Chiropractic Clinical Practice Guideline:
Evidence-based Treatment of Adult
Neck Pain Not Due to WhiplashThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Canadian Chiro Assoc 2005 (Sep); 49 (3): 158–209 ~ FULL TEXT
OPEN ACCESS Canadian Chiropractic Association;
Canadian Federation of Chiropractic Regulatory Boards;
Clinical Practice Guidelines Development Initiative;
Guidelines Development Committee (GDC) comprising,
Elizabeth Anderson-Peacock, BSc, DC, DICCP, Jean-Sébastien Blouin, PhD, DC,
Roland Bryans, BA, DC, Co-chair, Normand Danis, DC, Co-chair, Andrea Furlan, MD,
Henri Marcoux, DC, FCCS(C), DABCO, Brock Potter, BSc, DC, Rick Ruegg, BSc, PhD, DC,
Janice Gross Stein, BA, MA, PhD, and Eleanor White, MSc, DC
1. Introduction 2. Methods 3. Results: General 4. Results: Treatment 5. Results: Managing the Risk of Adverse Events 6. Results: Research Recommendations 7. Implementing the Recommendations 8. Conclusion Appendix 1: Spotlight on Dissection Appendix 2: Stroke: An Adverse Event of the Rotation Component of Manipulation? Appendix 3: Evidenced, Cervical Pain Benefit From Chiropractic Treatments Contributors ReferencesOBJECTIVE: To provide an evidence-based clinical practice guideline for the chiropractic cervical treatment of adults with acute or chronic neck pain not due to whiplash. This is a considerable health concern considered to be a priority by stakeholders, and about which the scientific information was poorly organized.
OPTIONS: Cervical treatments: manipulation, mobilization, ischemic pressure, clinic- and home-based exercise, traction, education, low-power laser, massage, transcutaneous electrical nerve stimulation, pillows, pulsed electromagnetic therapy, and ultrasound.
OUTCOMES: The primary outcomes considered were improved (reduced and less intrusive) pain and improved (increased and easier) ranges of motion (ROM) of the adult cervical spine.
EVIDENCE: An "extraction" team recorded evidence from articles found by literature search teams using 4 separate literature searches, and rated it using a Table adapted from the Oxford Centre for Evidence-based Medicine. The searches were 1) Treatment; August, 2003, using MEDLINE, CINAHL, AMED, MANTIS, ICL, The Cochrane Library (includes CENTRAL), and EBSCO, identified 182 articles. 2) Risk management (adverse events); October, 2004, identified 230 articles and 2 texts. 3) Risk management (dissection); September, 2003, identified 79 articles. 4) Treatment update; a repeat of the treatment search for articles published between September, 2003 and November, 2004 inclusive identified 121 articles.
VALUES: To enable the search of the literature, the authors (Guidelines Development Committee [GDC]) regarded chiropractic treatment as including elements of "conservative" care in the search strategies, but not in the consideration of the range of chiropractic practice. Also, knowledge based only on clinical experience was considered less valid and reliable than good-caliber evidence, but where the caliber of the relevant evidence was low or it was non-existent, unpublished clinical experience was considered to be equivalent to, or better than the published evidence.
REPORTED BENEFITS, HARMS AND COSTS: The expected benefits from the recommendations include more rapid recovery from pain, impairment and disability (improved pain and ROM). The GDC identified evidence-based pain benefits from 10 unimodal treatments and more than 7 multimodal treatments. There were no pain benefits from magnets in necklaces, education or relaxation alone, occipital release alone, or head retraction-extension exercise combinations alone. The specificity of the studied treatments meant few studies could be generalized to more than a minority of patients. Adverse events were not addressed in most studies, but where they were, there were none or they were minor. The theoretic harm of vertebral artery dissection (VAD) was not reported, but an analysis suggested that 1 VAD may occur subsequent to 1 million cervical manipulations. Costs were not analyzed in this guideline, but it is the understanding of the GDC that recommendations limiting ineffective care and promoting a more rapid return of patients to full functional capacity will reduce patient costs, as well as increase patient safety and satisfaction. For simplicity, this version of the guideline includes primarily data synthesized across studies (evidence syntheses), whereas the technical and the interactive versions of this guideline (http://ccachiro.org/cpg) also include relevant data from individual studies (evidence extractions).
RECOMMENDATIONS: The GDC developed treatment, risk-management and research recommendations using the available evidence. Treatment recommendations addressing 13 treatment modalities revolved around a decision algorithm comprising diagnosis (or assessment leading to diagnosis), treatment and reassessment. Several specific variations of modalities of treatment were not recommended. For adverse events not associated with a treatment modality, but that occur in the clinical setting, there was evidence to recommend reconsideration of treatment options or referral to the appropriate health services. For adverse events associated with a treatment modality, but not a known or observable risk factor, there was evidence to recommend heightened vigilance when a relevant treatment is planned or administered. For adverse events associated with a treatment modality and predicted by an observable risk factor, there was evidence to recommend absolute contraindications, and requirements for treatment modality modification or caution to minimize harm and maximize benefit. For managing the theoretic risk of dissection, there was evidence to recommend a systematic risk-management approach. For managing the theoretic risk of stroke, there was support to recommend minimal rotation in administering any modality of upper-cervical spine treatment, and to recommend caution in treating a patient with hyperhomocysteinemia, although the evidence was especially ambiguous in both of these areas. Research recommendations addressed the poor caliber of many of the studies; the GDC concluded that the scientific base for chiropractic cervical treatment of neck pain was not of sufficient quality or scope to "cover" current chiropractic practice comprehensively, although this should not suggest other disciplines are more evidence-based.
VALIDATION: This guideline was authored by the 10 members of the GDC (Elizabeth Anderson-Peacock, Jean-Sébastien Blouin, Roland Bryans, Normand Danis, Andrea Furlan, Henri Marcoux, Brock Potter, Rick Ruegg, Janice Gross Stein, Eleanor White) based on the work of 3 literature search teams and an evidence extraction team, and in light of feedback from a commentator (Donald R Murphy), a 5-person review panel (Robert R Burton, Andrea Furlan, Richard Roy, Steven Silk, Roy Till), a 6-person Task Force (Grayden Bridge, H James Duncan, Wanda Lee MacPhee, Bruce Squires, Greg Stewart, Dean Wright), and 2 national profession-wide critiques of complete drafts. Two professional editors with extensive guidelines experience were contracted (Thor Eglington, Bruce P Squires). Key contributors to the guideline included individuals with specialties or expert knowledge in chiropractic, medicine, research processes, literature analysis processes, clinical practice guideline processes, protective association affairs, regulatory affairs, and the public interest. This guideline has been formally peer reviewed.
From the FULL TEXT Article:
1. Introduction (details http://ccachiro.org/cpg)
Neck pain is an important cause of reduced quality of life (QoL) and carries a high economic cost. [1, 2] In a recent Toronto (Canada) clinic survey, [3] neck problems were the foremost reason for seeking chiropractic care. Overall, 25% of primary complaints among chiropractic patients likely involve neck pain. [3]
This clinical practice guideline (CPG) reflects evidence extracted from the published scientific literature about effective chiropractic cervical treatments for adult patients suffering from neck pain not due to whiplash. The CPG presents statements and recommendations synthesized from this evidence, and rates the “confidence” (strength) of each. It also identifies treatments for which evidence of ineffectiveness exists. This CPG does not provide a comprehensive overview of chiropractic treatment; any deficiency or omission directly reflects a deficiency or omission in the clinical literature.
This clinical practice guideline focuses on the treatment component of a chiropractic encounter that includes assessment, treatment and reassessment. Also, it does not address other areas of chiropractic care such as prevention.
1.1. Operational definitions
Chiropractic treatment was unanimously defined by the Guidelines Development Committee (GDC) as including the most common treatments employed by chiropractors but excluding: acupuncture; surgical procedures; invasive analgesic procedures, including nerve blocks, neuroablative procedures, epidural blocks, and facet and intramuscular injections; injections of botulinum toxin; systematic psychological interventions such as cognitive or behavioral therapies for anxiety or depression; and, prescription of over-the-counter or prescription drugs. Neck was unanimously defined by the GDC as the vertebral motion segments (vertebral muscles, ligaments, nerves, discs) that constitute the neck; that is, the portion of the spine located between the skull and the rib cage, the vertebrae of which are characterized by their relatively small size and the absence of ribs. [4] We deem chiropractic care to be of value across several age groups, but this CPG was limited to adults (18 or more years of age). Therefore, we searched and analyzed the literature related to adults only. This guideline only addresses pain originating in the neck. Also, it does not address pain referred from the neck to the cranium or to the upper extremities (below the acromioclavicular joint). Pain was defined as whichever assessment method a study used to evaluate it, be it of mechanical, non-mechanical, idiopathic, or pathologic origin. However, this CPG does not address pain resulting from whiplash injury, which we consider to be different from other pain (more details http://ccachiro.org/cpg); whiplash pain results from a unique, complex, coupled movement of the cervical spine involving simultaneous flexion, extension and axial compression, subjecting the cervical spine to an unnatural double curvature at the time of injury. [5] Also, this CPG does not address headache, which was considered outside of the practical scope of our work.
2. Methods
The operational methods to develop and deploy this CPG were articulated in a development, dissemination, implementation, evaluation, and revision plan (DevDIER). [6] The detailed development methods can be found in the technical version of this CPG (http://ccachiro.org/cpg); a summary follows.
The methods involved sequential contributions from literature search teams, an evidence-extraction team and a review panel – under the auspices of The Canadian Chiropractic Association and the Canadian Federation of Chiropractic Regulatory Boards, Clinical Practice Guidelines Development Initiative, GDC. Unanimity (complete agreement) within the relevant contributor groups was achieved at every stage of development. Two development drafts of the guideline were released for profession-wide critiques, the results of which were then incorporated.
2.1. Literature searches
Four electronic literature searches were undertaken:treatment (English and German, up to August, 2003);
risk management (managing the risk of non-dissection adverse events, English and French, up to October, 2004);
dissection risk management (the theoretic association between manipulation and dissection or stroke, English, up to September, 2003); and,
treatment update (English and French, for the period between September, 2003 and November, 2004 inclusive).The results of each search were downloaded into an electronic data set, and duplicates were manually removed. Some additional studies were added manually to each data set. The studies were retrieved and passed to the evidence-extraction team (TE, BPS).
2.2. Evidence extractions (data from individual studies)
Evidence extractors manually applied the operational definitions (Section 1.1) to reject studies or data that clearly did not respect these. Studies that contained both acceptable and unacceptable information were not rejected, but clearly unacceptable data within these studies were rejected. The 2 extractors recorded statistically significant results from the literature (p-value less or equal to 0.05), as well as non-significant findings where deemed appropriate. All relevant numeric data were extracted from studies into an electronic database; the extractors did not rely on study abstracts.

Table 1 Table 1 adapted from the Oxford Centre for Evidence-based Medicine (OCEBM) levels of evidence [7] was used to categorize results into rated evidence extractions. For treatment evidence, the extractors together used the OCEBM levels of evidence to rate the quality of each extraction, and then reached unanimity about all aspects of each extraction. For the evidence related to dissection, the 2 extractors reached unanimity about all aspects of each extraction. For evidence related to other risk-management concerns, because of the poor caliber of evidence, the 2 extractors were able to independently complete extraction work that was later amalgamated by one (TE).
For treatment evidence, the extractors accepted all Level 1 to 4 evidence, but Level 5 evidence only if it arose from a Level 1 to 4 study; e.g., if it was the study authors’ extrapolation from the study data (Table 1). For risk-management evidence, all Levels were accepted. Where applicable, the rating of extractions are cited in parentheses (e.g., {L-4}). Where relevant, the GDC’s interpretations of study results are included as Level 5 evidence extractions and cited as such (i.e., {L-5} or {L-5}GDC).
Evidence extractions were ultimately verified by the GDC in the course of recommendation-development workgroups (Section 2.4).
2.3. Evidence Syntheses
In this CPG, unless otherwise noted:
pain means neck pain not due to whiplash;
ranges of motion (ROM) means cervical ROM;
disability means neck disability;
manipulation and mobilization designate interventions localized to the cervical spine;
exercise designates clinic-based, supervised exercise programs;
and home exercise designates home-based, monitored or unmonitored exercise programs.
Also, International system of units (SI) abbreviations are used.The caliber of the studies precluded quantitative syntheses (e.g., statistical pooling). Therefore, topic related evidence extractions from individual studies were qualitatively summarized in evidence syntheses for ease of reading, by amalgamating related findings. The best quality evidence we could find in the extractions was used to make each pertinent point in the syntheses, and the quality of this evidence is cited.
For simplicity, this version of the CPG focuses on evidence syntheses, whereas the technical and the interactive versions of this CPG (http://ccachiro.org/cpg) also include all evidence extractions from individual studies. The extractions in those versions report all relevant study outcomes, but the syntheses included here focus on pain and ROM, because these two outcomes were the most consistently reported.
One extractor (TE) developed the treatment syntheses, and then the 2 extractors together examined them all to reach unanimity. One extractor (TE) completed all the risk-management syntheses.
Evidence syntheses were ultimately verified by the GDC in the course of recommendation-development workgroups (Section 2.4).
2.4. Formulating recommendations
The 10 members of the GDC qualitatively interpreted the clinical relevance of the evidence extractions and syntheses. Therefore, all recommendations should be considered to be a subjective extrapolation, “equivalent” to an OCEBM Level 5 rating.
Extractions and syntheses were used to formulate treatment, risk-management or research recommendations during collaborative work sessions. Risk-management and research recommendations incorporated a substantial amount of the GDC’s (unpublished) expertise, whereas treatment recommendations purposefully incorporated little.
The work-groups considered outcomes, the caliber of evidence, and an assessment of clinical relevance to reach unanimity about each recommendation. Clinical relevance included the deemed importance of the practice in chiropractic, the deemed over- or under-use of the practice in chiropractic, and the deemed importance of reported outcomes (calculated effect sizes were unavailable).
A 5-member panel reviewed a draft set of the treatment evidence syntheses and the treatment recommendations, and advised the GDC about these. The GDC determined with unanimity how to incorporate this advice into the CPG.
3. Results: general
Where applicable, the rating of an evidence extraction or synthesis is cited in parentheses (e.g., {L-4}). Where relevant, the GDC’s interpretations of study results are included as Level 5 (i.e., subjective extrapolation) and cited as such (i.e., {L-5} or {L-5}GDC).
For simplicity, this version of the CPG focuses on evidence syntheses, whereas the technical and the interactive versions of this CPG (http://ccachiro.org/cpg) also include all evidence extractions from individual studies.
The extractions in those versions report all relevant study outcomes, but the syntheses included here focus on pain and ROM, because these two outcomes were the most consistently reported.
3.1. Treatment evidence
The GDC concluded that this CPG reflects almost all the published, scientific clinical evidence directly addressing the chiropractic cervical treatment of neck pain not due to whiplash. The GDC also concluded that this evidence was not of sufficient scientific quality or scope to “cover” current chiropractic practice comprehensively, although this should not suggest other disciplines are more evidence-based.
No treatment data were drawn from the reviews or the CPGs we found because of a confounding mix of outcome or treatment data from back and neck pain patients, or confounding mix of outcome or treatment data from whiplash and non-whiplash patients. Also, single study results were excluded if they appeared to confound these data. Ultimately, treatment evidence was extracted from 90 studies for the development of treatment syntheses and recommendations (Section 4).
Studies were generally of medium quality, and many abstracts suggested the studies were of much higher quality than their methods illustrated. In general, considerable effort was required to extract pertinent results from needlessly ambiguous articles.We agreed with others [8] that a treatment effect size less than 0.5 was clinically unimportant {L-4},
and that an effect size from 0.5 to 0.79 was moderately important,
and 0.8 or more, important {L-5}. [9]
The effect size of treatments in the literature was infrequently reported, and where it was, it was usually unimportant or moderately important.
Section 7.1 addresses the practical issue [10] of what percentage of improvement in pain on numeric scales is clinically important.
Also, where clearly defined, individual modalities generally differed between studies (e.g., Tables 1 and 2 in the technical version [http://ccachiro.org/cpg]) and dramatically reduced our ability to synthesize results across studies into definitive, clinically applicable recommendations.

Table 2 Thirteen studies [9–21] reported whether adverse events occurred (Table 2) and 7 studies [10, 17, 18, 21–24] included placebo comparison groups. Twelve studies [22, 25–35] included a no-treatment comparison group; only for studies in which across-group comparisons were clear [22, 25–30] were we able to conclude that treatments were or were not better than no treatment.
3.2. Risk-management evidence
We were unable to extract useful information from many articles because the results were inconclusive, the conclusions were not self-evident, or the topic was considered outside of the scope of common chiropractic (e.g., interpreting blood assay or bone densitometry results).
We determined that 79 studies or reports were relevant to the hypothetical association between manipulation and dissection. Rated evidence extracted from these supported the development of syntheses and recommendations (Appendix 1).
The well-established predisposing risks for cardiovascular disease, atherosclerosis or stroke were summarized by extracting information from Wolf, [36] and those about the well-established physiologic parameters governing exercise treatments were summarized by extracting information from Kisner and Colby. [37] Of 230 articles about adverse events that did not deal with these 2 topics or dissection, evidence was extracted from 56 and rated, to support the development of syntheses and recommendations (Section 5, Appendix 2).
4. Results: treatment
Studies of adjustment were neither excluded nor overlooked. We concluded that studies of adjustment would have been identified in association with the search terms “chiropractic” or “neck pain” if they existed in the literature sources we searched. However, none of the treatment studies reported the outcomes of adjustments per se. All results likely related to this treatment modality were reported as outcomes of manipulation or mobilization; therefore, this nomenclature has been maintained in this CPG.
The extreme specificity of the studied treatments meant few studies could be generalized to more than a few patients, and, thus, recommendations required our interpretation of the evidence. Therefore, all recommendations should be considered to be our subjective extrapolation, “equivalent” to Level 5 evidence.
The limitations of this CPG reflect the limitations of current published evidence and the deliberate limitation of many recommendations to conclusions that studies directly supported, to the detriment of clinical scope.
Unless otherwise noted:
The term effect does not imply improvement. Many studies compared the effect of treatments across groups, but did not report whether any group showed improvement with treatment; a treatment may have a greater effect than another, but cause no significant improvement in patients.
The evidence did not clarify if the treatment was better than no treatment, which means that we do not know from the evidence whether a treatment is better than no treatment at all.
This CPG focuses on treatment, and does not explore fully the available systems of assessment, such as those listed in the 2003 summary of pre-manipulative assessment procedures by Hing et al. [ 38] The recommendations in this CPG were made with the caveat that it is each chiropractor’s responsibility to implement the appropriate risk-management procedures when implementing the treatment recommendations.
Where relevant, the statements below indicate the modality used and the approximate time effects were measured:immediate (less than a 1 day);
short term (1 day to 3 weeks);
medium term (3 weeks to 6 months);
or long term (more than 6 months).
Table 3 in the technical version (http://ccachiro.org/cpg) provides details of studies’ specific manipulation techniques, and Table 4 in the technical version (http://ccachiro.org/cpg), exercise.
Table 4. Aspects of treatment modalities that should be considered
to tailor treatment in response to modality-modifying risk factors
Aspects of modality that may reduce risk from risk factors
Manipulation (HVLA)
Force
Direction
Amplitude
Velocity
Patient position
Frequency
Duration
Location of treatment
Mobilization
Force
Direction
Amplitude
Velocity
Patient position
Frequency
Duration
Location of treatment
Ultrasound
Intensity
Duration
Wave frequency
Frequency of care
Duty cycle
Exercise
Repetition
Intensity
Frequency
Duration
Weight
Exertion
Type
Traction
Direction of force
Harness
Weight
Frequency
Duration
Manual vs mechanical
Intermittent vs continuous
Electro-therapies
Intensity
Duration
Frequency of care
Type of current or wave
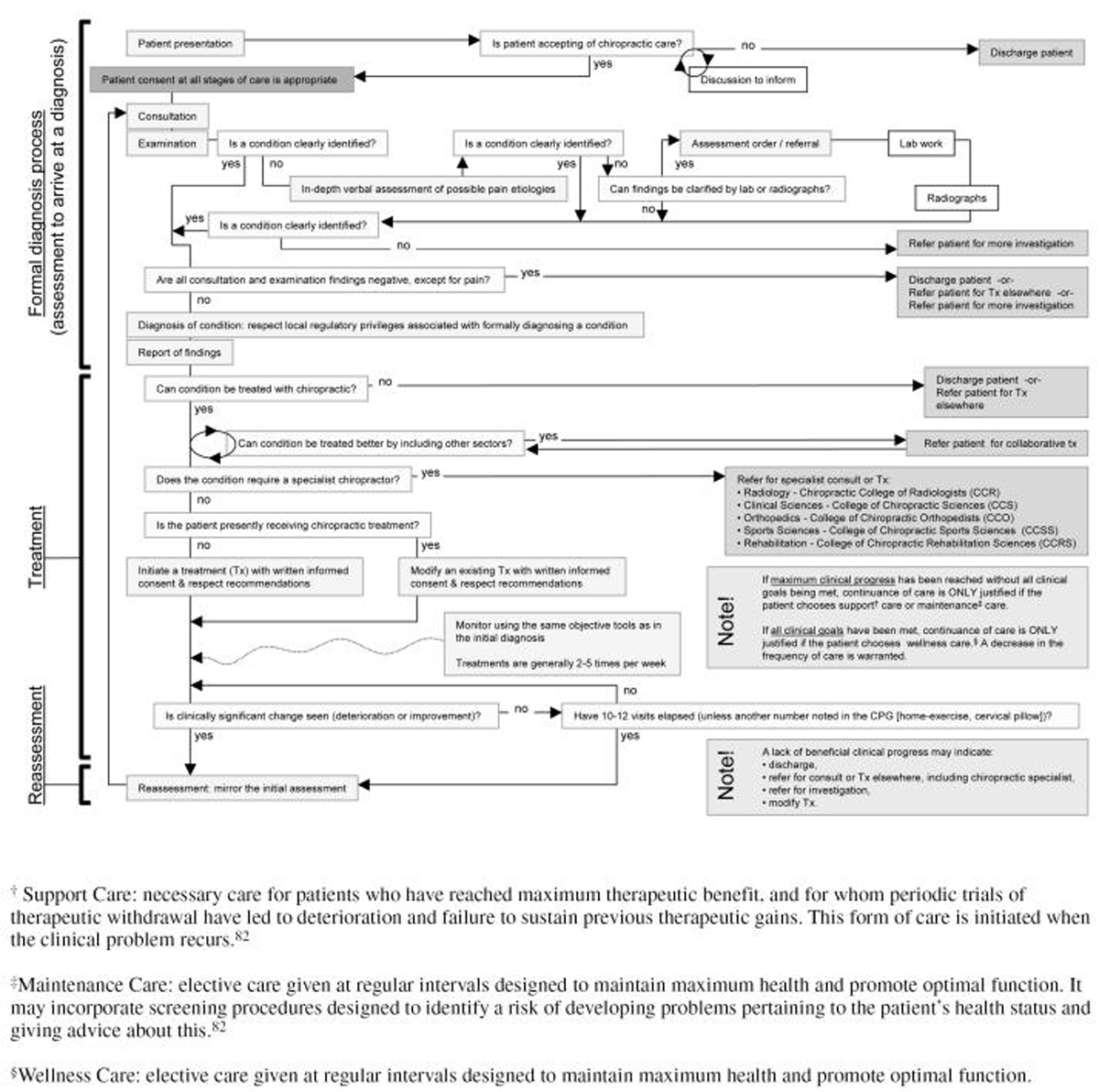
Figure 1 Treatment recommendation 1. Based on all the evidence below, we recommend the 3 sequential steps in the decision algorithm (Figure 1) – diagnosis (or assessment leading to diagnosis), treatment, reassessment – to treat patients with acute pain, an acute exacerbation of a recurrent pain, or chronic pain.
Similarly, we recommend the 3 sequential steps to treat patients with idiopathic pain or pain with an identified cause. The selection and dosages of treatment modalities will differentiate best practices for each unique combination of pain condition and patient. The selection and dosage of treatment modalities should respect recommendation 2.
Treatment recommendation 2. Based on all the evidence below, we also recommend manipulation, mobilization, ischemic pressure, clinic- and home-based exercise, traction, education, low-power laser, massage, transcutaneous electrical nerve stimulation (TENS), pillows, pulsed electromagnetic therapy, or ultrasound – for patients with acute or chronic pain, where the origin of pain is known or unknown, to improve pain and some ROM – in dosages and methods based on the practitioner’s experience and the patient’s specific situation, as there is insufficient published evidence to support or refute narrow generalizations about the use of these treatment modalities.
Treatment recommendation 3. Based on all the evidence below, in the absence of objective findings with neck pain not due to whiplash (e.g., ROM, muscle hypertonicity), we do not recommend that treatment be initiated. If, after a complete examination, all findings except for pain are normal, we recommend discharge of the patient from chiropractic care and, possibly, referral based on the practitioner’s experience.
Treatment recommendation 4. Based on all the evidence below, in addition to the details of the 3-step sequence in recommendation #1, if home exercise is prescribed, we recommend frequent monitoring of its quality and a reassessment of the quality and effect of the home exercise after 2 to 4 weeks.Immediate benefit from manipulation
Manipulation immediately improves pain and some ROM {L-4}, [11, 39–42] and a single manipulation ipsilateral to the location of pain is immediately better than a contralateral manipulation {L-2b}. [41] See Section 4.2 for the clinical importance of treatments with an immediate effect.
Short- and medium-term benefit from manipulation
Multiple manipulations improve pain in the short {L-4} [43–46] and medium {L-4} [43, 46–48] term, and some ROM in the short {L-4} [43, 44, 46, 48] and medium {L-4} [12, 43, 45–48] term. Manipulations in and opposite the direction of restriction may achieve greater ROM benefit than manipulation only in the direction of restriction {L-2b}. [44] Also, thoracic manipulations do not enhance the benefits from cervical manipulations in the short term {L-2b}. [48]Treatment recommendation 5. Based on the short- and medium-term benefit from manipulation, we do not recommend crossed bilateral transverse pisiform or anterior thoracic manipulations to be added to a course of cervical manipulations to improve pain and some ROM, unless where required for non-cervical benefits.
Short- and medium-term benefit from manipulation with stretching
Multiple manipulations, each preceded or followed by stretching of neck muscles, improve pain in the short and medium term {L-4}; [20] stretching either causes the relief or merely adds benefit to manipulation {L-5}.GDC Multiple manipulations with or without {L-2b} stretching improve ROM in the medium term {L-4}. [20] Additionally, multiple manipulations may be better than unsupervised stretching {L-4}. [49]
Medium- and long-term benefit from manipulation with 3-point traction
Multiple manipulations with 3-point traction improve pain in the medium and long term {L-4}, [25, 26] and are better than no treatment {L-2b} [26] {L-4}, [25] but should be preceded by a detailed disclosure of the risks of the 3-point traction {L-5}.GDC
Immediate benefit from mobilization
A single treatment of short mobilization sessions immediately improves pain {L-4}, [22] and is better than no treatment for an immediate effect {L-2b} (see Section 4.2).
Immediate benefit from ischemic pressure
Ischemic pressure applied to myofascial trigger points (TrgP) for 90 s immediately improves pain and some ROM {L-4} [50] (see Section 4.2).
Medium- and long-term benefit from exercise
Ongoing exercise improves: pain in the medium and long term {L-4}; [10] pain in the medium term for patients with unidentified conditions {L-4}, [32, 51–53] with strength exercise being the most consistently beneficial {L-1b}; [51] and pain and some ROM in the medium term for patients with cervical strain, herniated disc, degenerated discs {L-4}, [54] osteoarthritis, or “outset” resulting from minor injuries {L-4}. [31]
The evidence [27, 31, 32] is ambiguous about the advantage of exercise over no treatment, but suggests exercise is better than a placebo of clinical contact {L-1b}. [10] It is possible that subjectively perceived benefits such as pain reduction continue well beyond the treatment period, whereas objectively measured benefits do not {L-5}.GDC
Medium- and long-term benefit from intensive or light exercise
Ongoing intensive or light exercise equally {L-2b} improve {L-4} pain in the long term, and intensive exercise is better than light exercise for objective outcomes in the medium term {L-2b}. [55]
Medium- and long-term benefit from exercise with education
Ongoing exercise with education improves pain in the long term {L-4}, but may be less beneficial than more passive treatment that includes medications for patients with cervical spondylopathy, soft tissue rheumatism, humeral tendinitis, tension neck, cervical syndrome, or rhizopathy {L-5}. [56]
Also, for patients without defined conditions, exercise-education combinations improve worst pain in the medium term {L-4} and have significantly greater effect than education alone {L-2b}; [57] this suggests the critical ingredient in exercise-education combinations is the exercise {L-5}.GDC
Medium- and long-term benefit from exercise with education and home exercise
Ongoing exercise with home exercise and education improves pain and some ROM in the medium [13, 33, 34] and long [13] term {L-4}, and is better than extensive multi-modal treatments without exercise {L-2b}. [34]
Medium- and long-term benefit from exercise with multimodal treatments
Ongoing exercise with extensive multi-modal combinations improves pain and some ROM for patients with unspecified conditions in the medium [35, 58, 59] and long [58] term {L-4}.
It also improves pain and some ROM in the medium term for patients with osteoarthritis, cervical spondylopathy, strain, or cervical disc disease {L-4}. [60] In an exercise combination, exercise is likely most effective at improving objective outcomes, whereas the other modalities address subjective outcomes {L-5}.GDC
There is conflicting evidence whether exercise is better than general medical care for pain in the medium {L-5}GDC and long {L-2b} [33] term. Finally, the addition of more exercise to an exercise combination improves satisfaction and maintained benefit {L-5},GDC but is not as good as extending the scope of the multi-modal treatment {L-4}. [59]
The evidence [35] is ambiguous about exercise’s advantage over no treatment {L-5}.GDC
Summary exercise benefit statement
Multiple multi-modal treatments are the most effective and exercise, especially intensive exercise, is a critical element {L-5}.GDC Overall, in addition to pain and ROM outcomes, ongoing strength exercise increases strength, ongoing endurance exercise increases endurance, but the outcome of coordination exercise is ambiguous {L-5}.GDC
In addition, where it was reported, [28, 31, 32, 58] exercise was not consistently better than placebo or no-treatment groups {L-5};GDC the propensity for neck pain to resolve on its own may be a confounding factor (see Section 4.1).
Short-, medium- and long-term benefit from home exercise with or without education or ultrasound
Home exercise may improve pain and some ROM in the medium and long term {L-4}, [29, 61] but extensive tailoring of the home exercise to each patient is required {L-5}.GDC Some evidence {L-2b} [29] suggests that home exercise may be no better than no treatment for pain or ROM {L-5}.GDC
Home exercise with education or monitoring improves pain and some ROM in the medium term {L-4}. [16] Home exercise with ultrasound improves pain in the short and medium term {L-4}; ultrasound enhances the effect of home exercise alone {L-2b}, [62] but not home exercise with massage {L-5}.GDC However, home exercise with ultrasound is no better than no treatment for pain {L-2b}. [28]Treatment recommendation 6. Based on the summary exercise benefit statement and the short-, medium- and long-term benefit from home exercise with or without education or ultrasound, we do not recommend generic home exercise designed to improve pain or ROM that is not tailored to the individual patient.
We recommend tailored home exercise treatment, as rigorous as the patient can tolerate, if a loss of ROM, strength or endurance is found. It can be as frequent as once daily, with its rigor adjusted progressively.
Short- and medium-term benefit from ultrasound
Multiple ultrasound treatments improve pain and some ROM in the short and medium term {L-4}. [45]
Short- and medium-term benefit from low-power laser treatments
Multiple low-power laser treatments improve pain in the short and medium term {L-4}, [21, 23] and pain and some ROM in the short term for cervical osteoarthritis patients {L-4}. [63] The laser beam shows better results than placebo {L-1b} [21] {L-2b}, [23] which suggests that it causes the improvement {L-5}.GDC
Medium-term benefit from pillows
Use of a cervical pillow during sleep improves pain in the medium term {L-4}. [64]Treatment recommendation 7. Based on the medium-term benefit from pillows, in addition to the details of the 3-step sequence in recommendation #1, we recommend a cervical pillow as a secondary treatment that should be initiated only after at least one cycle of diagnosis (or assessment leading to diagnosis), treatment and reassessment–and if prescribed, the pillow should be used nightly.
Short- and medium-term benefit from pulsed electromagnetic field therapy
Multiple pulsed electromagnetic field treatments improve pain and some ROM in the short and medium term {L-4}. [17, 18, 24] The pulsed electromagnetic field quality of the various vectors used (collar, electrode-points) shows better results than placebo {L-1b} [24] {L-2b}, [17, 18] which suggests that it is causal {L-5}.GDCTreatment recommendation 8. Based on the short- and medium-term benefit from pulsed electromagnetic field therapy, in addition to the details of the 3-step sequence in recommendation #1, we recommend pulsed electromagnetic field treatment as an adjunctive, secondary treatment that should be initiated only after at least one cycle of diagnosis (or assessment leading to diagnosis), treatment and reassessment.
Immediate, medium- and long-term benefit from multimodal treatments
Multi-modal treatments improve pain and some ROM in the medium and long term {L-4}; [13, 15, 19, 34, 65] there is no discernable difference between combinations as long as they include home exercise, education, traction, 1 other secondary modality, and either manipulation, mobilization or clinic-based exercise {L-2b}. [13]
A single multi-modal treatment improves pain or pain and some ROM immediately {L-4}. [50] For example, one treatment of a 20-min hot-pack, active ROM, interferential current, and myofascial release (role of ischemic pressure undefined) is the first choice of 6 options of multi-modal treatments using secondary modalities to improve immediately: pain intensity, pain pressure tolerance, and pressure point thresholds (PPT) {L-5}GDC (see Section 4.2).The 6 treatments, from most effective to least, are:
- 20-min hot-pack, active ROM treatment, interferential current, myofascial release;
- 20-min hot-pack, active ROM treatment, stretch-and-spray, TENS;
- 20-min hot-pack, active ROM treatment, stretch-and-spray;
- 20-min hot-pack, active ROM treatment, ischemic pressure, TENS;
- 20-min hot-pack, active ROM treatment, ischemic pressure;
- 20 min hot-pack, active ROM treatment.
No additional benefit from magnets in necklaces
It is nearly certain that magnetic necklaces are no better than non-magnetic necklaces to improve pain in the short term {L-1b}, although improvement in pain follows the wearing of both {L-4}. [66]Treatment recommendation 9. Based on no additional benefit from magnets in necklaces, we do not recommend permanent magnet necklaces to improve pain, specifically because the monetary and lifestyle costs of a magnetic necklace do not appear to be counter-balanced by a clinical benefit.
No benefit from education or relaxation alone
Education alone does not improve pain [51, 57] or ROM [57] in the medium term {L-4}. Relaxation treatments are equal {L-2b} to advice to be active, in not improving pain or ROM in the medium or long term {L-4}. [27]Treatment recommendation 10. Based on no benefit from education or relaxation alone, we do not recommend education or relaxation alone to improve pain or ROM.
No immediate benefit from head retraction-extension exercise combinations alone
Head retraction-extension exercise combinations do not immediately improve pain {L-4}. [30]Treatment recommendation 11. Based on no immediate benefit from head retraction-extension exercise combinations alone, we do not recommend head retraction-extension exercise combinations to improve pain.
No immediate benefit from occipital release treatments alone
Occipital-release treatments do not immediately improve pain {L-4}. [30]Treatment recommendation 12. Based on no immediate benefit from occipital release treatments alone, we do not recommend occipital release treatments to improve pain.
4.1. Natural history of neck pain
Men and women may experience neck pain differently. More men have acute pain, whereas more women have chronic pain. [67] Men also gain more pain relief from chiropractic treatment. [68] We agree with at least five reports [69–73] that pain is consistently associated with functional loss (e.g., altered patterns of neck muscle activation, reduction in strength and endurance) {L-5}, which may be without outwardly obvious structural change such as degenerative disease {L-2c}. [74]
Acute neck pain in adults is generally regarded to be self-resolving. One report [75] on patients with acute and chronic back disorder suggested that 90% of neck or back pain cases had self-resolved at 6 weeks {L-5}, corroborating another report. [34] Also, a review [76] on patients with neck pain suggested that more than 50% of patients experience a decrease in pain 2 to 4 weeks after onset and 80% will be asymptomatic in 2 to 3 months {L-5}. Another study suggested that patients (defined as chronic) who spent 16 weeks on a waiting list for physiotherapy experienced improved pain, cortical control, and 6 of 22 elements on an examination and physiologic test index {L-4}. [33]
We caution that there can be a structural or functional detriment associated with some patients’ pain, and we agree with at least one author [69] that the resolution of the pain may not signal a complete resolution of the detriment {L-5}. Specifically for these patients, even if treatment does not result in a faster or greater improvement in pain, good treatment will also address non-pain problems, which left untreated, may have permanent sequelae {L-5}.GDC This justifies treatments that are expected to show less or slower improvement than the expected natural history of the treated pain in these patients {L-5}.GDC
Authors of a Saskatchewan study [77] suggested that complete resolution of pain or disability is difficult to achieve (treatment status undefined) {L-2c}, and other authors reported that half of patients treated with primary care did not experience complete resolution after 1 year [78] or 5 years [79] (specific treatment type or consistency over the period undefined) {L-2c}. For patients whose pain is not self-resolving, we agree with at least 3 reports [68, 80, 81] that there may be a treatment opportunity to halt the evolution of acute pain to a chronic condition {L-5}, perhaps specifically for patients predisposed to chronic pain for non-pathologic reasons (low self-rated health and high levels of psychologic stress) {L-5}. [80] Therefore, we concluded that good practice does not universally mean waiting to find out whose pain resolves, and whose does not, before proceeding with treatment {L-5}.
We therefore recommend:Treatment recommendation 13. We do not recommend treatments that are expected to show less or slower improvement than the expected natural history of the treated pain in a particular patient, unless: a) the treatment also addresses non-pain problems that, left untreated, may have permanent sequelae, or b) it is deemed that treatment will halt the evolution of acute pain to a chronic condition.
Treatment recommendation 14. If maximum clinical progress has been reached without all clinical goals being met, we recommend continuing care only if the patient chooses support or maintenance care. If all clinical goals have been met, we recommend continuing care only if the patient chooses “wellness” care.In the recommendation above; Support Care means the necessary care for patients who have reached maximum therapeutic benefit, and for whom periodic trials of therapeutic withdrawal have led to deterioration and failure to sustain previous therapeutic gains. This form of care is initiated when the clinical problem recurs. [82]
Maintenance Care means elective care given at regular intervals designed to maintain maximum health and promote optimal function. It may incorporate screening procedures designed to identify a risk of developing problems pertaining to the patient’s health status and giving advice about this. [82]
Wellness Care means elective care given at regular intervals designed to maintain maximum health and promote optimal function.
4.2. The role of focusing on immediate clinical outcomes
Seven treatment studies [11, 22, 39–42, 50] focused on improved pain or ROM immediately after a single treatment. These treatments do not reflect a typical treatment plan. However, it is our understanding that an immediate post-treatment reduction in pain or increase in ROM suggests that the particular patient’s pain or ROM responds to the treatment and that subsequent treatments will reap further short-, medium- or long-term benefits {L-5}.
In addition, we consider that a legitimate role for a single treatment that provides an immediate relief of pain, if administered without temporarily increasing pain, is to permit another intervention that would otherwise be too painful for the patient to bear {L-5}.Treatment recommendation 15. We recommend the planned one-time use of a treatment specifically and only to determine the utility of further treatments or to permit the immediate use of an otherwise painful intervention, both purposes therefore requiring an immediately-subsequent patient assessment. Thus, we do not recommend the planned one-time use of a treatment to merely achieve an immediate clinical effect.
4.3. Multi-sectoral care
A key to successful chiropractic treatment appears to be integrated care that accommodates clinical sectors that may fall outside of some chiropractors’ practice. For example, unimodal and multimodal treatments that incorporate behavior modification [16, 29, 83] or pharmacologic interventions [12, 19, 56, 62, 75, 83–92] have shown benefit.
It is our understanding that integrated care means the integration of all modalities of treatment into an optimal care plan for a particular patient, including those chiropractic modalities supported by the evidence that is the foundation of this CPG {L-5}.Treatment recommendation 16. We recommend a concerted effort to mesh chiropractic care into that of other health disciplines to maximize patients’ gains from their chiropractic treatments (recovery from pain, impairment and disability, reduced costs, increased patient safety, increased satisfaction among patients and health care payers).
5. Results: managing the risk of adverse events
The caliber of most studies in Section 5 was Level 5 (subjective extrapolation or observation, frequently based on a case study) and only a very few being Level 3 or better. Also, in general, the statistical significance of adverse event data was not reported. The results in Section 5 are of Level 5 caliber unless otherwise noted.
The treatment section (Section 4) of this CPG was based almost exclusively on evidence from the literature; recommendations did not extrapolate beyond what the evidence clearly supported (except for the algorithms). To accommodate the caliber of research in Section 5, we relied on a greater level of the GDC’s (unpublished) practice expertise and cited it accordingly ({L-5}GDC).Chiropractic regards the objective and subjective balance of benefits and the risk of adverse events to be especially important. The direct benefits of the treatment recommendations are expected to include those reported in the studies cited in Section 4, and reduced sick days and increased strength, endurance, flexibility, QoL, and ease of activities of daily living (ADL) – see technical version at http://ccachiro.org/cpg for details. Counter-balancing these benefits is the risk of adverse events.
For clarity, adverse events can be considered to be of 3 types:
adverse events (AE) not associated with a treatment modality, but that occur in the clinical setting (non-Tx-AE);
adverse events associated with a treatment modality, but not a known or observable risk factor (unforeseen-Tx-AE);
adverse events associated with a treatment modality and predicted by an observable risk factor (foreseen-Tx-AE).
These delineations are not static; for example, an adverse event that is associated with a treatment modality, but not predicted by a risk factor, may progress to being so (predicted by a factor) once this has been identified.
We deem that it is a chiropractor’s responsibility to understand and address all three types of adverse events (non-Tx-AE, unforeseen-Tx-AE, foreseen-Tx-AE) {L-5}.
Determination of the appropriate clinical response to an adverse event associated with a treatment, or to the relevant risk factor, is complicated by the manual nature of many chiropractic therapies. The risk of harm following treatment depends directly on the skill of the chiropractor {L-5}.GDC Jagbandhansingh [93] echoed this conclusion in a 1997 review of USA malpractice claims:
“Chiropractic care in itself may not pose a clinical risk. However, treatment in association with lack of good professional judgement [or] failure to properly assess a patient’s condition may” (p. 64).Risk-management, recommendation 17: To manage the risk of adverse events associated with a treatment modality, if a chiropractor is uncertain about the caliber of any aspect of his or her technique with a particular patient, we very strongly recommend discontinuance of care and referral to colleagues until this is addressed.
5.1. Managing the risk of adverse events not associated with a treatment modality, but that occur in the clinical setting (non-Tx-AE)
Chiropractors receive patients from all sectors of the population and may encounter any of the health problems that afflict everyone. Indeed, chiropractic patients are likely disproportionately ill compared with the general population. Côté et al. [94] reported that Saskatchewan patients seeking chiropractic care frequently presented with a wide array of serious comorbidities {L-2c}, such as heart disease.
When undiagnosed, these health problems may precipitate a non-Tx-AE before, during or after a treatment. Reports have described patients seeking care for symptoms treatable with chiropractic, but who have undiagnosed ailments outside of the scope of practice.
For example: the symptoms of various pathologies, such as:systemic lupus erythematosus, Bornholm disease, [95]
neoplasms [95, 96] (osteoblastoma), [97]
cervical fracture, [98]
or internal carotid artery dissection [99] (ICAD) can mimic mechanical neck pain;
the symptoms of myocardial infarct (MI) can masquerade as neck pain [100] (and vice versa); [101]
seizures or transient ischemic attacks (TIA) can mimic migraines; [102]
neck and jaw pain can result from Marfan syndrome without spinal involvement; [103]
undetected septic arthritis can be harbored in patients complaining of joint pain while taking glucocorticosteroid therapy; [104]
and pain coincident with trauma can be paralleled by undetected cervical fractures, [105]
or (later) acute respiratory distress syndrome (ARDS) leading to death. [106]
Comorbidities can also be missed when overt signs are present, as when surface lesions in acute scalp lymphangitis indicated an untreated infection masquerading as cervical pain in at least one case. [107]
Attempts to systematically address the identification of serious comorbidities in advance of care may include using a 15-item self-report tool developed by Côté et al. [94] It is our understanding that a set of 15 presenting symptoms (adapted from McMillin) [108] should raise suspicion that the presenting cervical pain is not of mechanical origin {L-5}:
Trunk or lower extremity neurologic symptoms, especially long-tract signs.
Bilateral upper extremity pain.
Remote symptoms with neck movements (lower extremity).
Signs of sphincter dysfunction, bowel or bladder dysfunction or incontinence.
Fever, unrelenting nocturnal pain, weight loss, chronic fatigue.
Recent infection or surgery.
Polyarthralgia.
Dysphagia.
Nuchal flexion or extension rigidity, especially in the absence of trauma.
Cranial neurologic deficit or central nervous system symptoms.
Cervical pain related to general exertion (i.e., after climbing stairs).
Symptoms unchanged or progressive, despite previous functional management.
Onset of cervical pain associated with direct head trauma, loss of consciousness.
Sudden onset of cervical pain without trauma or incident.
- (see Section 5.3.1).
Neck or occipital pain with a sharp quality and severe intensity, or severe and persistent headache, which is sudden and unlike any previously experienced pain or headache
When a relevant comorbidity or a non-Tx-AE is noted, chiropractors have a responsibility to act in the best interest of their patients by immediate consideration of the situation, and referral to the appropriate healthcare resource as necessary.Risk-management, recommendation 18: Before, during or after treatment, we recommend immediate, in-depth consideration of possible explanations, and reconsideration of treatment options or referral to the appropriate health services when an adverse event (not known to be associated with a treatment) is noted; i.e., when a patient demonstrates signs or symptoms of an undiagnosed condition, or signs or symptoms not known to be associated with a treatment.
5.2. Managing the risk of adverse events associated with a treatment modality, but not a known or observable risk factor (unforeseen-Tx-AE)
Managing the risk of unforeseen-Tx-AEs involves reacting appropriately if an event is noted. By definition, an unforeseen-Tx-AE follows the administration of a particular treatment, but there are no known factors differentiating patients at greater risk from others.
Thirteen [9–21] of the treatment studies reported about unforeseen-Tx-AEs (Table 2). Reports ranged from none [10–13, 16, 18, 20, 21] to those considered minor and self-resolving among a small fraction of study subjects, [14, 15, 16] or minor. [9, 19] The worsening of symptoms with treatment was also reported, [9, 10, 14, 64] but it was not possible to determine if this was as a result of treatment failure or an unforeseen-Tx-AE.
Richardson et al. [109] reported that about half of 98 patients had “some discomfort” following a 4-week trial of TENS {L-4}. About one-fifth had “unpleasant sensations” at or away from the TENS site, and a smaller number had headaches, muscle aches, nausea, bad temper, or dizziness. A systematic review [110] {L-1a} concluded that in a minority of patients, high-frequency (HF) TENS could cause skin rash whereas low-frequency (LF) TENS could cause a burning sensation over the area of electrodes, and HF-TENS or LF “train TENS” could cause skin irritation {L-5}.
Regarding manipulation; benign and transient unforeseen-Tx-AEs, or none, have been reported in a recent systematic review, [111] a comprehensive study [112] of more than 250 subjects, a prospective survey of 1,058 patients, [113] a prospective survey of 465 patients, [114] and 2 extensive literature reviews. [115, 116] In their 2003 study, Hurwitz et al. [112] reported that:“No serious complications from spinal manipulation or manual treatment have been reported... [in] any of the published clinical trials involving [spinal] manipulation or mobilization” (p. 21).
Hurwitz et al. [112] suggested that manipulation conferred a greater risk than mobilization for benign, transient unforeseen-Tx-AEs that usually occur within 24 hours of treatment {L-5}.
Indeed, about 5 of every 100 patients treated with manipulation will feel “very noticeable” discomforts, whereas about 20 will feel moderate discomfort, and about 15 will feel slight discomfort {L-4}. [117] This total of affected patients (40 of 100) approximates the 34% to 55% estimate by Cagnie et al. [114] In more than 80% of these cases, discomforts disappeared within 24 hrs, and in more than 85%, discomforts occurred on the day of the manipulation {L-4}. [117]
Rare, unforeseen-Tx-AEs include spinal cord compression, facet edema, disk herniation, [116] long thoracic nerve palsy, [118] ruptured cervical discs, [119] and diaphragm paralysis related to phrenic nerve trauma. [120] In addition, women and younger patients (27 to 46 years) have reported more complaints {L-2c}. [113]Risk-management, recommendation 19: During or after treatment, we recommend heightened vigilance for adverse events associated with a treatment modality, but not a known or observable risk factor (unforeseen-Tx-AE) when a relevant treatment is planned or administered–and immediate, in-depth consideration of possible explanations, and reconsideration of treatment options or referral to the appropriate health services when an event is noted.
Without considering the issue of dissection, patient differences in susceptibility to adverse events related to manipulation are apparent (being female, being older, smoking, regular medication use, history of migraine) {L-4}. [114] However, we agree with the conclusion suggested by the results of Cagnie et al. [114] that these risk factors have not been consistently predictive, and we question their clinical utility {L-5}.
5.3. Managing the risk of adverse events associated with a treatment modality and predicted by an observable risk factor (foreseen-Tx-AE)
Managing the risk of foreseen-Tx-AEs involves respecting risk factors and reacting appropriately if an adverse event is noted.
We extracted a list of factors from the risk management literature we found (more than 6,774 citations, more than 309 relevant articles), which likely constituted all reported foreseen-Tx-AEs. This list reflected our understanding of each relevant study’s determination of whether, strictly within the context of the study, the factor was a contraindication to the studied treatment modality, or merely indicated a requirement for caution {L-5}.GDC
We concluded that most of the contraindications we had listed were likely not contraindications outside of the specific study context or with patients other than the studied cases {L-5}.GDC Many of the comorbidities Côté et al. [94] reported in their survey of 1,131 Saskatchewan patients seeking chiropractic care {L-2c} were considered contraindications in this list.
These includedarthritis (26.8% of patients seeking a chiropractor only, 50% of patients seeking a chiropractor and medical physician),
cardiovascular disease (12.2%, 25%)
and significant depressive symptoms (22.5%, 38.5%).
Most of the ailments that mimic mechanical pain for which patients may seek chiropractic treatment (Section 5.1) were also considered contraindications in this list:systemic lupus arythmetous,
Bornholm disease [95] or
neoplasms [95, 96] (osteoblastoma), [97]
seizures, TIAs, [102]
Marfan syndrome, [103]
undetected septic arthritis, [104]
and cervical fractures. [105 ]
With most patients presenting with one or more of the factors we listed as a contraindication or caution, we deem that best practice rests on differentiating these factors into:(Section 5.3.1),absolute contraindications
factors requiring modification of a desired treatment modality (Section 5.3.2),
or factors suggesting caution (Section 5.3.3).
Risk-management, recommendation 20: We recommend respecting the absolute contraindications listed in Tables 3a to 3h, and the best-practice patterns of absolute contraindications, treatment modality modification and caution described in Sections 5.3.1, 5.3.2 and 5.3.3 of this CPG.
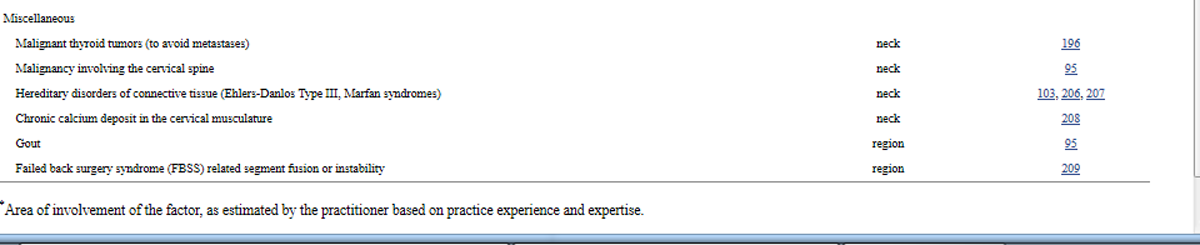
Table 3a. Risk factors that are absolute contraindications to all chiropractic cervical treatment

Table 3h. Risk factors that are absolute contraindications to cervical low-level laser therapy

5.3.1. Risk factors that are absolute contraindications
Where a risk factor signals a contraindication to an effective treatment modality, we deem that best practice comprises discontinuing care and immediate referral to emergency health services, or merely discontinuing the modality and an in-depth consideration of alternative modalities or referral.
Barring obvious medical emergencies (e.g., onset of myocardial infarct), we identified only 3 risk factors that are absolute contraindications that require immediate discontinuance of care and referral to emergency health services in the course of care:1) at least 1 of 4 signs or symptoms of neurovascular impairment (unilateral facial paresthesia, objective cerebellar signs, lateral medullary signs, visual field defects), or other signs or symptoms of neurovascular impairment with unknown cause;
2) neck or occipital pain with a sharp quality and severe intensity that is sudden and unlike any previously experienced pain (even when it is suspected the pain is of a musculoskeletal or neuralgic origin); and
3) severe and persistent headache that is sudden and unlike any previously experienced headache (even when it is suspected the pain is of a musculoskeletal or neuralgic origin) {L-5}.GDC
These are absolute contraindications to all treatment modalities {L-5}.GDCAll other risk factors we identified as absolute contraindications require merely discontinuing a specific treatment modality, and considering alternative modalities or referral.
Tables 3a to 3h list absolute contraindications based on evidence extracted from the articles we found. Tables 3a to 3h extensively incorporate the GDC’s (unpublished) practice expertise, and the Tables are thus of a Level 5 caliber.
In Tables 3a to 3h, conditions or syndromes are not expected to be diagnosed within the scope of chiropractic practice, unless otherwise noted. All other definitions cited in this CPG apply.
5.3.1.1. Risk factors that are absolute contraindications to an HVLA manipulation can also be absolute contraindications to a manipulation that is not HVLA or mobilization
Tables 3b and 3c list risk factors that are absolute contraindications to manipulation when defined as a high velocity, low amplitude (HVLA) thrust. The principle behind these absolute contraindications is that aspects of administering the manipulation (e.g., force, direction, amplitude, velocity, patient position, frequency, duration, location of treatment) may exacerbate the risk factor or other adverse events made possible by the factor (e.g., thrombi “resting” in a cervical aneurysm may be dislodged by an HVLA thrust and ascend to the brain, osteoporosis in the cervical vertebrae may weaken bone to the point where an HVLA thrust will cause fracture).
Table 3b. Risk factors that are absolute contraindications to cervical manipulation (and possibly mobilization)
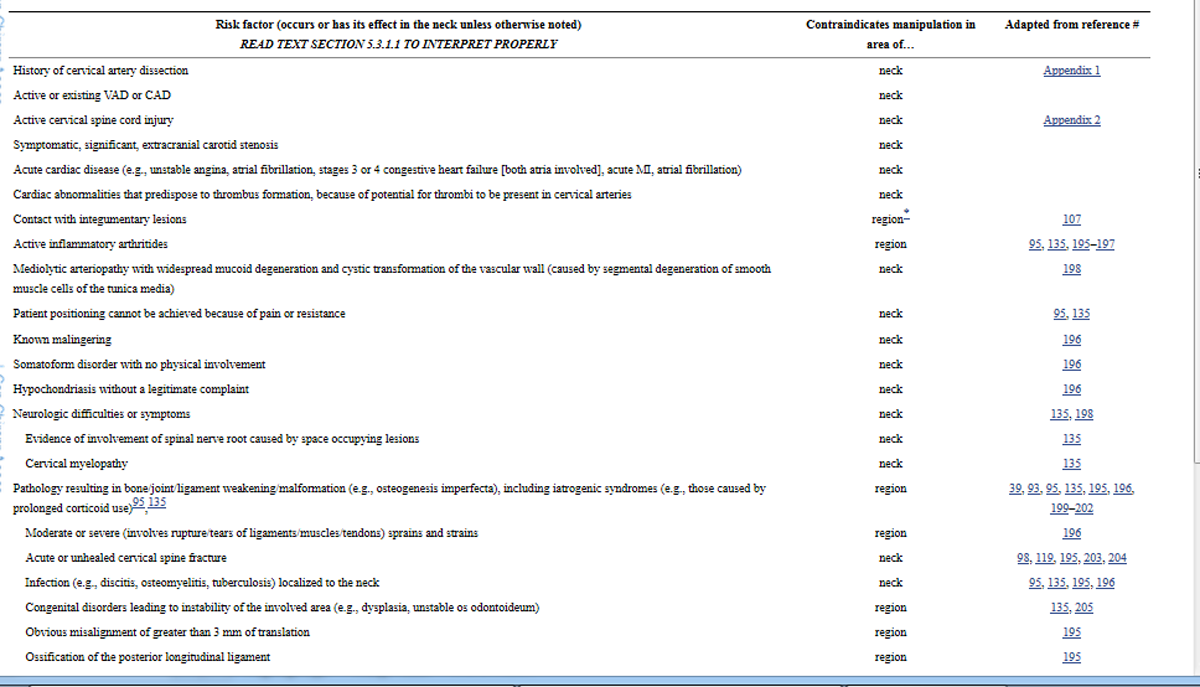
Table 3c. Risk factors that can be absolute contraindications to cervical manipulation in specific circumstances (and possibly to mobilization), or may merely require modality modification based on a learned practitioner’s practice experience and expertise
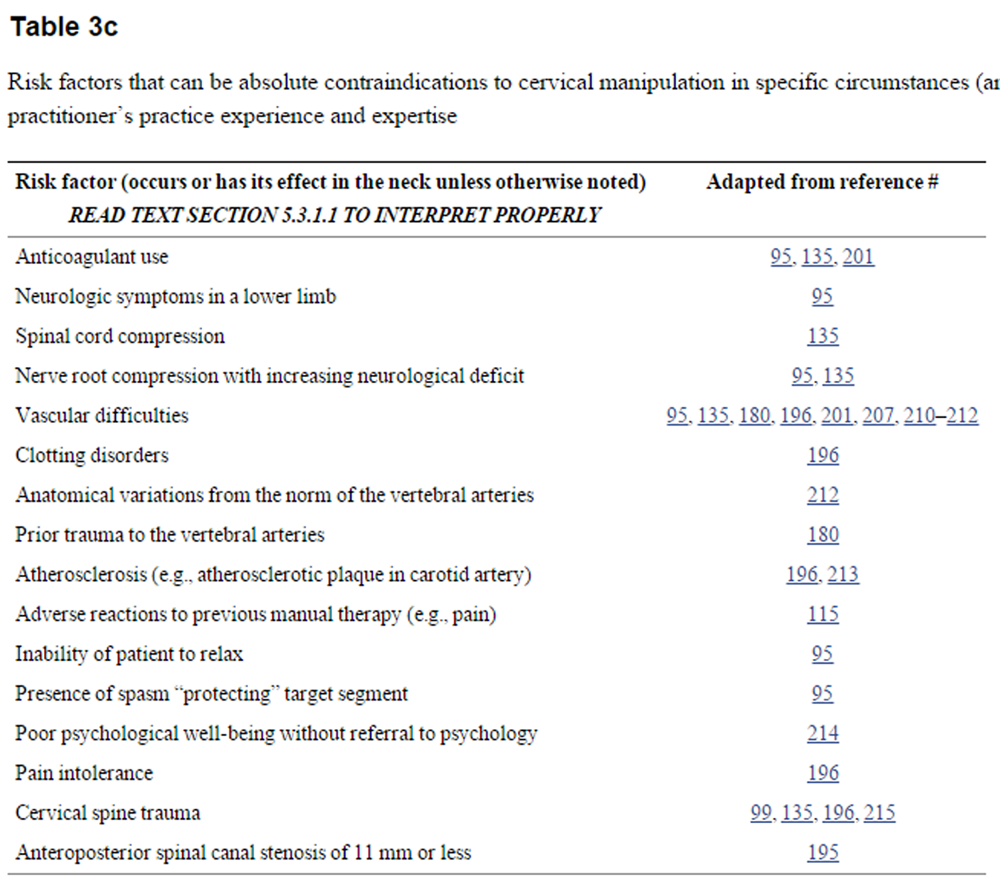
Manipulation that is not HVLA or mobilization are generally considered by the profession to be potential options where an HVLA thrust is contraindicated {L-5}.GDC However, we deem that where it is the mere movement of neck tissues that causes a risk factor to be an absolute contraindication to an HVLA thrust, manipulation that is not HVLA or mobilization are equally contraindicated by this factor {L-5}. We consider that it is a practitioner’s responsibility to acquire the practice experience and expertise to identify which factors are risks because of the mere movement of neck tissues {L-5}.
5.3.2. Risk factors that require treatment modality modifications
Compiling a list of all risk factors requiring modification of a desired treatment modality was beyond the scope of this CPG. Essentially every characteristic of a patient constitutes a factor; chiropractic uses treatment modalities specifically tailored in innumerable ways to each patient’s needs. This flexibility of treatment greatly expands treatment possibilities, allowing, for example, forceful or non-forceful maneuvers, or tangential or direct segment application.
Where a risk factor does not signal a contraindication to a treatment modality, but does signal a specific tailoring of the desired modality, we deem that best practice comprises a systematic approach to modifying the administration of the modality; the risk factor signals a “modality modification.”
Table 4 lists the aspects of core treatment modalities that we concluded should be considered by practitioners when faced with a modality-modifying risk factor, in the context of their practice experience and expertise {L-5}. Our literature search did not reveal any evidence supporting or refuting this list, and thus Table 4 incorporates solely the GDC’s (unpublished) practice expertise.
5.3.3. Risk factors that require treatment caution

Table 5 Compiling a list of all risk factors requiring caution in treatment was beyond the scope of this CPG. Essentially, caution should always be exercised as part of good practice {L-5}.GDC Thus, this CPG specifically identifies factors where we concluded there was a lack of clarity between these factors and those that indicate modality modifications or absolute contraindications {L-5}. Table 5 summarizes these “caution-indicating” risk factors.
We define caution as proceeding with a particular treatment modality only after an assessment that is as thorough as possible indicates the risk with administering this modality is not exacerbated.
In the context of good practice always involving caution, the clinical importance of the above is that in some cases this assessment will lead to the conclusion that a risk factor initially indicating caution is, in truth, an indicator for modality modification (Section 5.3.2) or a contraindication (Section 5.3.1). For example, with thorough assessment, multiple cautionindicating risk factors presenting in a patient may (together) indicate modification of, or a contraindication to the desired treatment modality.
6. Results: research recommendations
6.1. Compared groups
The GDC embarked on creating this CPG believing there was a large base of scientific studies available. The GDC has concluded from its work translating the results of these studies into a set of concise recommendations, and agrees with others’ conclusions, [120, 121] that the scientific base for chiropractic cervical treatment of neck pain is not of sufficient quality or scope to “cover” current chiropractic practice comprehensively, although this should not suggest other disciplines are more evidence based. In addition, the specificity of the studied treatments meant few studies could be directly generalized to more than a minority of patients.
We concluded that the result was an imbalance in the quantity and quality of our treatment evidence; that is, some infrequently used treatments have greater prominence than their use in practice warrants (e.g., 3-point traction, pulsed electromagnetic fields), and some frequently used modalities are not dealt with clearly and directly. Ultimately, this likely means that the clinical usefulness of the evidence and recommendations may be lower than we would like.Research, recommendation 21: We very strongly recommend the study of frequently used modalities or multi-modal treatments in studies of chiropractic treatments, to create a solid evidence base for future chiropractic.
As mentioned in Section 3, individual modalities of treatment generally differed between studies (see Tables 1 and 2). In addition, treatments were often vaguely defined (e.g., “manual therapy”). These factors dramatically reduced our ability to synthesize results across studies, and undermined the usefulness of these studies for practitioners.Research, recommendation 22: We very strongly recommend the inclusion of exact, reproducible descriptions of treatments in chiropractic studies, to ensure these are relevant and useful to the practicing chiropractor.
6.2. No-treatment comparison groups
Only 12 of the studies [22, 25–35] that supported the treatment recommendations (Section 4) included a no-treatment comparison group. The evidence that showed chiropractic treatment to be clearly better than no treatment was for very specific examples of manipulation with traction or mobilization, where the primary clinical goal was to reduce pain.
In light of the potential for self-resolution of pain complaints (Section 4.1), this weakness in a study is fatal to the utility of the study’s results in treating pain.Research, recommendation 23: Where ethical, we strongly recommend the inclusion of a no-treatment group in comparative studies of chiropractic treatments, to ensure these are useful to the front-line chiropractor.
6.3. Placebo comparison groups
In research, a placebo is generally considered an intervention that is used in across-group comparisons, to separate the direct benefits of the studied treatment from the effects of personal contact, the clinical milieu and patients’ belief in these benefits – thereby isolating the critical element of the studied treatment. Only 710, [17, 18, 21–24] of the treatment studies (Section 4) included what their authors considered to be placebo groups. Of these 7 studies, we consider only 3 studies [21, 22, 24] to have used effective placebos. The others used placebos that included more than the factors defined above (palpation, [23] physical contact with treatment device, [17, 18] infrared irradiation [10]).
This merely reflects how difficult it is to design valid placebos in a chiropractic practice laden with physical contact modalities that are, by design, tailored to each patient’s unique needs. [122] In addition, research is susceptible to including placebos that are an incremental part of the studied treatment (e.g., palpation compared with palpation plus acupuncture) [123] or an independent treatment modality (e.g., ”manual contact” with no segmental movement compared with mobilization). [22]
Pain is subjective [124, 125] and strongly influenced by psychologic, [80, 124, 126] cultural, [126] physiologic, [80, 124, 127] family, and work [127] factors, and the patient’s belief in the effectiveness of a treatment. [128] Pain is likely very susceptible to personal contact, the clinical milieu and belief in the benefits of a treatment. Several studies bear this out:
Pain intensity, pain frequency, disability, sick-leave taken, general health, distress, and risk improved after 8 weeks of placebo (twice-weekly, ”lowest level” ultrasound), and remained so at 6 and 12 months {L-4}. [58]
After 3 to 5 weeks of placebo (eighteen 30-min de-activated pulsed electromagnetic field [PEMF] “MT System” treatments, 3 to 5 per week) osteoarthritic patients experienced improved: pain, limitation of rotation and ease of ADL midway through treatment, at the end of treatment and 1 month later; tenderness midway through treatment and at the end of treatment; and pain on passive motion at the end of treatment {L-4}. [18]
Pain-related disability improved after 6 weeks of placebo (twice-weekly infrared irradiation), and remained so 6 months later {L-4}, equal to the same regimen with exercise at each treatment {L-1b}. [10]
The beneficial effect of placebo is likely illustrated in a study [66] of 3 weeks of continuous wearing of a magnetic or a non-magnetic (placebo) necklace. Both groups experienced significant improvement {L-4} on subjective evaluations of pain intensity and frequency, with no difference between the groups {L-1b}.
The above, plus our understanding of the holistic approach of chiropractic and the nature of chiropractic modalities, has led us to conclude that what is generally termed placebo effect (effect of contact, milieu, belief) is a valid and integral part of chiropractic treatment of neck pain. This suggests that best practice includes considering contact, milieu and belief as an active treatment modality that can be refined. One repercussion is that future chiropractic research should consistently and specifically examine this.
Research, recommendation 24: We very strongly recommend the inclusion of well-delineated treatment groups that isolate placebo effects (effect of personal contact, the clinical milieu and patients’ belief in the benefits of a treatment) in comparative studies of chiropractic treatments, to create a solid evidence base for future chiropractic.
7. Implementing the recommendations
The following information tools are presented to aid in implementing this CPG:a summary Table of the evidence-based cervical pain benefits of chiropractic treatment (Appendix 3);
algorithms that illustrate the process of individualizing care (Figure 1) and managing the risk of dissection (Figure 2, discussed in Appendix 1);
and a clinical question and answer list (Section 7.1 below).As well, this CPG is reinforced by the extensive dissemination, implementation, evaluation, and revision activities described in the development, dissemination, implementation, evaluation, and revision plan (DevDIER). [6]
Figure 2 Cervical spine manipulative therapy; decision algorithms coping with the theoretic risk of dissection
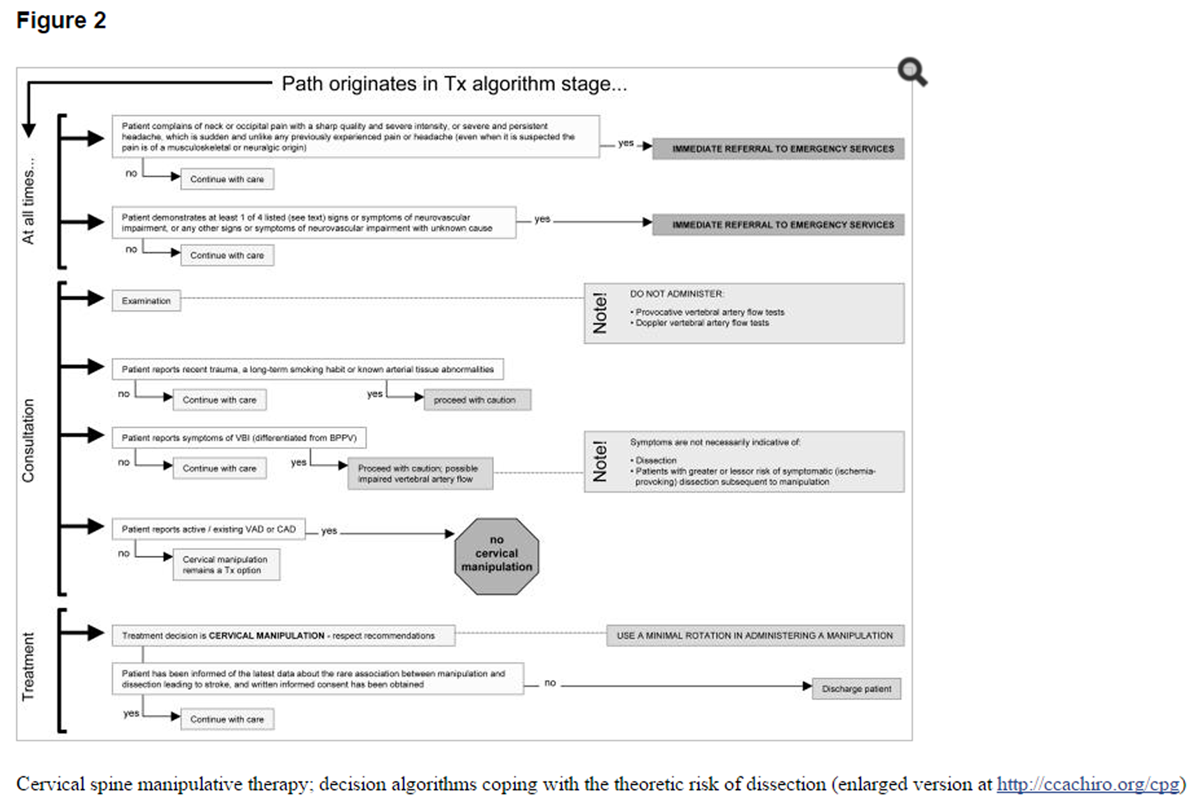
For researchers, a set of CPG development Q&As is included in the first and second Response to profession-wide feedback about the chiropractic clinical practice guideline: evidence-based treatment of adult neck pain not due to whiplash documents available at The CCA web-site.
7.1 Question and Answer List
How will the guidelines affect my care of, and my recommendations to patients?
In the context of supportive, maintenance, or wellness care, and preventive, intensive or stabilizing care, this CPG indicates modification and discontinuance points in care and assessment activities, and the evidence-based options to consider at each point.
Do I have to follow the guidelines “to the letter”?
Although CPGs can link the best available evidence to good clinical practice, they are only one component of a well-informed approach to providing good care. CPGs are not standards that dictate practice, but rather guides and tools for chiropractors and their patients. Each CPG The CCA/CFCRB-CPG is developing and deploying will reflect a well-substantiated consensus about treatment options based on current available evidence. As such, although the CPG is not a standard, it is reasonable to expect chiropractors will need to justify interventions outside this consensus.
If a treatment is not present in the guidelines, does that mean I should not use it?
If a treatment is not mentioned in the guidelines, it is because we did not find any clinically important evidence to comment about it. You should use your clinical judgement and the patient’s best interest to decide whether and how to use the treatment.
I have a subluxation-based practice; what use are neck pain guidelines to me?
The sequence of diagnosis (or assessment leading to diagnosis), treatment and reassessment are relevant to your management of the patient regardless of your focus. However, within a subluxation-based practice, some of the clinical outcomes you rely on to assess your patient may differ from those reported in the literature (and hence also in this CPG), and your assessment may be termed an “analysis” that parallels establishing a separate, formal diagnosis. The priority topics for future CPGs listed in the development, dissemination, implementation, evaluation, and revision plan (DevDIER)6 are directed by the stakeholders from each region of Canada.
Do these guidelines mean that every patient must have 10 to 12 treatments before reassessment?
No. Any significant change in the patient's condition demands reassessment.
Should I apply this guideline to my patients who have a whiplash-associated disorder (WAD)?
No. A separate guideline for whiplash-associated disorders (including pain) is planned.
What if my patient has associated comorbid conditions? Should I use this guideline?
While respecting the guideline recommendations, if the comorbidity falls within your scope of practice, use your clinical judgement and knowledge of the patient’s best-interest to determine treatment. If the comorbidity falls outside your scope of practice, make sure that the patient is seen by the appropriate professional.
What should I do if my treatment does not fit well with the categories that you describe?
The sequential process of diagnosis (or assessment leading to diagnosis), treatment and reassessment does not change. The specific treatments chosen must be adapted to each patient, reflecting the idiosyncratic nature of pain, using your clinical judgement and knowledge of the patient’s best interest.
How can I evaluate how successful the recommendations are?
In the day-to-day clinical context, the main clinical effects of this CPG’s recommendations can be evaluated using numerical pain scales (0–10 [unbearable pain]) or visual analogue pain scales (0–100 mm [max pain]), and goniometric ROM assessment tools. One study [129] determined that a change of 3 points on a 0–10 pain scale (i.e., an 11-point scale) indicated clinically important improvement {L-2c}, corroborating other work in non-chiropractic patients with acute or chronic pain at various locations and from various causes, [130-133] although another study [134] about chronic neck pain suggested that a 1-point change was sufficient {L-5}. Consistent use of the same scales or tools across one patient’s treatments, or across all patients will help in understanding the clinical effect of this CPG.
As well, outcome measurements common in the literature (Table 9 in technical version at http://ccachiro.org/cpg) [135] are appropriate to assess the impact of the treatment recommendations, and monitor patients’ progress. Recent advances include multilingual adaptations of English pain scales (including Chinese, [10] French [136] and Turkish [137]), and attempts to predict the impact of treatment using a 17-variable model [139] or the prognostic indicators of age, concomitant low back pain, [78, 138] cycling, or psychological distress. [78]
The predictors of functional detriment can also be useful monitoring variables apart from pain per se; Luo et al. [140] reported that the predictors in patients with neck pain were (in order of importance):neck pain,
work status,
back pain,
education,
stress,
arm or shoulder pain,
depression,
smoking,
and anxiety {L-2c}.For those exploring the boundaries of practice, reports such as those of White et al [141] and Childs et al. [142] can provide leading-edge methods of monitoring based on experimental pain-classification systems. Finally, for researchers, at least one study [8] has attempted to synthesize a rigorous method to tease out clinically important outcomes from those that are merely statistically significant.
Can I be sued more easily if I don’t follow this guideline?
This guideline is not a standard tacitly “set” by others or a standard that is set by your regulatory board. This guideline describes treatment practices directly supported by the current evidence. This guideline clearly states that, because of the lack of studies, it does not cover the full extent of chiropractic treatment related to the cervical spine in dealing with neck pain. Thus, the GDC considers that this guideline cannot be used to legitimately limit practice, even though not all practice elements are covered in this CPG.
How are you going to ensure that these guidelines won’t be abused by practitioners or third-party payers?
We cannot.
Where can I find a definition of what chiropractic is, or does, in the guideline?
Chiropractic treatment of neck pain is clarified in Section 1.1. In addition, the principles of chiropractic are summarized in the Appendix 1 of the development, dissemination, implementation, evaluation, and revision plan (DevDIER). [6]
I’m concerned about the lack of evidence supporting my treatment. What can the profession do about this?
We very strongly recommend that the profession continue to invest effort and money in high-quality, clinically applicable research.
Why do we need this guideline? With other guidelines already in place, why do we need these guidelines?
This CPG fills a gap in the foundation of evidence-based chiropractic practice by recommending only treatments that are substantiated in the literature. This CPG also draws precise distinctions usually lacking in other chiropractic CPGs, such as separating whiplash and non-whiplash patients. In addition, it was developed and is being deployed in accordance with evidence-based principles; this is discussed at length in the associated document entitled the development, dissemination, implementation, evaluation, and revision plan (DevDIER). [6]
Does this CPG’s limitation to those over 18 years of age mean that chiropractic treatment of those under the age of 18 is inappropriate?
No. This guideline does not intend to restrict chiropractic care of neck pain to those over 18. Our recommendations are based on an analysis of research about people older than 18 years of age, and thus no recommendations for or against the treatment of those under 18 are made.
The evidence is largely dealing with manipulation based on a fixation model with little to no consideration of the neurologic consequences – was neurology of the syndrome ignored?
We understand pain to be a neurologic symptom. Additionally, it should be noted that all reported outcomes were included in the evidence extractions; extractions were not purposefully limited to non-neurologic outcomes (detailed outcomes in technical version of this CPG at http://ccachiro.org/cpg).
The dissection evidence suggests that we cannot be confident that a positive (impaired) provocative “flow test” is predictive of impaired flow, but even so, what is the relationship between a positive test and the risk for dissection associated with manipulation?
Some reports suggested that vertebrobasilar insufficiency (VBI) tests were clinically useful {L-5}. [143] However Haldeman et al. [144] concluded, after using a physical examination technique that included placing the neck in extension and rotation (a commonly advocated pre-manipulative test of the patency of the vertebral arteries), that they were unable to identify any factors that indicated a greater risk for cerebral ischemia after manipulation {L-4}.
Carey [145] and, later, Magarey et al. [146] echoed this theme, suggesting that pre-manipulative tests do not identify all patients at risk (corroborating the ambiguity of results from research about alterations of blood flow associated with insufficiencies within the vertebrobasilar system) and that there is no method for assessing the anatomy of the in-vivo vertebral artery {L-5}. Licht et al. [147] affirmed the uncertainty of functional tests, stating that the literature indicated a test could be negative in the presence of a vertebral artery occlusion, and suggested a negative test did not preclude the occurrence of cerebrovascular “accidents” (accident undefined) {L-5}.
In sum, although some reports suggested otherwise {L-5}, [143] other reports [144-148] suggested that it is not now possible to identify physical factors that indicate a greater {L-4} [144] or lessor {L-5} [147] risk of experiencing a symptomatic (ischemia-provoking) dissection associated with manipulation.
8. Conclusion
Well executed research, and the high caliber evidence this can produce, can objectively quantify outcomes subjectively observed in daily practice. This research can be used to confidently predict the response to a treatment for a clearly delimited problem. Thus, evidence-based practice permits the administration of interventions that are objectively known to produce a favorable clinical response in patients sharing the same problem.
This CPG examined the evidence for treatments designed to alleviate neck pain. Pain is fundamentally qualified by its multi-factor origin and the idiosyncratic way it demonstrates itself as a set of symptoms. Thus, we agree with at least one literature review, [125] examining the use of research in chiropractic practice, that it is likely impossible for the subjects of any studied group to be experiencing the “same” pain condition – homogeneously susceptible to a tested treatment {L-5}.
One repercussion is that a study assessing the impact of a treatment’s effectiveness is unlikely to satisfactorily direct good practice on its own, because it is likely other patients’ pain (and thus treatment needs) will differ from the studied sample. The GDC concluded this is a unique confounder in using evidence-based chiropractic to treat pain.
Faced with this, we consider that the most effective method of using the scientific literature in practice includes the application of “individualizing” algorithms. These illustrate the optimal decision path used to tailor objectively-determined treatments, which benefit the average patient, to particular patients – the care that is ultimately offered is thereby unique to each patient.Research recommendation 25: We recommend, in future research about the chiropractic treatment of pain, a concerted effort to develop evidence-based algorithms that illustrate the optimal decision path used to individualize care; our recommended method to incorporate evidence about treatments that are objectively known to benefit the average patient.
Table 3d Risk factors that are absolute contraindications to cervical exercise
Table 3e Risk factors that can be absolute contraindications to cervical exercise in specific circumstances, or may merely require modality modification based on a learned practitioner’s experience and expertise
Table 3f Risk factors that are absolute contraindications to cervical traction
Table 3g Risk factors that can be absolute contraindications to cervical traction in specific circumstances, or may merely require modality modification based on a learned practitioner’s experience and expertise
Appendix 1: Spotlight on dissection (more detail in Appendix 16)
In this CPG, unless otherwise noted: dissection means cervical (carotid or vertebral) artery dissection. Where not noted, the results in Appendix 1 are of Level 5 caliber.
The small sample sizes of the clinical trials supporting this CPG are unlikely to have encountered the rare adverse event of a cervical artery disturbance following manipulation, ranging from arterial insufficiency to vertebral artery dissection. [112, 144, 147, 149–155] We consider dissection to be independent of stroke; dissection was differentiated from stroke in several articles, and it was reported that not all dissection led to stroke, and not all stroke was the result of dissection. [148, 149, 156–160]
The term “spontaneous” was used often in the literature to describe dissections “without a cause.” However, we agree with several authors161 that it is unlikely that dissection occurs spontaneously, and we do not consider this distinction to be clinically useful. We use the term idiopathic (i.e., unknown cause) to denote dissections reported in the literature as being spontaneous or of unknown cause.
The evidence suggests that manipulation is not associated with carotid artery dissection (CAD) {L-4} [149, 153] {L-5}, [162, 163] and supports our consideration that there is no anatomic basis for an association between manipulation and CAD {L-5}.
The remainder of Appendix 1 deals with results reported for dissection in general or vertebral artery dissection (VAD) only, unless discussion of the carotid or basilar arteries is warranted. Some of the literature we retrieved considered “vertebrobasilar” dissection without distinguishing which artery the dissection was in. This imprecision weakens the usefulness of these reports {L-5}.GDCResearch, recommendation 26. In studies of the association between manipulation and dissection, we recommend distinction between the carotid, vertebral and basilar arteries to ensure findings are relevant to drawing conclusions. In addition, we recommend a focus on VAD in future studies of the association between manipulation and dissection to ensure findings are as relevant as possible to drawing conclusions.
1.1. Incidence of dissection or VAD or VAD-related stroke associated with manipulation
The evidence [149, 156, 161, 164–167] suggests that VAD following manipulation occurs very rarely (1 per million manipulations [Appendices 16a, 16b in the technical version of this CPG available at http://ccachiro.org/cpg]), and that most VAD arise from ordinary ADL, but none of the evidence is of sufficient quality to support objective conclusions {L-5}.GDC The core difficulty appears to be that if the rate of manipulation-associated VAD is 1 per million manipulations, 12 million manipulations would have to be studied to reach a scientifically confident conclusion {L-5} [167] – a logistically impractical proposition at this time {L-5}.GDC
1.2. Role of manipulation
The evidence suggests that more manipulations do not mean more complications {L-4} [148] and manipulation does not exacerbate the outcome of dissections {L-4}. [149] However, the evidence also suggests there is a statistical association between manipulation and bilateral VAD [149, 153, 156, 168] (with VAD symptoms moments to 10 days later), but not unilateral VAD {L-4}, [149] and between manipulation and VAD within 30 days {L-4}. [153]
Contrasting one study {L-4} [149] but supported by another {L-4}, [168] we deem that it is unlikely (based on anatomic principles) that manipulation can be responsible for bilateral VAD but not unilateral {L-5}, and thus an association between manipulation and bilateral VAD is likely not clinically important {L-5}.GDC
Also, as suggested by Hufnagel’s results {L-4}, [156] it is unlikely (based on physiologic principles) that a manipulation can be responsible for a VAD more than 3 weeks later {L-5};GDC thus, a 30-day association between manipulation and VAD is likely not clinically important {L-5}.GDC
Considering the possibility that manipulation may cause dissection directly, we strongly agree with Sackett’s [167] suggestion that none of the available evidence shows causation. However, the evidence does suggest that patients with impaired vertebral artery flow may seek manipulative care for symptoms of these impairments {L-4} [148] {L-5}. [149, 150, 154]Research, recommendation 27. In studies of the association between manipulation and dissection, we very strongly recommend discriminating between realistic and unrealistic post-manipulation periods to ensure findings are relevant to drawing conclusions.
1.3. Managing the issue of dissection
Irrespective of cause, it appears there may be a remote risk of VAD occurring subsequent to manipulation {L-5}.GDC We agree with Magarey et al. [146] that this risk is a clinical factor to be managed, much as drug side effects are in pharmacotherapy and anesthetic adverse events are in surgery {L-5}. This risk does not suggest that manipulation should be excluded from the armamentarium of practice as several authors [169, 170] have hinted {L-5}.GDC
The diagnosis (or assessment leading to diagnosis), treatment and reassessment steps of the decision algorithm in Figure 1 guided our description of the factors relevant to managing the risk of dissection:informed consent (Section 1.3.1 of Appendix 1);
pre-disposing history (Section 1.3.2 of Appendix 1);
predisposing factors in physical assessment (Section 1.3.3 of Appendix 1);
and the occurrence of dissection (Section 1.3.4 of Appendix 1).
1.3.1. Informed consent
The evidence suggests that informed consent is as important for younger and healthier patients as it is for others, because these patients bear a disproportionately high burden of dissection-related stroke {L-3b} [157] {L-4}. [153]Risk-management, recommendation 28 We very strongly recommend obtaining informed consent based on current evidence, and respecting the 3 sequential steps in the decision algorithm (Figure 1) – diagnosis (or assessment leading to diagnosis), treatment, reassessment – when caring for any patient.
1.3.2. Predispositions to dissection in a patient’s history
Trauma The evidence suggests that a recent history of trauma may predispose to dissection {L-4}. [149]
Lifestyle The evidence suggests that among lifestyle factors, smoking may predispose to VAD [149] among those who are vulnerable to tobacco’s negative, cumulative vascular effects [153] {L-4}. The factor governing this vulnerability is unknown {L-5}.GDC
Sex The evidence suggests that a person’s sex is not a determinant of non-traumatic VAD {L-4}. [168]
Non-vascular illnesses The evidence suggests that a history of non-vascular illness is not a definitive determinant of VAD {L-4}. [153, 156]
Vascular pathologies (excluding dissection or impaired vertebral artery flow) The evidence suggests that a history of vascular pathologies is not a definitive determinant of VAD {L-4}, [156] although it may be suggestive of risk; of the vascular pathologies that have been proposed as risks for dissection, the most clearly demonstrated have been tissue abnormalities of the cervical arteries {L-3b} [171] {L-4}, [172] but the link between these abnormalities and dissection, and the practicality of pre-manipulatively identifying these remains elusive {L-5}.GDC Patients predisposed to dissection by these factors may seek chiropractic care for neck pain {L-5}.GDC
Hyperhomocysteinemia and cervical artery dissection The evidence suggests that hyperhomocysteinemia is associated with CAD - [173] or dissection - [174] related stroke {L-3b}, but not CAD per se {L-3b}, [173] contradicting at least one extensive review. [175] The role of 5, 10-methylenetetrahydrofolate reductase (MTHFR, a deficient enzyme form associated with hyperhomocysteinemia) is ambiguous {L-5};GDC it is not associated with dissection-related stroke (a mix of VAD and CAD) {L-3b} [174] or non-dissection stroke {L-5}, [175] but it is associated with CAD-related stroke {L-3b}. [174] Hyperhomocysteinemia may be associated with VAD, but results are too inconclusive at this time for this to have clinical importance {L-5}.GDC
Historical predispositions; caution or contraindication? Together, the current evidence [144, 148–150, 153, 156, 158, 169, 171–179] suggests that none of the predisposing factors hypothesized in the literature definitively predict a dissection-related “cerebrovascular ischemic event” {L-4}, [144] and, therefore, none is a contraindication to manipulation {L-5}.GDC However, other evidence suggests that caution should be exercised in treating patients with a history of trauma {L-4}, [149] a smoking habit {L-4} [149] (especially among patients vulnerable to tobacco’s negative, cumulative vascular effects {L-4}), [153] or known arterial tissue abnormalities {L-3b} [171] {L-4}. [172]Risk-management, recommendation 29 We recommended caution in treating a patient with trauma, a smoking habit or known arterial tissue abnormalities to manage the risk for dissection, but the evidence does not warrant that these be contraindications to manipulation.
1.3.3. Noting predispositions during physical examination; impaired vertebral artery flow
Doppler identification of impaired vertebral artery flow The evidence of an extensive review of the literature suggests that a positive (impaired) Doppler “flow test” does not predict impaired vertebral artery blood flow {L-5}. [146]
Provocative identification of impaired vertebral artery flow Pre-manipulative vertebral artery function tests to identify patients with impaired flow have been part of practice knowledge since Smith and Eldridge introduced a test in 1962. [147] Since then, several tests have been developed, and the vertebral artery flow effects [180] of these are moderately well understood: Barré-Leiou’s sign test, George’s cerebrovascular craniocervical functional test, Maigne’s test, Hautant’s test, Underberg’s test, Dix-Hallpike maneuver (also known as Nylen’s or Barany’s maneuver), and deKleyn’s test. [147] The most common is deKleyn’s test. [147]
The evidence suggests that a positive (impaired) provocative “flow test” rarely indicates changes in vertebral artery blood flow {L-4} [147] and, consequently, deKleyn’s test is neither sensitive nor specific. Thus a positive test should not be an absolute contraindication to manipulation {L-5}. [147] Other results {L-4} have also suggested pre-manipulative flow testing is unlikely to identify patients with flow impedances {L-5}. [146]
Observational identification of impaired vertebral artery flow (overt symptoms of VBI) We agree with the evidence defining the signs and symptoms of VBI (nystagmus, nausea, numbness, diplopia, drop attacks, dysphagia, dysarthria, ataxia), [181] as differentiated from benign paroxysmal positional vertigo (BPPV) {L-5}. [146] It is our understanding that these differentiated signs and symptoms may indicate the vertebral artery has an anatomic predisposition to dissection, has a narrow safety margin for the impairment of flow, or is dissected {L-5}. This suggests caution in proceeding with treatment when overt signs or symptoms of VBI are noted {L-5}.GDCRisk-management, recommendation 30. We recommend an assessment for signs and symptoms of unprovoked VBI (differentiated from BPPV) to identify the possibility of impaired vertebral artery flow (signs and symptoms are: nystagmus, nausea, numbness, diplopia, drop attacks, dysphagia, dysarthria, and ataxia), because we recommend caution in treating a patient with suspected impairment of flow. However, the evidence does not warrant this being a contraindication to manipulation.
Risk-management, recommendation 31. We do not recommend an assessment for signs or symptoms of unprovoked VBI (differentiated from BPPV) to identify the presence of dissection, or to identify patients with greater or lessor risk of symptomatic (ischemia-provoking) dissection subsequent to manipulation; the assessment lacks predictive value.
Risk-management, recommendation 32. We do not recommend Doppler or provocative pre-manipulative vertebral artery function tests (e.g., deKleyn’s test) to identify impaired vertebral artery flow, the presence of dissection, or patients with greater or lesser risk of symptomatic (ischemia-provoking) dissection subsequent to manipulation; the assessment lacks predictive value.
1.3.4. Dissection in the chiropractic clinic
Identifying the occurrence of dissection before or during a visit We agree with at least 3 other reports [153, 162, 182] that manipulation is contraindicated for patients with an active or existing VAD or CAD, although there have been uneventful case reports of manipulation being used with benefit in treating patients who have “recovered” from dissections. [169, 183]Risk-management, recommendation 33. We do not recommend manipulation for patients who present with active or existing VAD or CAD.
This is relevant because impaired vertebral artery flow can exhibit symptoms [149, 150, 154] that may lead to the patient seeking chiropractic care {L-5};GDC VAD and CAD are associated with neck-pain and headache {L-4}, [99, 149] although asymptomatic ICAD has been reported {L-5}. [162]
The evidence suggests that symptoms of impaired vertebral artery flow can lead patients to seek chiropractic care {L-4}; [149, 156] and that in most patients with dissection-precipitated cerebral ischemia, the event of dissection [161] – or the presence of dissection leading to stroke [148, 153] (specifically VAD) [149, 168, 184] – is associated with neck or occipital pain with a sharp quality and severe intensity, or a severe and persistent headache, which is sudden and unlike any previously experienced pain or headache {L-4}. However, this pain may not be clearly distinguished from musculoskeletal or neuralgic pain {L-5}. [144, 153]Risk-management, recommendation 34. We recommend caution in treating a patient who reports a recent (but not ongoing) neck or occipital pain with a sharp quality and severe intensity, or a severe and persistent headache, which was sudden and unlike any previously experienced pain or headache (even when it is suspected the pain was of a musculoskeletal or neuralgic origin).
Risk-management, recommendation 35. We recommend immediate discontinuance of treatment and referral to emergency health services when a patient complains in the course of care (diagnosis [or assessment leading to diagnosis], treatment, reassessment) of neck or occipital pain with a sharp quality and severe intensity, or a severe and persistent headache, which is sudden and unlike any previously experienced pain or headache (even when it is suspected the pain is of a musculoskeletal or neuralgic origin).
Mitigating the harm of VAD; a stroke The full extent of identifying and managing the risk of stroke subsequent to VAD is beyond the scope of this CPG. However, we conclude that any sign of VAD should precipitate immediate referral to emergency health services {L-5}.GDC
The evidence suggests that progression from VAD to stroke may be indicated by specific neurovascular signs or symptoms:[168]unilateral facial paresthesia,
objective cerebellar signs,
lateral medullary signs,
and visual field defects {L-4}.
Vertigo is also cited, [168] but we consider this to be a prevalent symptom of various less alarming syndromes, such as those with inner ear involvement {L-5}.GDC We agree with the text by Goetz [185] that provides a more complete list of signs and symptoms {L-5}.
Risk-management, recommendation 36. We recommend immediate discontinuance of treatment and referral to emergency health services when, in the course of care (diagnosis [or assessment leading to diagnosis], treatment, reassessment), a patient demonstrates at least 1 of 4 signs or symptoms of neurovascular impairment (unilateral facial paresthesia, objective cerebellar signs, lateral medullary signs, visual field defects) or other signs or symptoms of neurovascular impairment with unknown cause, irrespective of complaints of neck or head pain. In addition, we recommend immediate investigation for these 4 signs or symptoms of neurovascular impairment whenever a patient demonstrates vertigo – if none are present, we recommend caution in treating the patient because of the continued risk for neurovascular impairment.
1.3.5 Recommendation summary
We recommend management of the risk of VAD subsequent to manipulation in keeping with the decision algorithm of Figure 2, to maximize the demonstrated benefits and minimize the theoretic harms associated with manipulation.
We recommend a risk-management approach that includes:
1) informed consent;
2) caution in treating a patient with trauma, a smoking habit, or known arterial tissue abnormalities;
3) caution in treating a patient with signs or symptoms of vertebro-basilar insufficiency differentiated from benign paroxysmal positional vertigo;
4) caution in treating a patient reporting a recent (but not ongoing) neck or occipital pain with a sharp quality and severe intensity, or a severe and persistent headache, which was sudden and unlike any previously experienced pain or headache (even when it is suspected the pain was of a musculoskeletal or neuralgic origin);
5) no manipulation for patients who present with active or existing VAD or CAD;
6) immediate discontinuance of treatment and referral to emergency health services when a patient complains in the course of care of neck or occipital pain with a sharp quality and severe intensity, or a severe and persistent headache, which is sudden and unlike any previously experienced pain or headache (even when it is suspected the pain is of a musculoskeletal or neuralgic origin);
7) immediate discontinuance of treatment and referral to emergency health services in the course of care when a patient demonstrates at least 1 of 4 signs or symptoms of neurovascular impairment (unilateral facial paresthesia, objective cerebellar signs, lateral medullary signs, visual field defects) or other signs or symptoms of neurovascular impairment with unknown cause, irrespective of complaints of neck or head pain.
In addition, we recommend immediate investigation for these 4 signs or symptoms of neurovascular impairment whenever a patient demonstrates vertigo – if none are present, we recommend caution in treating the patient because of the continued risk for neurovascular impairment. We do not recommend provocative vertebral artery function tests. We define caution as proceeding with a particular treatment modality only after an assessment that is as thorough as possible indicates the risk with administering this modality is not exacerbated.
Appendix 2: Stroke; an adverse event of the rotation component of manipulation?
In this CPG, unless otherwise noted: dissection means cervical (carotid or vertebral) artery dissection. |
Stroke has been associated with chiropractic manipulation in the news media for some time. Reports associating chiropractic treatment with stroke rest on accepting a hypothetical 5-link chain:
1) manipulation may be associated with dissection or cervical artery intima injury with
2) possible clot formation at the site of injury,
3) from which emboli or thrombi may be shed,
4) which in-turn may occlude arteries feeding the brain,
5) possibly causing a full stroke. However, not all dissection leads to stroke, and not all stroke is the result of dissection.148,149,156–160
The evidence suggests that rotary manipulation is very popular but its role in precipitating cerebrovascular (CV) accidents remains unclear {L-5}.GDC On one hand, no particular manipulation technique predicts stroke {L-4}, [148] and no particular technique is disproportionately prevalent among manipulation-associated stroke patients {L-4}. [156]
On the other hand, rotation techniques applied to the upper-cervical spine are more associated with CV accidents than non-rotation or rotation to the mid- or lower-cervical spine {L-4}, [186] and upper-cervical manipulations of any type are 4 times more associated with cerebrovascular incidents (CVIs, transitional signs of possible CV accident) than lower-cervical manipulations {L-4}. [187]
Risk-management, recommendation 37. Although the role (alleviating, neutral, exacerbating, causative) of manipulation in CV accidents is unclear, we recommend using a minimal rotation in administering an upper-cervical spine manipulation until better information is available, to maximize the benefit to harm balance.
Risk-management, recommendation 38. Extrapolating from our recommendation to use a minimal rotation in administering an upper-cervical spine manipulation, we also recommend the use of a minimal rotation in administering any modality of upper-cervical spine treatment.
2.1. Hyperhomocysteinemia as a risk for stroke or TIA
The evidence that elevated plasma homocysteine concentrations (and the associated, reduced plasma concentrations of folate, vitamin B12 or vitamin B6 {L-1b} [192] {L-2c}) [188-191] correlates with an increased risk of stroke is not conclusive {L-5}GDC – although some evidence directly suggests that specifically sub-optimal levels of vitamin B6, and not hyperhomocysteinemia, are associated with hypothesized increases in the risk of stroke or TIA {L-5}. [193] Although the evidence is inconclusive, this suggests that patients presenting with known hyperhomocysteinemia should be treated with caution {L-5},GDC but not that an assay of homocysteine levels should be part of an assessment with healthy patients.
2.2. Stroke; predictors of this adverse event subsequent to manipulation
The link between rotation manipulation and stroke is tenuous (above), and at least one study [194] has reported that the maximal angular difference between the head and neck or trunk in the course of a rotation manipulation does not exceed the active physiologic range of motion {L-4}.
However, we concluded that the risk factors for stroke should be accounted for in best practice patterns (Section 5). See Tables 3a to 3h for all identified risk factors that are absolute contraindications, Table 5 for all identified factors that require treatment caution, and Table 6 for stroke-specific risk factors that require treatment modality modification {L-5}. Section 5.3.2 clarifies the clinical meaning of modality modification.
Table 6 Risk factors for stroke requiring modification of manipulation or mobilization
Higher than optimal blood pressure (120/80 mm Hg [systolic/diastolic], particularly > 140/90 mm Hg) Guez M, Hildingsson C, Nilsson M, Toolanen G. Cote P, Cassidy JD, Carroll L. Waalen DP, White TP, Waalen JK. Eliot DJ. Barnsley L, Lord SM, Bogduk N. The Canadian Chiropractic Association and the Canadian Federation of Chiropractic Regulatory Boards Clinical Practice Guideline Development Initiative (The CCA/CFCRB-CPG) development, dissemination, implementation, evaluation, and revision (DevDIER) plan. Oxford Centre for Evidence-based Medicine. Hurst H, Bolton J. Gert Bronfort DC, PhD; Roni Evans DC; Brian Nelson MD; Peter D. Aker DC, MSc; et al. Chiu TT, Lam TH, Hedley AJ. Cassidy JD, Lopes AA, Yong-Hing K. Lynton G. F. Giles; Reinhold Muller Jordan A, Bendix T, Nielsen H, Hansen FR, Host D, Winkel A. Hoving JL, Koes BW, de Vet HC, van der Windt DA, Assendelft WJ, van
Mameren H, et al. Korthals-deBos IBC, et al. Taimela S, Takala EP, Asklof T, Seppälä K, Parviainen S. Foley-Nolan D, Barry C, Coughlan RJ, O’Connor P, Roden D. Trock DH, Bollet AJ, Markoll R. Brodin H. Allan M, Brantingham JW, Menezes A. Gur A, Sarac AJ, Cevik R, Altindag O, Sarac S. Sterling M, Jull G, Wright A. Ceccherelli F, Altafini L, Lo Castro G, Avila A, Ambrosio F, Giron GP. Smania N, Corato E, Fiaschi A, Pietropoli P, Aglioti SM, Tinazzi M. Harrison DE, Cailliet R, Harrison DD, Janik TJ, Holland B. Harrison DE, Harrison DD, Betz JJ, Janik TJ, Holland B, Colloca CJ, et al. Viljanen M, Malmivaara A, Uitti J, Rinne M, Palmroos P, Laippala P. Gam AN, Warming S, Larsen LH, Jensen B, Høydalsmo O, Allon I, et al. Horneij E, Hemborg B, Jensen I, Ekdahl C. Hanten WP, Barrett M, Gillespie-Plesko M, Jump KA, Olson SL. Revel M, Minguet M, Gergoy P, Vaillant J, Manuel JL. Takala EP, Viikari-Juntura E, Tynkkynen EM. Lundblad I, Elert J, Gerdle B. Levoska S, Keinänen-Kiukaanniemi S. Wang WTJ, Olson SL, Campbell AH, Hanten WP, Gleeson PB. Wolf PA. Kisner C, Colby LA. Hing WA, Reid DA, Monaghan M. Cassidy JD, Quon JA, LaFrance LJ, Yong-Hing K. Vernon HT, Aker PD, Burns S, Viljakaanen S, Short L. Pikula JR. Yurkiw D, Mior S. Wood TG, Colloca CJ, Mathews R. Cilliers K, Penter C. Moodley M, Brantingham JW. van Schalkwyk R, Parkin-Smith GF. Wallace HL, Jahner S, Buckle K, Desai N. Parkin-Smith GF, Penter CS. Rogers RG. Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Ahlgren C, Waling K, Kadi F, Djupsjobacka M, Thornell LE, Sundelin G. Berg HE, Berggren G, Tesch PA. Kadi F, Ahlgren C, Waling K, Sundelin G, Thornell LE. Highland TR, Dreisinger TE, Vie LL, Russell GS. Randløv A, Ostergaard M, Manniche C, Kryger P, Jordan A, Heegaard S, et al. Ekberg K, Björkvist B, Malm P, Bjerre-Kiely B, Axelson O. Waling K, Sundelin G, Ahlgren C, Järvholm B. Kjellman GV, Öberg BE. Vasseljen O, Jr, Johansen BM, Westgaard RH. Zylbergold RS, Piper MC. Klemetti M, Santavirta N, Sarvimaki A, Bjorvell H. Esenyel M, Caglar N, Aldemir T. Özdemir F, Birtane M, Kokino S. Hagino C, Boscariol J, Dover L, Letendre R, Wicks M. Ylinen J, Ruuska J. Hong CZ, Lin JC, Bender LF, Schaeffer JN, Meltzer RJ, Causin P. Bovim G, Schrader H, Sand T. Verhoef MJ, Page SA, Waddell SC. Falla D. Vogt MT, Simonsick EM, Harris TB, Nevitt MC, Kang JD, Rubin SM, et al. Moseley GL. Fishbain DA, Cutler RB, Cole B, Lewis J, Smets E, Rosomoff HL, et al. Clair D, Edmondston S, Allison G. Peterson C, Bolton J, Wood AR, Humphreys BK. Koes BW, Bouter LM, van Mameren H, Essers AH, Verstegen GJ, Hofhuizen DM, et al. Borenstein DG. Côté P, Cassidy JD, Carroll LJ, Kristman V. Hill J, Lewis M, Papageorgiou AC, Dziedzic K, Croft P. Enthoven P, Skargren E, Öberg B. Liebenson C. Nordemar R, Thörner C. Henderson, D, Chapman-Smith, D, Mior, S, and Vernon, H. Graff-Radford SB, Reeves JL, Jaeger B. Chok B, Wong WP. Evans R, Bronfort G, Bittell S, Anderson AV. Krabak BJ, Borg-Stein J, Oas JA. Linton SJ, Hellsing AL, Andersson D. Mathias BJ, Dillingham TR, Zeigler DN, Chang AS, Belandres PV. Sloop PR, Smith DS, Goldenberg E, Dore C. Walko EJ, Janouschek C. West DT, Mathews RS, Miller MR, Kent GM. Howe DH, Newcombe RG, Wade MT. Jagbandhansingh MP. Cote P, Cassidy JD, Carroll L. Grieve GP. Roug IK. Stern PJ, Dzus A, Cassidy JD. King SW, Hosler BK, King MA, Eiselt EW. Bonkowsky V, Steinbach S, Arnold W. Erfanian P. Mork AA, Haufe SM, Yancey WB. Schimp DJ. McBain AA. Gompels BM, Darlington LG. Regelink GA, de Zoete A. Mirtz TA. Boudreau LA, Pinto A. McMillin AD. Richardson CD, Maciver K, Wright M, Wiles JR. Carroll D, Moore RA, McQuay HJ, Fairman F, Tramèr M, Leijon G. G ross AR, Hoving JL, Haines TA, Goldsmith CH, Kay T, Aker P, et al.; Hurwitz EL, Morgenstern H, Vassilaki M, Chiang LM. Senstad O, Leboeuf-Yde C, Borchgrevink C. Cagnie B, Vinck E, Beernaert A, et al. Atchison JW. Stevinson C, Ernst E. Ernst E. Oware A, Herskovitz S, Berger AR. Tseng SH, Lin SM, Chen Y, Wang CH. Meeker WC, Mootz RD, Haldeman S. Swezey RL. Hawk, C, Long, CR, Reiter, R, Davis, CS, Cambron, JA, and Evans, R. Irnich D, Behrens N, Molzen H, Konig A, Gleditsch J, Krauss M, et al. Merskey H, Bogduk N, editors. Busse JW, Guyatt GH, Bhandari M, Cassidy JD. Rodriguez AA, Bilkey WJ, Agre JC. Jordan A, Oostergard K. van den Heuvel SG, de Looze MP, Hildebrandt VH, Thé KH. Bolton JE. Farrar JT, Berlin JA, Strom BL. Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole MR. Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Farrar JT. Erfanian P. Gibbons P, Tehan P. Wlodyka-Demaille S, Poiraudeau S, Catanzariti JF, Rannou F, Fermanian J, Revel M. Bicer A, Yazici A, Camdeviren H, Erdogan C. Hoving JL, de Vet HC, Twisk JW, Devillé WL, van der WD, Koes BW, et al. Michaelson P, Sjölander P, Johansson H. Luo X, Edwards CL, Richardson W, Hey L. White P, Lewith G, Prescott P. Childs MJ, Fritz JM, Piva SR, Whitman JM. Barker S, Kesson M, Ashmore J, Turner G, Conway J, Stevens D. Haldeman S, Kohlbeck FJ, McGregor M. Carey PF. Magarey ME, Rebbeck T, Coughlan B, Grimmer K, Rivett DA, Refshauge K. Licht PB, Christensen HW, Høilund-Carlsen PF. Haldeman S, Kohlbeck FJ, McGregor M. Dziewas R, Konrad C, Dräger B, Evers S, Besselmann M, Lüdemann P, et al. Nadgir RN, Loevner LA, Ahmed T, Moonis G, Chalela J, Slawek K, et al. Haldeman S, Carey P, Townsend M, Papadopoulos C. Ernst E. Smith WS, Johnston SC, Skalabrin EJ, Weaver M, Azari P, Albers GW, et al. Licht PB, Christensen HW, Høilund-Carlsen PF. Young YH, Chen CH. Hufnagel A, Hammers A, Schönle PW, Böhm KD, Leonhardt G. Rothwell DM, Bondy SJ, Williams JI. Schievink WI. Giroud M, Fayolle H, Andre N. Biller J, Hingtgen WL, Adams HP, Smoker WRK, Godersky JC, Toffol GJ. Norris JW, Beletsky V, Nadareishvili ZG. Haneline MT. Haneline MT. Canadian Stroke Consortium. Haneline MT, Lewkovich G. Inquest into the death of Lana Dale Lewis; Inquest into the death of Lana Dale Lewis; Bin Saeed A, Shuaib A, Al Sulaiti G, Emery D. Michaud TC. Refshauge KM, Parry S, Shirley D, Larsen D, Rivett DA, Boland R. Brandt T, Orberk E, Weber R, Werner I, Busse O, Muller BT, et al. Brandt T, Hausser I, Orberk E, Grau A, Hartschuh W, Anton-Lamprecht I, et al. Pezzini A, Del Zotto E, Archetti S, Negrini R, Bani P, Albertini A, et al. Gallai V, Caso V, Paciaroni M, Cardaioli G, Arning E, Bottiglieri T, et al. Rosner AL. Haneline MT, Croft AC, Frishberg BM. Grond-Ginsbach C, Klima B, Weber R, Striegel J, Fischer C, Hacke W, et al. Brandt T, Grond-Ginsbach C. Schievink WI, Thompson RC, Yong WH. Mann T, Refshauge KM. van der Velde GM. Grant R. Rubinstein AM, Haldeman S. Sturzenegger M. Vertebrobasilar stroke syndromes. Klougart N, Leboeuf-Yde C, Rasmussen LR. Klougart N, Leboeuf-Yde C, Rasmussen LR. Sehub J, Jacques PF, Bostom AG, D’Agostino RB, Wilson PW, Belanger AJ, et al. Brattstrom L. Jacob RA, Wu M, Henning SM, Swendseid ME. Bazzano LA, He J, Ogden LG, Loaria C, Vupputuri S, Myers L, et al. Toole JF, Malinow MR, Chambless LE, Pettigrew LC, Howard VJ, Sides EG, et al. Kelly PJ, Shih VE, Kistler JP, Barron M, Lee H, Mandell R, et al. Klein P, Broers C, Feipel V, Salvia P, Van Geyt B, Dugailly PM, et al. Shekelle PG, Coulter I. Gatterman MI. McDermaid C, Mior SA. Kukurin GW. Ziv I, Rang M, Hoffman HJ. Schmidley JW, Koch T. McCarthy KA. Ea H-K, Weber A-J, Yon F, Liote F. Brynin R, Yomtob C. Hadida C, Lemire JJ. Gran DF, McLean I, Scuderi D, Cooperstein R, Rowell RM. Di Duro JO. North KN, Whiteman DAH, Pepin MG, Byers PH. Kier A, McCarthy P. Polkinghorn BS, Colloca CJ. Simnad VI. Horowitz M, Jovin T, Balzar J, Welch W, Kassam A. Sédat J, Dib M, Mahagne MH, Lonjon M, Paquis P. Jumper JM, Horton JC. Mathew KM, Ravichandran G, May K, Morsley K. Heffner JE. Alberstone CD, Benzel EC. Simmers TA, Bekkenk MW, Vidakovic-Vukic M. Constantoyannis C, Konstantinou D, Kourtopoulos H, Papadakis N.
History of stroke or TIA
Being medically treated for lupus
Appendix 3:
Evidenced, cervical pain benefit from chiropractic treatments
Formatting too complex to include here... please click on
Appendix 3
Footnotes
Contributing advisors:
Literature Search Team (treatment and dissection, all at CMCC, Toronto, ON):
Carol Hagino, BSc, MBA; Janet Hayes RN, CCRP; Kim Humphreys, PhD, DC; Anne Taylor-Vaisey, MLS; Howard Vernon DC, PhD, FCCS(C).
Literature Search Team (adverse events):
Andrea Furlan, MD; Anne Taylor-Vaisey, MLS.
Literature Search Team (treatment update):
Anne Taylor-Vaisey, MLS.
Evidence Extraction Team:
Thor Eglington, MSc, RN (Ottawa, ON); Bruce P Squires, PhD, MD (Ottawa, ON).
Critical commentary:
Donald R Murphy, DC, DACAN (Rhode Island Spine Center, Department of Community Health, Brown University School of Medicine, Providence, RI, USA).
Review Panel:
Robert R Burton, BSc, DC, FCCRS(C), DACRB (St John’s, NL); Andrea Furlan, MD; Richard Roy, DC (Université du Québec à Trois Rivières, Trois Rivières, QC ); Steven Silk, BSc, DC (Wiarton, ON); Roy Till, DC, FCCRS(C) (Stoney Creek, ON).
Task Force:
Grayden Bridge, DC (President, The Canadian Chiropractic Association [The CCA]); H James Duncan, BFA, ex-officio (The CCA); Wanda Lee MacPhee, BSc, DC (President, Canadian Federation of Chiropractic Regulatory Boards [CFCRB]); Bruce Squires, BA, MBA (ex-officio, Ontario Chiropractic Association [OCA]); Greg Stewart, BPE, DC (The CCA); Keith Thomson, DC (CFCRB); Dean Wright, DC (ex-officio, President, OCA).
Sources of support and sponsoring agency:
This work was funded by an unrestricted grant provided by the Ontario Ministry of Health and Long-Term Care to the Ontario Chiropractic Association. This work was sponsored by The CCA and the CFCRB.
Conflicts of interest:
The contributing individuals declared no conflicts of interest. GDC members Andrea Furlan and Janice Gross Stein received a per diem for their participation. The literature search and evidence extraction teams were contracted.
Contributor Information
Elizabeth Anderson-Peacock, Barrie, ON.
Jean-Sébastien Blouin, School of Human Kinetics, University of British Columbia, Vancouver, BC.
Roland Bryans, Clarenville, NL.
Normand Danis, Montreal, PQ.
Andrea Furlan, Evidence-Based Practice Co-ordinator, Institute for Work & Health, Toronto, ON.
Henri Marcoux, Winnipeg, MB.
Brock Potter, North Vancouver, BC.
Rick Ruegg, Associate Dean, Clinics, Canadian Memorial Chiropractic College [CMCC], Toronto, ON.
Janice Gross Stein, Belzberg Professor of Conflict Management and Negotiation, Department of Political Science, Director of the Munk Centre for International Studies, University of Toronto, Toronto, ON.
Eleanor White, Markham, ON.
REFERENCES:
The prevalence of neck pain: a population-based study from northern Sweden.
Acta Orthop Scand. 2002;73:455–9
The Saskatchewan Health and Back Pain Survey.
The Prevalence of Neck Pain and Related Disability in Saskatchewan Adults
Spine (Phila Pa 1976). 1998 (Aug 1); 23 (15): 1689–1698
Demographic and clinical characteristics of chiropractic patients: a five year study of patients treated at the Canadian Memorial Chiropractic College.
J Can Chiropr Assoc. 1994;38:75–82.
Functional anatomy of the cervical spine.
In: Murphy DR, editor. Conservative management of cervical spine syndromes.
New York: McGraw-Hill; 2000. p. 3–24.
The pathophysiology of whiplash.
Spine: State of the art reviews. 1998;12(2):209–42.
J Can Chiropr Assoc. 2003;48(1):56–72
Oxford Centre for Evidence-based Medicine Levels of Evidence (May 2001).
Oxford (UK). 2001 [cited 2005 May 17].
Available from: http://www.cebm.net/levels_of_evidence.asp
Assessing the clinical significance of change scores recorded on subjective outcome measures.
J Manipulative Physiol Ther. 2004;27(1):26–35
A Randomized Clinical Trial of Exercise and Spinal Manipulation
for Patients with Chronic Neck Pain
Spine (Phila Pa 1976). 2001 (Apr 1); 26 (7): 788–797
A Randomized controlled trial on the efficacy of exercise for patients with chronic neck pain.
Spine. 2005;30(1):E1–7
The immediate effect of manipulation versus mobilization on pain and range of motion in the cervical spine: a randomized controlled trial.
J Manipulative Physiol Ther. 1992;15(9):570–5
Chronic Spinal Pain: A Randomized Clinical Trial Comparing Medication,
Acupuncture, and Spinal Manipulation
Spine (Phila Pa 1976) 2003 (Jul 15); 28 (14): 1490–1502
Intensive training, physiotherapy or manipulation for patients with chronic neck pain. A prospective, single-blinded, randomized clinical trial.
Spine. 1998;23:311–8. discussion 319
Manual Therapy, Physical Therapy, or Continued Care by a General Practitioner
for Patients with Neck Pain. A Randomized, Controlled Trial
Annals of Internal Medicine 2002 (May 21); 136 (10): 713–722
Cost Effectiveness of Physiotherapy, Manual Therapy, and General Practitioner Care
for Neck Pain: Economic Evaluation Alongside a Randomised Controlled Trial
British Medical Journal 2003 (Apr 26); 326 (7395): 911
Active treatment of chronic neck pain: a prospective randomized intervention.
Spine. 2000;25:1021–7
Pulsed high frequency (27MHz) electromagnetic therapy for persistent neck pain. A double blind, placebo-controlled study of 20 patients.
Orthopedics. 1990;13:445–51
The effect of pulsed electromagnetic fields in the treatment of osteoarthritis of the knee and cervical spine. Report of randomized, double blind, placebo controlled trials.
J Rheumatol. 1994;21:1903–11
Cervical pain and mobilization.
Man Med. 1985;2:18–22.
Stretching as an adjunct to chiropractic manipulation of chronic neck pain – before, after or not at all? A prospective randomized controlled clinical trial.
Eur J Chiropractic. 2003;50(2):41–52.
Efficacy of 904 nm gallium arsenide low level laser therapy in the management of chronic myofascial pain in the neck: a double-blind and randomize-controlled trial.
Lasers Surg Med. 2004;35(3):229–35
Cervical mobilisation: concurrent effects on pain, sympathetic nervous system activity and motor activity.
Man Ther. 2001;6(2):72–81
Diode laser in cervical myofascial pain: a double-blind study versus placebo.
Clin J Pain. 1989;5:301–4
Therapeutic effects of repetitive peripheral magnetic stimulation on myofascial pain syndrome.
Clin Neurophysiol. 2003;114:350–8
A new 3-point bending traction method for restoring cervical lordosis and cervical manipulation: a nonrandomized clinical controlled trial.
Arch Phys Med Rehabil. 2002;83(4):447–53
Increasing the cervical lordosis with chiropractic biophysics seated combined extension-compression and transverse load cervical traction with cervical manipulation: nonrandomized clinical control trial.
J Manipulative Physiol Ther. 2003;26:139–51
Effectiveness of dynamic muscle training, relaxation training, or ordinary activity for chronic neck pain: randomised controlled trial.
BMJ. 2003;327:475
Treatment of myofascial trigger-points with ultrasound combined with massage and exercise – a randomised controlled trial.
Pain. 1998;77(1):73–9
No significant differences between intervention programmes on neck, shoulder and low back pain: a prospective randomized study among home-care personnel.
J Rehabil Med. 2001;33:170–6
Effects of active head retraction with retraction/extension and occipital release on the pressure pain threshold of cervical and scapular trigger points.
Physiother Theory Pract. 1997;13:285–91.
Changes in cervicocephalic kinesthesia after a proprioceptive rehabilitation program in patients with neck pain: a randomized controlled study.
Arch Phys Med Rehabil. 1994;75:895–9
Does group gymnastics at the workplace help in neck pain? A controlled study.
Scand J Rehabil Med. 1994;26(1):17–20
Randomized controlled trial of physiotherapy and Feldenkrais interventions in female workers with neck-shoulder complaints.
J Occup Rehabil. 1999;9(3):179–94.
Active or passive physiotherapy for occupational cervicobrachial disorders? A comparison of two treatment methods with a 1-year follow-up.
Arch Phys Med Rehabil. 1993;74(4):425–30
Effectiveness of physical therapy for patients with neck pain: an individualized approach using a clinical decision-making algorithm.
Amer J Phys Med Rehabil. 2003;82:203–18
Prevention of stroke (cardiology rounds). 2001; 5(8) [cited 2004 Dec 22].
Available from: http://www.cardiologyrounds.org/crus/cardious_1001.pdf
Therapeutic exercise: foundations and techniques. 4th ed.
Philadelphia, Pa: FA Davis Co; 2002.
p. 36, 95–9, 102, 103, 163–9, 198–200, 258.
Manipulation of the cervical spine.
Man Ther. 2003;8(1):2–9
The effect of manipulation on pain and range of motion in the cervical spine: a pilot study.
J Manipulative Physiol Ther. 1992;15(8):495–500
Pressure pain threshold evaluation of the effect of spinal manipulation in the treatment of chronic neck pain: a pilot study.
J Manipulative Physiol Ther. 1990;13(1):13–6
The effect of spinal manipulative therapy (SMT) on pain reduction and range of motion in patients with acute unilateral neck pain: a pilot study.
J Can Chiropr Assoc. 1999;43:111–9.
Comparison of two chiropractic techniques on pain and lateral flexion in neck pain patients: a pilot study.
Chiropr Tech. 1996;8(4):155–62.
A Pilot Randomized Clinical Trial on the Relative Effect of Instrumental
(MFMA) Versus Manual (HVLA) Manipulation in the Treatment
of Cervical Spine Dysfunction
J Manipulative Physiol Ther 2001 (May); 24 (4): 260–271
Relative effectiveness of two different approaches to adjust a fixated segment in the treatment of facet syndrome in the cervical spine.
J Neuromusculoskel Sys. 1998;6:1–5.
The relative effectiveness of spinal manipulation and ultrasound in mechanical pain: pilot study.
Chiropr Tech. 1999;11(4):164–8
A clinical trial investigating the possible effect of the supine cervical rotatory manipulation and the supine lateral break manipulation in the treatment of mechanical neck pain: a pilot study.
J Manipulative Physiol Ther. 2000;23:324–31
The relationship of changes in cervical curvature to visual analog scale, neck disability index scores and pressure algometry in patients with neck pain.
J Chiropr Res Clin Invest. 1994;9(1):19–23.
A clinical trial investigating the effect of two manipulative approaches in the treatment of mechanical neck pain: a pilot study.
J Neuromusculoskel Sys. 1998;6(1):6–16.
The effects of spinal manipulation on cervical kinesthesia in patients with chronic neck pain: a pilot study.
J Manipulative Physiol Ther. 1997;20(2):80–5
Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity.
Arch Phys Med Rehabil. 2002;83:1406–14
Effects on physical performance and pain from three dynamic training programs for women with work-related trapezius myalgia.
J Rehabil Med. 2001;33:162–9
Dynamic neck strength training effect on pain and function.
Arch Phys Med Rehabil. 1994;75:661–5
The effects of different training programs on the trapezius muscle of women with work-related neck and shoulder myalgia.
Acta Neuropathol. 2000;100(3):253–8
Changes in isometric strength and range of motion of the isolated cervical spine after eight weeks of clinical rehabilitation.
Spine. 1992;17(6 Suppl):S77–82
Intensive dynamic training for females with chronic neck/shoulder pain. A randomized controlled trial.
Clin Rehabil. 1998;12(3):200–10
Controlled two year follow up of rehabilitation for disorders in the neck and shoulders.
Occup Environ Med. 1994;51:833–8. [PMC free article] [PubMed]
Perceived pain before and after three exercise programs – a controlled clinical trial of women with work-related trapezius myalgia.
Pain. 2000;85:201–7
A randomized clinical trial comparing general exercise, McKenzie treatment and a control group in patients with neck pain.
J Rehabil Med. 2002;34(4):183–90
The effect of pain reduction on perceived tension and EMG-recorded trapezius muscle activity in workers with shoulder and neck pain.
Scand
Cervical spine disorders: a comparison of three types of traction.
Spine. 1985;10:867–71
Tension neck and evaluation of a physical training course among office workers in a bank corporation.
J Adv Nurs. 1997;26:962–7
Treatment of myofascial pain.
Am J Phys Med Rehabil. 2000;79(1):48–52
The clinical efficacy of low-power laser therapy on pain and function in cervical osteoarthritis.
Clin Rheumatol. 2001;20:181–4
Before/after study to determine the effectiveness of the align-right cylindrical cervical pillow in reducing chronic neck pain severity.
J Manipulative Physiol Ther. 1998;21(2):89–93
Clinical use of neck isometric strength measurement in rehabilitation.
Arch Phys Med Rehabil. 1994;75:465–9
Magnetic necklace: its therapeutic effectiveness on neck and shoulder pain.
Arch Phys Med Rehabil. 1982;63:462–6
Neck pain in the general population.
Spine. 1994;19:1307–9
The chiropractic outcome study: pain, functional ability and satisfaction with care.
J Manipulative Physiol Ther. 1997;20(5):235–40
Unravelling the complexity of muscle impairment in chronic neck pain.
Manual Ther. 2004;9(3):125–33
Neck and shoulder pain in 70- to 79-year-old men and women: findings from the health, aging and body composition study.
Spine J. 2003;3(6):435–41
Impaired trunk muscle function in sub-acute neck pain: etiologic in the subsequent development of low back pain?
Manual Ther. 2004;9(3):157–63
Are patients with chronic low back pain or chronic neck pain fatigued?
Pain Med. 2004;5(2):187–95
Variability in pain intensity, physical and psychological function in non-acute, non-traumatic neck pain.
Physiother Res Int. 2004;9(1):43–54
A cross-sectional study correlating degeneration of the cervical spine with disability and pain in United kingdom patients.
Spine. 2003;28(2):129–33
A randomized clinical trial of manual therapy and physiotherapy for persistent back and neck complaints: subgroup analysis and relationship between outcome measures.
J Manipulative Physiol Ther. 1993;16:211–9
Management of neck pain: a primary care approach.
Hosp Pract. 1998;33(10):147–4. 160
The annual incidence and course of neck pain in the general population: a population-based cohort study.
Pain. 2004;112(3):267–73
Predicting persistent neck pain: a 1-year follow-up of a population cohort.
Spine. 2004;29(15):1648–54
Clinical course in patients seeking primary care for back or neck pain: a prospective 5-year follow-up of outcome and health care consumption with subgroup analysis.
Spine. 2004;29(21):2458–65
Functional reactivation for neck pain patients.
J Bodywork Movement Ther. 2002;6(1):59–66.
Treatment of acute cervical pain – a comparative group study.
Pain. 1981;10:93–101
Clinical Guidelines for Chiropractic Practice in Canada
Canadian Chiropractic Association, Toronto, ON; 1993
Management of chronic head and neck pain: effectiveness of altering factors perpetuating myofascial pain.
Headache. 1987;27:186–90
Treatment of unilateral upper thoracic vertebral pain using an eclectic approach.
Physiother Res Int. 2000;5:129–34
A pilot study for a randomized clinical trial assessing chiropractic care, medical care, and self-care education for acute and subacute neck pain patients.
J Manipulative Physiol Ther. 2003;26:403–11
Chronic cervical myofascial pain syndrome: improvement in dizziness and pain with a multi-disciplinary rehabilitation program: a pilot study.
J Back Musculoskel Rehabil. 2000;15:83–7
A controlled study of the effects of an early intervention on acute musculoskeletal pain problems.
Pain. 1993;54:353–9
Topical capsaicin for chronic neck pain. A pilot study.
Am J Phys Med Rehabil. 1995;74:39–44
Manipulation for chronic neck pain. A double-blind controlled study.
Spine. 1982;7:532–5
Effects of osteopathic manipulative treatment in patients with cervicothoracic pain: pilot study using thermography.
J Am Osteopath Assoc. 1994;94(2):135–41
Effective management of spinal pain in one hundred seventy-seven patients evaluated for manipulation under anesthesia.
J Manipulative Physiol Ther. 1999;22:299–308
Manipulation of the cervical spine: a pilot study.
J R Coll Gen Pract. 1983;33:574–9
Most common causes of chiropractic malpractice lawsuits.
J Manipulative Physiol Ther. 1997;20(1):60–4
The Treatment of Neck and Low Back Pain: Seeks Care? Who Goes Where?
Med Care. 2001 (Sep); 39 (9): 956–967
Contra-indications to spinal manipulation and allied treatments.
Physiotherapy. 1989;75(8):445–53.
Brain metastases and neck pain.
Eur J Chiropractic. 1996;44(3):51–7.
Cervical spine osteoblastoma presenting as mechanical neck pain: a case report.
J Can Chiropr Assoc. 1994;38(3):146.
Missed cervical spine fracture-dislocations: the importance of clinical and radiographic assessment.
J Manipulative Physiol Ther. 2002;25(4):263–9
Vertigo and cranial nerve palsy caused by different forms of spontaneous dissections of internal and vertebral arteries.
Eur Arch Otorhinolaryngol. 2002;259(7):365–8
Patient with signs and symptoms of myocardial infarction presenting to a chiropractic office: a case report.
J Can Chiropr Assoc. 2001;45(1):35.
Sometimes (what seems to be) a heart attack is (really) a pain in the neck.
J Am Board Fam Pract. 2004;17:74–7
Atypical sensory phenomenon: how to differentiate migraine, seizure, and transient ischemic attack.
Top Clin Chiropractic. 1995;2(3):29–33.
Marfan’s Syndrome: an unusual case of neck, shoulder, and jaw pain in a former college basketball player.
J Sports Chiro Rehabil. 1999;13(1):12–5.
Septic arthritis in rheumatoid disease causing bilateral shoulder dislocation: diagnosis and treatment assisted by grey scale ultrasonography.
Ann Rheum Dis. 1981;
A missed Jefferson fracture in chiropractic practice.
J Manipulative Physiol Ther. 2001;24(3):210–3
Acute respiratory distress syndrome: clinical recognition and preventive management in chiropractic acute care practice.
J Manipulative Physiol Ther. 2001;24(7):467–73
Acute lymphangitis mimicking mechanical neck pain.
J Manipulative Physiol Ther. 2001;24(7):474–6
Back to basics – clinical considerations in the mechanical assessment of the cervical spine.
Top Clin Chiropractic. 1995;2(3):1–18.
Patient reports of the effects and side-effects of TENS for chronic non-malignant pain following a four week trial.
The Pain Clinic. 2002;13(3):265–76.
Transcutaneous electrical nerve stimulation (TENS) for chronic pain.
The Cochrane database of systematic reviews 2000. Issue 4.
Art. No.: CD003222. DOI: 10.1002/14651858. CD003222, 2000
Cervical Overview Group. Manipulation and mobilisation for mechanical neck disorders.
The Cochrane database of systematic reviews 2002, Issue 3.
Art. No.: CD004249.pub2. DOI: 10.1002/14651858. CD004249.pub2, 2002
Adverse reactions to chiropractic treatment and their effects on satisfaction and clinical outcomes among patients enrolled in the UCLA Neck Pain Study.
J Manipulative Physiol Ther. 2004;27:16–25
Predictors of side effects to spinal manipulative therapy.
J Manipulative Physiol Ther. 1996;19(7):441–4
How Common Are Side Effects of Spinal Manipulation And Can These Side Effects Be Predicted?
Manual Therapy 2004 (Aug); 9 (3): 151–156
Manipulation efficacy: upper body.
J Back Musculoskeletal Rehabil. 2000;15(1):3–15
Risks associated with spinal manipulation.
Am J Med. 2002;112(7):566–71
Prospective investigations into the safety of spinal manipulation.
J Pain Symptom Manage. 2001;21(3):238–42
Long thoracic nerve palsy following cervical chiropractic manipulation.
Muscle Nerve. 1995;18(11):1351
Ruptured cervical disc after spinal manipulation therapy: report of two cases.
Spine. 2002;27(3):E80–2
The state of chiropractic research.
Top Clin Chiropr. 2002;9:1–13.
Chronic neck pain.
Rheum Dis Clin North Am. 1996;22:411–37
Issues in Planning a Placebo-controlled Trial of Manual Methods: Results of a Pilot Study
J Altern Complement Med 2002; 8 (1) Feb: 21–32
Randomised trial of acupuncture compared with conventional massage and “sham” laser acupuncture for treatment of chronic neck pain.
BMJ. 2001;322:1574
IASP Task Force on Taxonomy. Classification of Chronic Pain:
Descriptions of Chronic Pain Syndromes and Definition of Pain Terms. 2nd ed.
Seattle: IASP Press; 1994. p. 209–14 [cited 2004 May 1]
Available from: http://www.iasp-pain.org/terms-p.html#Pain
User’s guide to the chiropractic literature-1B: how to use an article about therapy.
J Manipulative Physiol Ther. 2003;26(8):525–32
Therapeutic exercises in chronic neck and back pain.
Arch Phys Med Rehabil. 1992;73:870–5
Rehabilitation of neck/shoulder patients in primary health care clinics.
J Manipulative Physiol Ther. 1996;19:32–5
Effects of software programs stimulating regular breaks and exercises on work-related neck and upper-limb disorders.
Scand J Work Environ Health. 2003;29(2):106–16
Sensitivity and specificity of outcome measures in patients with neck pain: detecting clinically significant improvement.
Spine. 2004;29(21):2410–17
Clinically important changes in acute pain outcome measures: a validation study.
J Pain Symptom Manage. 2003;25(5):406–11
Clinical Importance of Changes in Chronic Pain Intensity
Measured on an 11-point Numerical Pain Rating Scale
Pain 2001 (Nov); 94 (2): 149-158
Defining the clinically important difference in pain outcome measures.
Pain. 2000;88(3):287–94
What is clinically meaningful: outcome measures in pain clinical trials.
Clin J Pain. 2000;16(2 Suppl):S106–12
Assessing effects of a semi-customized experimental cervical pillow on symptomatic adults with chronic neck pain with and without headache.
J Can Chiropr Assoc. 2004;48(1):20–8
Spinal manipulation: indications, risks and benefits.
J Bodywork Mov Ther. 2001;5(2):110–9
The ability to change of three questionnaires for neck pain.
Joint Bone Spine. 2004;71(4):317–26
Assessment of pain and disability in patients with chronic neck pain: reliability and construct validity of the Turkish version of the neck pain and disability scale.
Disabil Rehabil. 2004;26(16):959–62
Prognostic factors for neck pain in general practice.
Pain. 2004;110(3):639–45
Factors predicting pain reduction in chronic back and neck pain after multimodal treatment.
Clin J Pain. 2004;20(6):447–54
Relationships of clinical, psychologic, and individual factors with the functional status of neck pain patients.
Value Health. 2004;7(1):61–9
The core outcomes for neck pain: validation of a new outcome measure.
Spine. 2004;29(17):1923–30
Proposal of a classification system for patients with neck pain.
J Orthop Sports Phys Ther. 2004;34(11):686–96
Guidance for pre-manipulative testing of the cervical spine (professional issue)
Man Ther. 2000;5(1):37–40
Unpredictability of Cerebrovascular Ischemia Associated with Cervical Spine Manipulation Therapy:
A Review of Sixty-four Cases After Cervical Spine Manipulation
Spine (Phila Pa 1976) 2002 (Jan 1); 27 (1): 49–55
A suggested protocol for the examination and treatment of the cervical spine: managing the risk.
J Can Chiropr Assoc. 1995;39(1):35–60.
Pre-manipulative testing of the cervical spine review, revision and new clinical guidelines.
Man Ther. 2004;9:95–108. [cited 2005 May 22]
Available from:
http://www.udel.edu/PT/manal/spinecourse/Journalclub205/premaniptestingcspine.pdf
Is There a Role for Premanipulative Testing Before Cervical Manipulation?
J Manipulative Physiol Ther 2000 (Mar); 23 (3): 175–179
Stroke, Cerebral Artery Dissection, and Cervical Spine Manipulation Therapy
Journal of Neurology 2002 (Jul); 249 (8): 1098–1104
Cervical artery dissection – clinical features, risk factors, therapy and outcome in 126 patients.
J Neurol. 2003;250(10):1179–84
Simultaneous bilateral internal carotid and vertebral artery dissection following chiropractic manipulation: case report and review of the literature.
Neuroradiol. 2003;45:311–4
Clinical Perceptions of the Risk of Vertebral Artery Dissection After Cervical Manipulation:
The Effect of Referral Bias
Spine J 2002 (Sep); 2 (5): 334–342
Manipulation of the cervical spine: a systematic review of case reports of serious adverse events, 1995–2001.
Med J Aust. 2002;176:376–80
Spinal manipulative therapy is an independent risk factor for vertebral artery dissection.
Neurology. 2003;60(9):1424–8
Is Cervical Spinal Manipulation Dangerous?
J Manipulative Physiol Ther 2003 (Jan); 26 (1): 48–52
Acute vertigo following cervical manipulation.
Laryngoscope. 2003;113:659–62
Stroke following chiropractic manipulation of the cervical spine.
J Neurol. 1999;246(8):683–8
Chiropractic Manipulation and Stroke:
A Population-based Case-control Study
Stroke 2001 (May); 32 (5): 1054-1060
Spontaneous dissection of the carotid and vertebral arteries.
N Engl J Med. 2001;344(12):898–906
Incidence of internal carotid artery dissection in the community of Dijon.
J Neurol Neurosurg Pshyciatry. 1994;57:1443
Cervicocephalic arterial dissections: a ten year experience.
Arch Neurol. 1986;43:1234–8
Sudden Neck Movement and Cervical Artery Dissection
CMAJ. 2000 (Jul 11); 163 (1): 38–40
Identification of Internal Carotid Artery Dissection in Chiropractic Practice
J Can Chiropr Assoc. 2004 (Sep); 48 (3): 206–210
Internal carotid artery dissection following chiropractic manipulation: clinical features and mechanisms of injury.
J Am Chiropractic Assoc. 2003;40(5):20–4.
SPONTADS (spontaneous vs traumatic arterial dissection)
May 2001 update [cited 2004 Jun 21].
Available from:
http://www.strokeconsortium.ca/PG-08iii.sp-update.html
Critique of the Canadian Stroke Consortium’s spontaneous vs traumatic arterial dissection study (SPONTADS)
J Am Chiropr Assoc. 2004 May;:18–21.
Testimony transcript of Dr JW Norris.
Jun 25, 2002. Toronto, Ontario.
Testimony transcript of Dr D Sackett.
Nov 18, 2002. Toronto, Ontario.
Vertebral Artery Dissection: Warning Symptoms, Clinical Features and Prognosis in 26 Patients
Canadian Journal of Neurological Sciences 2000 (Nov); 27 (4): 292–296
Uneventful Upper Cervical Manipulation in the Presence of a Damaged Vertebral Artery
J Manipulative Physiol Ther 2002 (Sept); 25 (7): 472–483
Professional responsibility in relation to cervical spine manipulation [review]
Aust J Physiother. 2002;48(3):171–9. response 180–5
Pathogenesis of cervical artery dissections.
Neurology. 2001;57(1):24–30
Ultrastructural connective tissue abnormalities in patients with spontaneous cervicocerebral artery dissections.
Ann Neurol. 1998;44:281–5
Plasma homocysteine concentration, C677T MTHFR genotype, and 844ins68bp CBS genotype in young adults with spontaneous cervical artery dissection and atherothrombotic stroke.
Stroke. 2002;33(3):664–9
Mild hyperhomocyst(e)inemia: a possible risk factor for cervical artery dissection.
Stroke. 2001;32(3):714–8
Spontaneous Cervical Artery Dissections and Implications for Homocysteine
J Manipulative Physiol Ther 2004 (Feb); 27 (2): 124–132
Association of internal carotid artery dissection and chiropractic manipulation.
Neurolog. 2003;9(1):35–44
Exclusion mapping of the genetic predisposition for cervical artery dissections by linkage analysis.
Ann Neurol. 2002;52:359–64
Spontaneous cervical artery dissections.
Stroke. 2002;33:657–70
A syndrome of spontaneous cerebral and cervical artery dissections with angiolipomatosis: report of two cases.
J neurosurg. 2003;98(5):1124–7
Causes of complications from cervical spine manipulation.
Aust J Physiother. 2001;47(4):255–66
Benign paroxysmal positional vertigo part I: background and clinical presentation.
J Can Chiropr Assoc. 1999;43(1):31–40.
Influence of vertebral artery blood flow research outcomes on clinical judgment [letter]
Aust J Physiother. 2001;47(3):167.
Cervical manipulation to a patient with a history of traumatically induced dissection of the internal carotid artery: a case report and review of the literature on recurrent dissections.
J Manipulative Physiol Ther. 2001;24:520–5
Headache and neck pain: the warning symptoms of vertebral artery dissection.
Headache. 1994;34(4):187–93
In: CG Goetz, editor. Textbook of Clinical Neurology 2nd ed.
Philadelphia, Pa: WB Saunders; 2003. p. 413.
Safety in Chiropractic Practice, Part I; The Occurrence of Cerebrovascular Accidents
After Manipulation to the Neck in Denmark from 1978-1988
J Manipulative Physiol Ther 1996 (Jul); 19 (6): 371–377
Safety in Chiropractic Practice Part II: Treatment to the Upper Neck
and the Rate of Cerebrovascular Incidents
J Manipulative Physiol Ther 1996 (Nov); 19 (9): 563–569
Association between plasma homocysteine concentrations and extracranial carotid artery stenosis.
N Engl J Med. 1995;332:285–9.
Vitamins as homocysteine-lowering agents.
J Nutr. 1996;126(Suppl 4):S1276–80
Homocysteine increases as folate decreases in plasma of health men during short term dietary folate and methyl group restriction.
J Nutr. 1994;124:1072–80
Dietary intake of folate and risk of stroke in US men and women.
Stroke. 2002;33:1183–9
Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial.
JAMA. 2004;291:565–75
193.
Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification.
Stroke. 2003;34:E51–4
Global 3D head-trunk kinematics during cervical spine manipulation at different levels.
Clin Biomech (Bristol, Avon) 2003;18(9):827–31
Cervical spine manipulation: summary report of a systematic review of the literature and a multidisciplinary expert panel.
J Spinal Disord. 1997;10(3):223–8
Standards of practice relative to complications of and contraindications to spinal manipulative therapy.
J Can Chiropr Assoc. 1991;35(4):232.
Ankylosing spondylitis presenting to a chiropractic office: a report of two cases.
J Can Chiropr Assoc. 2000;44(2):87–97.
The amelioration of symptoms in cervical spinal stenosis with spinal cord deformation through specific chiropractic manipulation: a case report with long-term follow-up.
J Manipulative Physiol Ther. 2004;27(5):366.e1–7
Paraplegia in osteogenesis imperfecta. A case report.
J Bone Joint Surg Br. 1983;65(2):184–5
The noncerebrovascular complications of chiropractic manipulation.
Neurology. 1984;34(5):684–5
Template for developing a clinical impression of the aging spinal pain patient.
Top Clin Chiropractic. 2002;9(2):60–7.
Osteoporotic fracture of the dens revealed by cervical manipulation.
Joint Bone Spine. 2004;71(3):246–50
Missed cervical spine fracture: chiropractic implications.
J Manipulative Physiol Ther. 1999;22(9):610–4
Missed upper cervical spine fracture: clinical and radiological considerations.
J Can Chiropr Assoc. 1997;41(2):77–85.
Neck pain in a 29-year-old female with congenital anomalies of the cervical spine (grand rounds)
JNMS. 2001;9(4):135–41.
Dural tear and intracranial hypotension in a chiropractic patient.
J Neurol Neurosurg Psychiatry. 2004;75(2):346–7
Cerebrovascular complications in Ehlers-Danlos Syndrome Type IV.
J Chiro Technique. 1997;9(1):40.
Retropharyngeal tendinitis.
Eur J Chiropractic. 2003;48(2):39–45.
Chiropractic treatment of postsurgical neck syndrome with mechanical force, manually assisted short-lever spinal adjustments.
J Manipulative Physiol Ther. 2001;24(9):589–95
Acute onset of painful ophthalmoplegia following chiropractic manipulation of the neck: initial sign of intracranial aneurysm.
West J Med. 1997;166(3):207–10
Bow hunter’s syndrome in the setting of contralateral vertebral artery stenosis: evaluation and treatment options.
Spine. 2002;27(23):E495–8
Stroke after chiropractic manipulation as a result of extracranial postero-inferior cerebellar artery dissection.
J Manipulative Physiol Ther. 2002;25(9):588–90
Central retinal artery occlusion after manipulation of the neck by a chiropractor.
Am J Ophthalmol. 1996;121(3):321–2
The biopsychosocial model and spinal cord injury.
Spinal Cord. 2001;39(12):644–9
Diaphragmatic paralysis following chiropractic manipulation of the cervical spine.
Arch Intern Med. 1985;145(3):562–4
Cervical spine complications in rheumatoid arthritis patients.
Postgrad Med. 2000;107(1):199–208
Internal jugular vein thrombosis after cervical traction.
J Internal Med. 1997;241(4):333–5
Intermittent cervical traction for cervical radiculopathy caused by large-volume herniated disks.
J Manipulative Physiol Ther. 2002;25(3):188–92

Return to CHRONIC NECK PAIN
Return to NECK DISORDER GUIDELINES
Return to the GUIDELINES Articles Section
Since 9-06-2015


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |