPs occurs in primates when the dorsal columns of the upper thoracic, or mid cervical aspects of the spinal cord are ablated. [24] No SEP anomalies occur when there are lesions to other parts of the spinal cord but the dorsal columns are left intact. This finding suggests the dorsal column tracts are essential in the mediation of SEPs. [16] Additionally, SEP peaks have been shown to result mainly from stimulation of large myelinated sensory afferents such as 1a muscle afferents and, possibly, cutaneous afferents. [18, 19] The low intensity of stimulation applied, which is just above motor threshold, means that large myelinated sensory afferents which are also the most rapidly conducting afferents are preferentially excited, and reach the cortex prior to other afferent fibres.
The presence of a specific pathology is another factor that impacts SEP peak amplitude, latency or total absence. Peaks may be delayed or absent in pathology cases with an etiology that is degenerative, traumatic or congenital. [16] Degenerative pathologies such as multiple sclerosis (MS) [25–28], spinal cord tumours affecting the posterior columns [29] amyotrophic lateral sclerosis (ALS), Freidrich’s ataxia [30, 31] and Guillain-Barré Syndrome [32] will alter SEP waveforms. Traumatic or compressive pathologies including focal nerve lesions [33, 34], brachial plexus lesions/nerve root avulsion [35–37], meralgia paresthetica [34], or nervous system lesions from a traumatic brain injury (TBI) [38] or surgery [16] are visible in the presence or absence of SEP components. Congenital pathology such as achondroplasia with associated foramen magnum stenosis will yield an abnormal SEP study. [39] SEPs can even be used to conclusively identify brain death. A peak at N13/N14 with no peaks of further latency indicate that signals are reaching the cervical spine close to the medulla, but with no cerebral activity. [16]
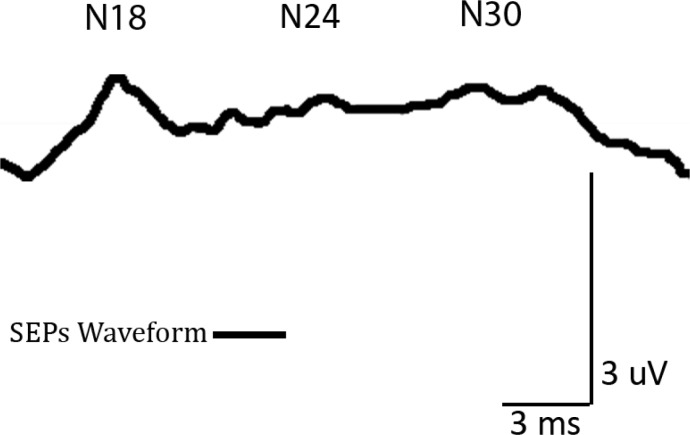
Figure 3 It is possible to identify many SEP peaks, their origin, and significance (Figure 3). For the purpose of brevity, this review will focus on the origin of the P14–N18 complex for N18, N20–P27 complex for N20, P22–N24 complex for N24, and P22–N30 complex for N30. These peaks have different possible implications for the study of tactile information processing (N1840; N2041; N2442; N3043). Tactile information processing is particularly relevant in the study of chiropractic and other manual therapies. Clinicians use their tactile sense to both assess (ex. joint and muscle palpation) and treat patients. Patients receive tactile input (ex. manipulative thrust, soft tissue mobilization) delivered with therapeutic intent from the clinician.
The N18 Peak
The far field, widespread, N18 component is distinct in SEP traces. It has the broadest elevation from baseline following the P13–14 potential. [44] Mauguiere [15] suggested that there are multiple generators of the N18 scalp-recorded potential. Clinical evidence indicates that the N18 component is generated in the brain stem at the level of the midbrain-pontine region. [45] Such brainstem lesions significantly attenuate the N18 amplitude. [45] Noel, Ozaki and Desmedt [46] suggested that the N18 peak originates in the lower medulla nuclei including the accessory inferior olives and dorsal column nuclei. Noel, Ozaki and Desmedt [46] presented three patients whose N18 component remained intact although they had lesions at the medial lemniscus levels including the midbrain and upper medulla. The finding that N18 is related to the dorsal column nuclei is also supported by Manzano, Negrao and Nobrega [47] who found N18 as the only SEP component resistant to tactile cutaneous vibratory changes. Sonoo, Sakuta, Shimpo, Genba, and Mannen [48], and later Sonoo et al. [49] concluded that the cuneate nucleus was likely responsible for the N18 potential based on several observed cases of patients with deep sensation disturbance and high cervical brain stem, thalamic, and pontine lesions. A review by Sonoo [50] expanded on the mechanism for the N18 peak. He concluded it is likely generated in the cuneate nuclei through primary afferent depolarization. Specifically, by collaterals from dorsal column afferents to cuneate nuclei interneurons that synapse on dorsal column fibers’ pre-synaptic terminals that become depolarized and function as presynaptic inhibition. [50]
The N20 Peak
The primary somatosensory cortex lies in the posterior bank of the rolandic fissure representing Brodmann’s area 3b in the parietal lobe. This is the site of N20 peak generation. [51] It is known to respond to contralateral tactile stimuli. [52] The parietal N20 peak is consistent and occurs contralateral to the site of stimulation. [15] Brodmann’s area 3b (the primary somatosensory cortex) responds to cutaneous inputs, but not joint movement input. Desmedt and Osaki [53] confirmed this N20 cutaneous response, and not joint movement, in a study on passive finger movement. In healthy normal participants the N20 peak is the earliest cortical processing in the primary somatosensory cortex.
The N24 peak
The origin of peak N24 is located close to the location of N20. N24 is a frontal lobe negativity that appears on the ascending slope of peak N30. Garcia Larrea, Bastuji and Mauguiere [54] found that N24 is best revealed at higher stimulus rates (greater than 3 Hz) that selectively decrease the N30 peak. As discussed previously in this review, Haavik and Murphy [21] have recently shown that 5 Hz stimulation is sufficient to enhance the N24 recording while ensuring detection of changes subsequent to a motor training task. Due to its mild variability in latency the N24 peak has also been referred to as N2355, or N2543. Waberski et al. [43] used source localization to identify to the posterior wall of the central sulcus in area 3b of the somatosensory cortex as the site of N24 generation. In order for this pathway to continue the input sent to the somatosensory cortex travels through the cerebellar cortex and deep cerebellar nuclei. [56] The N24 amplitude is enhanced if the cerebellar cortex is disrupted. N24 is reduced or absent, but all preceding peaks are left intact if the cerebellar cortex and deep cerebellar nuclei are lesioned. [42] The characteristics of N24 are linked directly to the integrity of the cerebellum through its cortex and its deep nuclei. In summary, when a deep structure is lesioned the peak is obliterated, while if only the cortex is disrupted the peak is enhanced. The aforementioned findings provide evidence of the possibility that the deep structures generate increased activity in an attempt to relay signals to the cortex in the event that the cortex is damaged and fails to appropriately received signals.
The N30 Peak
The N30 frontal lobe peak reflects sensory integration. [57] This peak is negatively impacted by imagined or actual voluntary muscle contraction. Cheron and Borenstein [55] demonstrated that both imagined and actual finger movements attenuated the N30 peak. As a result this peak is believed to reflect complex cortical and subcortical loops that link the basal ganglia, thalamus, pre-motor areas, and primary motor cortex. [58–61] Parkinson’s disease (PD) is known to degrade components of the basal ganglia, including but not limited to the internal globus pallidus, and the subthalamic nucleus. A PD patient population has demonstrated a decreased N30 peak compared to a control population. [62, 63] Muscle tone rigidity decreases and N30 amplitude increases in PD patients when the neuromuscular junction is blocked. [63] Basal ganglia deep brain stimulation also produces increased N30 amplitude, which is attributed to improved supplementary motor area (SMA) activity. [62] Basal ganglia efferents are anatomically found to terminate in the ventrolateral thalamus, from where they project to the SMA. [64, 65] Waberski et al. [43] employed a mathematical technique known as “source localisation” to suggest that primary motor cortex or more specifically the pre-central motor cortex is the N30 peak generator. Primate [66, 67], and subsequently human [68] intracortical recordings support that N30 is generated at the motor cortex.
The neural generators of the N30 SEP peak have recently been explored using novel technology. Cebolla, Palmero-Soler, and Cheron [69] used swLORETA (standardized weighted Low Resolution Brain Electromagnetic Tomography) and determined that the N30 is generated by network activity in the motor, premotor and prefrontal cortex. This finding sheds light on the role N30 plays as a marker of neural processing relevant to sensorimotor integration. The role of the prefrontal cortex is a finding of particular interest since it is a site of executive function including cognitive planning and decision-making. The prefrontal cortex receives somatosensory input and other internal and external sources of information that can be used to inform decision-making. Clinicians who deliver manual therapies use their tactile sense via palpation of muscles and joints to make clinical decisions.
Utilization and Application of SEPs
Clinical
SEP recording is an objective technique and is often more sensitive than the traditional neurological component of physical examination. [7] For example, SEPs can be used in comatose, anesthetised patients. [7] Interpretation of the presence and absence of specific waveforms can be utilized to predict comatose patient prognosis. When SEPs are recorded within 72 hours of entering the comatose state prediction of prognosis is >99% accurate. [70]
Based on the reliability of SEP peaks, it is increasingly accepted for use in the operating room. Operating room monitoring of SEP peaks is done to correct spinal cord ischaemia, prior to it becoming a debilitating issue. SEPs are used in repetition to continuously monitor for detection of neurological impairment during scoliosis surgery. This technique has resulted in a 50–60% decrease in paraplegia post surgery. [71]
Surface recording electrodes, while relatively non invasive, cause the spatial accuracy of SEP recording to be decreased compared to other direct neuromeasurement techniques. SEPs are regarded as having high temporal and low spatial resolution. [72] The meaningfulness of the interpretation of SEP waveforms is established enough that is has been used as a pre-screening tool for inclusion or exclusion of participants in scientific research. SEPs were collected prior to selection for experimental inclusion in a traumatic brain injury (TBI) study by Sarno, Erasmu, Lipp and Schlaegel. [38] This technique allowed the reduction and refinement of a pool of participants for a reaction time study. Understanding limitations and performance of a TBI population can otherwise be problematic to test due to the possible heterogeneity of symptoms. Examination of the quality of the N20 peak allowed the exclusion of participants with severe sensory impairment, thus yielding an objective test to produce a more homogenous experimental group. SEPs may be used as a neurophysiological outcome measure when behavioural findings are absent (clinically silent). [16] Whether or not SEPs also have the potential to reveal clinically silent musculoskeletal lesions is an area that requires further research.
SEPs Concurrent with an Experimental Task and at Baseline/Control/Pretest
Buchner et al. [41] measured immediate cortical plasticity related to attention and anesthesia. They first elicited SEPs at base line, then again concurrent with conditions of directed attention. They found that an immediate cortical reorganization occurs at peak N20 when partial deafferentation was present. They used an electrical stimulation attention task on fingers 1, 3 and 5. Temporary deafferentation was achieved via injection of 1.5–2 ml of a 2% Meaverin solution to digits 2–4. They found that when participants were anesthetized directed attention to the dorsal hand increased the accessibility of neighboring cortical areas. Waberski, Gobbele, and Buchner [73] found similar results before and during air puff stimulation of the anesthetised thumb. Cortical representation of the thumb decreased in the presence of anesthesia compared to a preanesthetic condition. They interpreted this finding to indicate that anesthesia yields an immediate cortical reorganization of the representation of the affected and adjacent digits. From a clinical perspective even an acute peripheral injury or sensory perturbation may cause immediate cortical reorganization measurable using SEPs.
Psychophysical literature that pertains to tactile stimulation raises concerns regarding the generation and recording of SEPs concurrent with perception or performance related to another task. It is possible that concurrent SEPs stimulation could negatively impact accurate performance when responding to multiple tactile stimuli, or distractors, leading to unintended masking or enhancement. For example Giblin [13] determined that SEP peaks are attenuated or masked in the presence of additional tactile stimulation meant to be irrelevant to SEPs technique recording. The phenomenon is now known as “sensory gating”. Morita, Petersen, and Nielsen [74], cautioned that SEPs gating can occur with concurrent motor activation in the lower extremity.
When designing a movement study with concurrent SEPs recording, experimentors need to be aware that factors leading to gating can result in the decreased amplitude of an expected waveform signal. For example, in as few as 60 ms post contraction tibial nerve SEPs would become attenuated when either foot was plantar or dorsi flexed concurrent with SEPs recording. In as few as 60 ms post contraction tibial nerve SEPs would become attenuated when either foot was plantar or dorsi flexed concurrent with SEPs recording. [74] If such factors are not controlled for the misinterpretation of results is possible.
SEPs Pain Studies
Tinazzi et al. [75] explored the impact of tactile sensory disruption using a passive tactile stimulus (no other cognitive, perceptual or motor intervention), in a within-participant SEPs study. Spinal (N14) and subcortical (N18) peaks remained unchanged. The parietal lobe N20 and frontal lobe N30 cortical SEP amplitudes were increased during anesthetic block of the ipsilateral ulnar nerve. This anesthesia, which the authors termed “transient deafferentation” was induced via injection of a 2% lidocaine solution. The amplitudes differed significantly during anesthesia compared to baseline, and following when anesthesia was worn off. The authors interpreted that increased peak amplitudes reflected increased activity that may be intracortical in origin, specifically in subareas of the somatosensory cortex. Clinicians need to be aware that peripheral changes in sensation, may lead to amplified changes in central (cortical) activity.
Unilateral radicular pain from the C–6 nerve root level demonstrates SEP amplitude differences compared to both the unimpaired side and to healthy controls. [76] Ten participants with a cervical disc protrusion compressing the C–6 nerve root, and ten healthy age matched controls were recruited. SEPs were recorded in a between-limb, and between-participants design. Amplitudes of peaks N13, P14, N20, P27, N30 were all significantly amplified in the limb with the presence of pain. This suggests that peak enhancement can reflect a positive correlation between the presence of pain and SEP amplitude. Tinazzi et al. [75] concluded that SEPs might be a sensitive neuro-physiological tool to investigate physiopathological changes in humans before the appearance of hard neurological (absent reflex, or motor impairment) symptoms. The same experimental design was used earlier to examine a population with EMG evidence of chronic unilateral carpal tunnel syndrome (Tinazzi et al., 1998). [76] Identical to the radiculopathy study, peaks N13, P14, N20, P27 were all increased in amplitude when generated from the pathological limb compared to the healthy limb, and to an asymptomatic healthy age-matched control group. While all pain and function loss in patient participants impacted the median nerve, ulnar nerve stimulation was used to generate and record the SEPs. Based on their finding Tinazzi et al. concluded that changes associated with chronic pain detected by peripheral nerves may cause plastic changes that can be detected in the brainstem prior to reaching the cortex. Limitations to both studies are the inability to completely homogenize the onset, duration, and intensity of the symptoms in the pain-participant population. Future research is needed to explore the possible neuro-physiological quantification of unilateral pain. Studies on clinical interventions that decrease self-reported musculoskeletal pain could utilize a pre- post-intervention SEPs design with the predication that peaks will attenuate as pain decreases.
The issue of standardizing pain delivered to participants has been overcome using an experimentally-induced pain model. [77] Rossi et al. [57] built on their foundation to understand how their induced perturbation impacted behavioural, specifically motoric and imagined movement findings in a subsequent study. The induced tonic hand pain using a Levo-Ascorbic solution injection in the first dorsal interosseous muscle. They found that the N18 SEP peak was significantly increased when the pain was present. There was a significant decrease in N30 amplitude when asked to imagine finger movement during the pain condition. The attenuation of N30 was even more pronounced during actual motor recruitment. The strength of this study is the consideration of neurophysiological measurement, and behavioural or imagined movement. A weakness is that no behavioural outcome measures were recorded to quantitatively assess motor task performance.
SEPs Design (Pre and Post) and Neural Reorganization/Neuroplasticity
SEPs when recorded at baseline and compared to SEPs recorded following a separate perceptual, sensory or motor task reflect the neuroplasticity associated with a perceptual [78] or motor task [79]. A pre-test and post-test experimental design can be used to avoid inadvertently masking the tactile system while utilizing the SEPs technique. Pellicciari, Miniussi, Rossini and De Gennaro [78] compared SEP recordings in the elderly and in a young population, pre- and post-exposure to paired-associative stimuli. In their study paired-associative stimuli were the combination of median nerve electrical stimulation, and 20 ms later transcranial magnetic stimulation (TMS) of the S1 region. The 20 ms time delay reflects the time needed for the afferent signal from the Median nerve to arrive at S1. Essentially it is the reason for the N20 latency SEPs peak. The limitation of TMS is that it is not focal to a single structure and is a gross activation or inhibition. While neuroplasticity may take place in both populations with learning, the patterns and underlying structures reflecting plastic changes may differ. This suggests possible compensatory changes to accommodate the abilities of the elderly population. Murphy, Haavik-Taylor, Wilson, Oliphant, and Mathers [79] used pre- and post-task SEPs as a neurophysiological measure for plasticity related to motor output. In a within-participants design 10 individuals had SEPs recorded at baseline, then immediately after a 20–minute repetitive-typing task. Attenuation of the N13 peak, N14–18 complex, and N30–P40 complex all occurred immediately following the typing task. Had Murphy et al. [79] attempted to concurrently record SEPs while performing the typing task, the stimulus intended to be used to stimulate the somatosensory system may have served as an attentional, cognitive, or peripheral perturbation to motor performance that could have masked changes in the targeted SEP peaks. To ensure accurate interpretation, appropriate control groups are an asset to pre- and post-task designs.
Haavik-Taylor and Murphy [1] used a pre- and post-SEPs design to consider plasticity associated with the clinical intervention of spinal manipulation. Prior to the intervention, in a between-participant design, 24 individuals were pseudorandomized to receive either manipulation, or passive head motion. Only the spinal manipulation group yielded a significant attenuation of peaks N20 and N30, for about 20 minutes post-manipulation. This plasticity effect provides evidence for altered cortical somatosensory processing and sensorimotor integration following spinal manipulation. The authors concluded that their findings may aid in the further study of the understanding of mechanisms for functional restoration and pain relief following spinal manipulation. An understanding at the mechanistic level, would aid clinicians in communicating the clinical significance of their intervention to patients, and colleagues from other healthcare disciplines.
A more recent somatosensory evoked potential (SEP) study investigated patients with a history of reoccurring neck pain or stiffness. SEPs were elicited via 3 methods. First from the median nerve and second from the ulnar nerve. The third method included simultaneous median and ulnar nerve stimulation. The ratios of the individual sum were compared to the dual simulatanous SEPs. [80] In a pre- post-task design participants had baseline SEPs recordings, performed a thumb tapping task on a single key for 20–minutes at a rate of 180 strikes per minute, then had post-task recordings. There was a significant increase in the dual SEP ratio for the N20–P25 complex, and the P22–N30 SEP cortical SEP components after a 20–minute motor task. However this increase did not occur when the motor training task was preceded with spinal manipulation. Spinal manipulation prior to the motor training task actually caused a significant decrease in the dual SEP ratio for the P22–N30 SEP component, most likely due to changes in the ability to appropriately filter somatosensory information at the cortical level.
SEPs and Future Chiropractic Research
The future usefulness of SEPs for the chiropractor or other manual therapists can be viewed from 2 distinct vantage points. First, SEPs can by used to measure if changes are present in the patient pre- compared to post-intervention. Hypothetically, a patient with concussive symptoms of mechanical origin may demonstrate central changes associated with a course of chiropractic intervention. A patient with a peripheral nerve entrapment, may yield changes in peripheral, central, or a combination of regions following a course of care compared to baseline. There is precedent for using a pre- post-intervention SEPs design with a clinical population. For example as mentioned in the previous section Haavik Taylor and Murphy recorded SEPs on a population with neck pain [80], they have also previously studied SEPs in patients with reoccuring neck stiffness [1], and pain-free people with a history of cervical spine issues [2] pre- and post-spinal manipulation. In a recent review regarding their work related to SEPs and spinal manipulation, Haavik and Murphy hypothesize that spinal manipulation leads to appropriate joint movement, which in turn yields normal afferent input allowing for appropriate somatosensory processing and integration to occur. [3] Second, SEPs could in the future also be used to measure if there are changes in the clinician, either:a) with learning the motor skill of spinal manipulation delivery; or
b) if the clinician suffers an injury or pathology but is still trying to deliver manual therapies.When measuring changes in the clinician it would be most useful to use SEPs in tandem with a behavioural performance measure (reaction or movement time, and with kinetic or kinematic data) in order to determine if there is a correlation between behavioural and neurophysiological measures. The addition of behavioural measures allows for the intrepretation of not just the neurological regions impacted by clinical intervention, but also the functional performance differences that are possible.
Conclusion
Somatosensory evoked potential recording has been established as a meaningful neurophysiological measurement technique in both clinical and research contexts. Specific parameters for eliciting and measuring SEPs have been created as recommendations for uniform testing conditions. Obliteration and pathology studies have allowed understanding of the significance and origin of several peaks. Changes in activity resulting in peak latency and amplitude modulation allow the visualization and quantification of precognitive neural plasticity associated with perceptual, cognitive, and motor tasks or phenomena. SEPs have also been used to show changes with both transient and chronic pain, and changes following spinal manipulation. Future studies should extend the work on altered sensory input, including pain, joint dysfunction, paresthesia, as well as their interaction with motor training and sensory perception.
REFERENCES:
Haavik-Taylor H, Murphy B.
Cervical Spine Manipulation Alters Sensorimotor Integration:
A Somatosensory Evoked Potential Study
Clin Neurophysiol. 2007 (Feb); 118 (2): 391–402Haavik-Taylor H, Murphy B.
Altered sensorimotor integration with cervical spine manipulation.
JMPT. 2008;31:115–126Haavik, H and Murphy, B.
The Role of Spinal Manipulation in Addressing Disordered Sensorimotor Integration and
Altered Motor Control
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 768–776Brodal P.
The central nervous system: structure and function.
New York: Oxford University Press; 1998.Arezzo JC, Schaumburg HH, Spencer PS.
Structure and function of the somatosensory system: a neurotoxicological perspective.
EHP. 1982;44:23–30Leeman SA.
SSEPs: from limb to cortex.
Am J Electroneurodiagnostic Technol. 2007;47:165–177Walsh P, Kane N, Butler S.
The clinical role of evoked potentials.
JNNP. 2005;76(Supplementary II):ii16–ii22Lew HL, Lee EH, Pan SSL, Chiang JYP.
Electrophysiologic assessment techniques: evoked potentials and electroencephalography.
In: Zasler ND, Katz DI, Zafonte RD, editors.
Brain injury medicine: principles and practice.
New York, NY: Demos Publishing; 2007. pp. 157–166.Heinbecker P, Bishop GH, O’Leary J.
Analysis of sensation in terms of the nerve impulse.
Arch Neurol Psychiatry. 1934;31:34–53.Bartley SH, Heinbecker P.
The response of the somatosensory cortex to stimulation of a peripheral nerve.
Am J Physiol. 1937;121:21–31.Dawson GD.
Cerebral responses to electrical stimulation of peripheral nerve in man.
JNNP. 1947;10:134–140Eccles JC.
Interpretation of action potentials evoked in the cerebral cortex.
Electroencephalogr Clin Neurophysiol. 1951;3:449–464Giblin DR.
Somatosensory evoked potentials in healthy subjects and in patients with lesions of the nervous system.
Annals New York Academy of Sciences. 1964;112:93–142Nuwer MR, Aminoff M, Desmedt J, Eisen AA, Goodin D,
Matsuoka S, Mauguiere F, Shibasaki H, Sutherling W, Vibert JF.
IFCN recommended standards for short latency somatosensory evoked potentials: report of an IFCN committee – International Federation of Clinical Neurophysiology.
Electroencephalogr Clin Neurophysiol. 1994;91:6–11Mauguiere F.
Somatosensory evoked potentials: normal responses, abnormal waveforms, and clinical applications in neurological diseases.
In: Niedermeyer E, Lopes da Silva FH, editors.
Electroencephalography: basis principles, clinical applications, and related fields.
Philidelphia: Lippincott, Williams & Walkin; 2005. p. 1067.Aminoff MJ, Eisen AA.
AAEM minimonograph 19: somatosensory evoked potentials.
Muscle Nerve. 1998;21:277–290Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F,
Rossini PM, Treede RD, Garcia-Larrea L.
Recommendations for the clinical use of somatosensory-evoked potentials.
Clin Neurophysiol. 2008;119:1705–1719Gandevia SC, Burke D.
Projection to the cerebral cortex from proximal and distal muscles in the human limb.
Brain. 1988;111:389–403Gandevia SC, Burke D, McKeon B.
The projection of muscle afferents from the hand to cerebral cortex in man.
Brain. 1984;107:1–13Yamada T, Yeh M, Kimura J.
Fundamental principles of somatosensory evoked potentials.
Phys Med Rehabil Clin N Am. 2004;15:19–42Haavik H, Murphy B.
Selective changes in cerebellar-cortical processing following motor training.
Exp Brain Res. 2013;231:397–403Turker KS, Miles TS, Le HT.
The lip-clip: a simple, low-impedance ground electrode for use in human electrophysiology.
Brain Res Bull. 1988;21:139–141Allison T, McCarthy G, Wood CC, Jones SJ.
Potentials evoked in human and monkey cerebral cortex by stimulations of the median nerve: a review of scalp and intracranial recordings.
Brain. 1991;114:2465–2503Cusick JF, Myklebust JB, Larson SJ, Sances A.
Spinal cord evaluation by cortical evoked potentials.
Arch Neurol. 1979;36:140–143Ganes T.
Somatosensory evoked responses and central afferent conduction times in patients with multiple sclerosis.
JNNP. 1980;43:948–953Mastaglia FL, Black JL, Collins DWK.
Visual and spinal evoked potentials in diagnosis of multiple sclerosis.
Br Med J. 1976;2:732Trojaborg W, Petersen E.
Visual and somatosensory evoked cortical potentials in multiple sclerosis.
JNNP. 1979;42:323–330Small DG, Beauchamp M, Matthews WB.
Spinal evoked potentials in multiple sclerosis.
Electroencephalogr Clin Neurophysiol. 1977;42:141.Linden D, Berlit P.
Spinal arteriovenous malformations: clinical and neurophysiological findings.
J Neurol. 1996;243:9–12Mastaglia FL, Black JL, Edis R, Collins DWK.
The contribution of evoked potentials in the functional assessment of the somatosensory pathway.
In: Tyrer JH, Eadie MJ, editors.
Clinical and Experimental Neurology:
Proceedings of the Australasian Association of Neurologists.
Sydney: Adis Press; 1978. p. 279Jones SJ, Baraitser M, Halliday AM.
Peripheral and central somatosensory nerve conduction defects in Friedreich’s ataxia.
JNNP. 1980;43:495–503Brown WF, Feasby TE.
Sensory evoked potentials in Guillain-Barré polyneuropathy.
JNNP. 1984;47:288–291Desmedt JE.
Somatosensory cerebral evoked potentials in man.
In: Cobb WA, editor. Handbook of Electroencephalography and Clinical Neurophysiology.
Amsterdam: Elsevier; 1971. p. 55.Synek VM.
Assessing sensory involvement in lower limb nerve lesions using somatosensory evoked potential techniques.
Muscle Nerve. 1985;8:511–515Synek VM, Cowan JC.
Somatosensory evoked potentials in patients with supraclavicular brachial plexus injuries.
Neurology. 1982;32:1347–1352Synek V.
Somatosensory evoked potentials from musculocutaneous nerve in the diagnosis of brachial plexus injuries.
JNS. 1983;61:443–452Jones SJ, Wynn Parry CB, Landi A.
Diagnosis of brachial plexus traction lesions by sensory nerve action potentials and somatosensory evoked potentials.
Injury. 1981;12:376–382Sarno S, Erasmu LP, Lipp B, Schlaegel W.
Multisensory integration after traumatic brain injury: a reaction time study between pairings of vision, touch and audition.
Brain Inj. 2003;17:413–426Nelson FW, Goldie WD, Hecht JT, Butler IJ, Scott CI.
Short latency somatosensory evoked potentials in the management of patients with achondroplasia.
Neurology. 1984;34:1053–1058Jones SJ.
An “interference” approach to the study of somatosensory evoked potentials in man.
Electroencephalogr Clin Neurophysiol. 1981;52:517–530Buchner H, Reinartz U, Waberski TD, Gobbele R, Noppeney U, Scherg M.
Sustained attention modulates the immediate effect of de-afferentation on the cortical representation of the digits: source localization of somatosensory evoked pontentials in humans.
Neurosci Lett. 1999;260:57–60Restuccia D, Valeriani M, Barba C, Le Pera D,
Capecci M, Filippini V, Molinari M.
Functional changes of the primary cerebral cortex in humans.
Brain. 2001;124:757–768Waberski TD, Buchner H, Perkuhn M, Gobbele R,
Wagner M, Kucker W, Silny J.
N30 and the effect of explorative finger movements: a model of the contribution of the motor cortex to early somatosensory potentials.
Clin Neurophysiol. 1999;110:1589–1600Desmedt JE, Cheron G.
Non-cephalic reference recording of early somatosensory potentials to finger stimulation in adult or aging normal man: differentiation of widespread N18 and contralateral N20 from the prerolandic P22 and N30 components.
Electroencephalogr Clin Neurophysiol. 1981;52:553–570Urasaki E, Wada S, Kadoya C, Tokimura T, Yokota A,
Yamamoto S, Fukumura A, Hamada S.
Amplitude abnormalities in the scalp far-field N18 of SSEPs to median nerve stimulation in patients with midbrainpontine lesions.
Electroencephalogr Clin Neurophysiol. 1992;84:232–242Noel P, Ozaki I, Desmedt JE.
Origin of N18 and P14 far-fields of median nerve somatosensory evoked potentials studied in patients with a brain-stem lesion.
Electroencephalogr Clin Neurophysiol. 1996;98:167–170Manzano GM, Negrao N, Nobrega JAM.
The N18 component of the median nerve SEP is not reduced by vibration.
Electroencephalogr Clin Neurophysiol. 1998;108:440–445Sonoo M, Sakuta M, Shimpo T, Genba K, Mannen T.
Widespread N18 in median nerve SEP is preserved in a pontine lesion.
Electroencephalogr Clin Neurophysiol. 1991;80:238–240Sonoo M, Genba K, Zai W, Iwata M, Mannen T, Kanazawa I.
Origin of the widespread N18 in median nerve SEP.
Electroencephalogr Clin Neurophysiol. 1992;84:418–425Sonoo M.
Anatomic origin and clinical application of the widespread N18 potential in median nerve somatosensory evoked potentials.
J Clin Neurophysiol. 2000;17:258–268Desmedt JE, Cheron G.
Central somatosensory conduction in man: neural generators and interpeak latencies of the far-field components recorded from neck and right or left scalp and earlobes.
Electroencephalogr Clin Neurophysiol. 1980;50:382–403Hlushchuk Y, Hari R.
Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation.
J Neurosci. 2006;26:5819–5824Desmedt JE, Osaki I.
SEPs to finger joint input lack the N20-P20 response that is evoked by tactile inputs: contrast between cortical generators in areas 3b and 2 in humans.
Electroencephalogr Clin Neurophysiol. 1991;80:513–521Garcia-Larrea L, Bastuji H, Mauguiere F.
Unmasking of cortical SEP components by changes in stimulus rate: a topographic study.
Electroencephalogr Clin Neurophysiol. 1992;84:71–83Cheron G, Borenstein S.
Gating of the early components of the frontal and parietal somatosensory evoked potentials in different sensory-motor interference modalities.
Electroencephalogr Clin Neurophysiol. 1991;80:522–530Molinari M, Restuccia D, Leggio MG.
State estimation, response prediction, and cerebellar sensory processing for behavioural control.
Cerebellum. 2009;8:399–402Rossi S, della Volpe R, Ginanneschi F, Ulivelli M,
Bartalini S, Spidalieri R, Rossi A.
Early somatosensory processing during tonic muscle pain in humans: relation to loss of proprioception and motor ‘defensive’ strategies.
Clin Neurophysiol. 2003;114:1351–1358Kanovský P, Bare M, Rektor I.
The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: evidence from intracerebral recordings.
Clin Neurophysiol. 2003;114:981–991Mauguiere F, Desmedt JE, Courjon J.
Astereognosis and dissociated loss of frontal or parietal components of somatosensory evoked potentials in hemispheric lesions: detailed correlations with clinical signs and computerized tomographic scanning.
Brain. 1983;106:271–311Rossini PM, Babiloni F, Bernardi G, Cecchi L,
Johnson PB, Malentacca A, Stanzione P, Urbano A.
Abnormalities of short-latency somatosensory evoked potentials in parkinsonian patients.
Electroencephalogr Clin Neurophysiol. 1989;74:277–289Rossini PM, Gigli GL, Marciani MG, Zarola F, Caramia M.
Non-invasive evaluation of input-output characteristics of sensorimotor cerebral areas in healthy humans.
Electroencephalogr Clin Neurophysiol. 1987;68:88–100Pierantozzi M, Mazzone P, Bassi A, Rossini PM,
Peppe A, Altibrandi MG, Stefani A, Bernardi G, Stanzione P.
The effect of deep brain stimulation on the frontal N30 component of somatosensory evoked potentials in advanced Parkinson’s disease patients.
Clin Neurophysiol. 1999;110:1700–1707Pierantozzi M, Sabato AF, Leonardis F, Marciani MG,
Cicardi C, Giacomini P, Bernardi G, Stanzione P.
Curariform peripheral block of muscular tone selectively increases precentral N30 somatosensory evoked potentials component. A pharmacological study carried out on healthy subjects and parkinsonian syndromes.
Exp Brain Res. 2000;133:368–376Schell GR, Strick PL.
The origin of thalamic inputs to the arcuate premotor and supplementary motor areas.
J Neurosci. 1984;4:539–560Wiesendanger R, Wiesendanger M.
The thalamic connections with medial area 6 (supplementary motor cortex) in the monkey (macaca fascicularis)
Exp Brain Res. 1985;59:91–104Strick PL, Preston JB.
Two representations of the hand in area 4 of a primate II: somatosensory input organization.
J Neurophysiol. 1982;48:150–159Tanji J, Wise SP.
Submodality distribution in sensorimotor cortex of the unanesthetized monkey.
J Neurophysiol. 1981;45:467–481Balzamo E, Marquis P, Chauvel P, Regis J.
Short-latency components of evoked potentials to median nerve stimulation recorded by intracerebral electrodes in the human pre- and postcentral areas.
Clin Neurophysiol. 2004;115:1616–1623Cebolla AM, Palmero-Soler E, Cheron DB.
Frontal phasic and oscillatory generators of the N30 somatosensory evoked potential.
NeuroImage. 2011;54:1297–1306Wang JT, Young GB, Connolly JF.
Prognostic value of evoked responses and event-related brain potentials in coma.
Can J Neurol Sci. 2004;31:438–450Nuwer MR, Dawson EG, Carlson LG, Kanim L, Sherman JE.
Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey.
Electroencephalogr Clin Neurophysiol. 1995;96:6–11Legon W, Staines WR.
Predictability of the target stimulus for sensory-guided movement modulates early somatosensory cortical potentials.
Clin Neurophysiol. 2006;11:1345–1353Waberski TD, Gobbele R, Kawohl W, Cordes C, Buchner H.
Immediate cortical reorganization after local anesthetic block of the thumb: source localization of somatosensory evoked potentials in human subjects.
Neurosci Lett. 2003;347:151–154Morita H, Petersen N, Nielsen J.
Gating of somatosensory evoked potentials during voluntary movement of the lower limb in man.
Exp Brain Res. 1998;120:143–152Tinazzi M, Fiaschi A, Rosso T, Faccioli F, Grosslercher J, Aglioti SM.
Neuroplastic changes related to pain occur at multiple levels of the human somatosensory system: a somatosensory-evoked potentials study in patients with cervical radicular pain.
Journal of Neuroscience. 2000;20:9277–9283Tinazzi M, Zanette G, Polo A, Volpato D, Manganotti P,
Bonato C, Testoni R, Fiaschi A.
Transient deafferentation in humans induces rapid modulation of primary sensory cortex not associated with subcortical changes: a somatosensory evoked potential study.
Neurosci Lett. 1997;223:21–24Rossi A, Decchi B, Groccia V, Della Volpe R, Spidalieri R.
Interactions between nociceptive and non-nociceptive afferent projections to somatosensory cortex in patients with unilateral cerebellar lesions.
Neurosci Lett. 1998;248:155–158Pellicciari MC, Miniussi C, Rossini PM, De Gennaro L.
Increased cortical plasticity in the elderly: changes in the somatosensory cortex after paired associative stimulation.
Neuroscience. 2009;163:266–276Murphy BA, Haavik-Taylor H, Wilson SA, Oliphant G, Mathers KM.
Rapid reversible changes to multiple levels of the human somatosensory system following the cessation of repetitive contractions: a somatosensory evoked potential study.
Clin Neurophysiol. 2003;114:1531–1537Taylor HH, Murphy B.
The Effects of Spinal Manipulation on Central Integration of Dual Somatosensory Input
Observed After Motor Training: A Crossover Study
J Manipulative Physiol Ther. 2010 (May); 33 (4): 261–272
Return to CHRONIC NECK PAIN
Since 10-23-2015


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |