Recent Considerations in Nonsteroidal
Anti–inflammatory Drug GastropathyThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: American Journal of Medicine 1998 (Jul 27); 105 (1B): 1S–38S ~ FULL TEXT
Gurkirpal Singh, MD
Department of Medicine, ARAMIS Postmarketing Surveillance Program,
Stanford University of Medicine, Palo Alto, California 94303, USA
Conservative calculations estimate that approximately 107,000 patients are hospitalized annually for nonsteroidal anti-inflammatory drug (NSAID)-related gastrointestinal (GI) complications (internal bleeding) and at least 16,500 NSAID-related deaths occur each year among arthritis patients alone.
The figures for all NSAID users would be overwhelming, yet the scope of this problem is generally under-appreciated.
In the following year (June 1999) the prestigious New England Journal of Medicine published a similar statement:
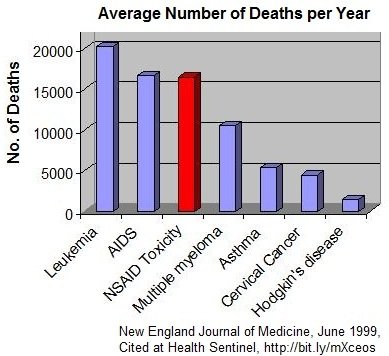
“It has been estimated conservatively that 16,500 NSAID-related deaths occur among patients with rheumatoid arthritis or osteoarthritis every year in the United States. This figure is similar to the number of deaths from the acquired immunodeficiency syndrome and is considerably greater than the number of deaths from multiple myeloma, asthma, cervical cancer, or Hodgkin’s disease.
If deaths from gastrointestinal toxic effects from NSAIDs were tabulated separately in the National Vital Statistics reports, these effects would constitute the 15th most common cause of death in the United States. Yet these toxic effects remain mainly a “silent epidemic,” with many physicians and most patients unaware of the magnitude of the problem.
Furthermore these mortality statistics do not include deaths ascribed to the use of over-the-counter NSAIDS.”
Another statement that just happened to catch my eye:On the basis of these conservative figures and ARAMIS data, the annual number of hospitalizations in the United States for serious gastrointestinal complications is estimated to be at least 103,000. At an estimated cost of $15,000 to $20,000 per hospitalization, the annual direct costs of such complications exceed $2 billion. [14]
Thanks to the American Nutrition Association
for access to this picture!The Arthritis, Rheumatism, and Aging Medical Information System (ARAMIS) Post-Marketing Surveillance Program (PMS) has prospectively followed patient status and outcomes, drug side effects, and the economic impact of illness for >11,000 arthritis patients at 8 participating institutions in the United States and Canada.
Analysis of these data indicates that:.
osteoarthritis (OA) and rheumatoid arthritis (RA) patients are 2.5—5.5 times more likely than the general population to be hospitalized for NSAID-related GI events;
the absolute risk for serious NSAID-related GI toxicity remains constant and the cumulative risk increases over time;
there are no reliable warning signals- >80% of patients with serious GI complications had no prior GI symptoms;
independent risk factors for serious GI events were age, prednisone use, NSAID dose, disability level, and previous NSAID-induced GI symptoms; and
antacids and H2 antagonists do not prevent NSAID-induced gastric ulcers, and high-risk NSAID users who take gastro-protective drugs are more likely to have serious GI complications than patients not taking such medications
The author concludes:
Currently, limiting NSAID use is the only way to decrease the risk of NSAID-related GI events.
No one has yet calculated the total cost for hospitalizing the 107,000 patients, or the pain and suffering experienced by the families of those 16,500 individuals who die, simply for using NSAIDs for pain relief.
Research has clearly demonstrated that Omege-3 Fatty acids provides the same level of pain relief as NSAIDs, and investigators have reported that rheumatoid arthritis patients, consuming omega-3 dietary supplements, were able to either lower or discontinue their background doses of nonsteroidal antiinflammatory drugs.
Considering that patients with arthritis are one of the leading group-users of NSAIDs, perhaps it's time for medical guidelines to recommend Omega-3 fatty acids FIRST for pain relief, before resorting to the more dangerous NSAIDs.
From the FULL TEXT Article:
FROM: American Journal of Medicine 1998 (Jul 27); 105 (1B): 31S–38S ~ FULL TEXT
Gurkirpal Singh, MD
Department of Medicine, ARAMIS Postmarketing Surveillance Program,
Stanford University of Medicine, Palo Alto, California 94303, USA
The Abstract
Conservative calculations estimate that approximately 107,000 patients are hospitalized annually for nonsteroidal anti-inflammatory drug (NSAID)-related gastrointestinal (GI) complications and at least 16,500 NSAID-related deaths occur each year among arthritis patients alone. The figures for all NSAID users would be overwhelming, yet the scope of this problem is generally under-appreciated. The Arthritis, Rheumatism, and Aging Medical Information System (ARAMIS) Post-Marketing Surveillance Program (PMS) has prospectively followed patient status and outcomes, drug side effects, and the economic impact of illness for >11,000 arthritis patients at 8 participating institutions in the United States and Canada. Analysis of these data indicates that: (1) osteoarthritis (OA) and rheumatoid arthritis (RA) patients are 2.5-5.5 times more likely than the general population to be hospitalized for NSAID-related GI events; (2) the absolute risk for serious NSAID-related GI toxicity remains constant and the cumulative risk increases over time; (3) there are no reliable warning signals- >80% of patients with serious GI complications had no prior GI symptoms; (4) independent risk factors for serious GI events were age, prednisone use, NSAID dose, disability level, and previous NSAID-induced GI symptoms; and (5) antacids and H2 antagonists do not prevent NSAID-induced gastric ulcers, and high-risk NSAID users who take gastro-protective drugs are more likely to have serious GI complications than patients not taking such medications. Currently, limiting NSAID use is the only way to decrease the risk of NSAID-related GI events. Ongoing ARAMIS research is aimed at developing a simple point-score system for estimating individual risks of developing serious NSAID-related GI complications.
Background
Gastrointestinal (GI) symptoms are the most common adverse events associated with nonsteroidal anti-inflammatory drug (NSAID) therapy. Because patients with arthritis are frequent users of NSAIDs and often have other risk factors, this patient population is particularly at risk for serious GI complications. To better characterize NSAID-related GI complications in patients with rheumatic disease and to determine methods for reducing their frequency, a series of studies was begun several years ago by the Arthritis, Rheumatism, and Aging Medical Information System (ARAMIS).

Table 1 ARAMIS, which has been funded by the National Institutes of Health for the past 25 years, is a prospective observational data bank system that systematically collects data on individuals with chronic rheumatic disease. [1] Data are reported by both physicians and patients; the database currently contains information on >36,000 rheumatic disease patients with >300,000 patient-years of follow-up from 17 centers in the United States and Canada. [2] Within this larger population are >11,000 arthritis patients that comprise the patient population of the ARAMIS Post-Marketing Surveillance Program (PMS; Table 1). This program prospectively follows patient status and outcomes, drug side effects, and the economic impact of illness for consecutively diagnosed patients at each participating institution.
Patient characteristics and study design have been described in detail elsewhere. [3–5] Patients in the ARAMISPMS program come from a wide variety of sources, including academic institutions and urban and community populations, and are generally representative of rheumatology patients in North America. [1]
Diagnosis of rheumatoid arthritis (RA) and osteoarthritis (OA) is made and documented according to American College of Rheumatology (ACR) criteria. Additionally, a random 10% subgroup of the Santa Clara County community sample was examined to confirm the diagnosis.
Enrolled patients complete the Stanford Health Assessment Questionnaire (HAQ) every 6 months. The Stanford HAQ is a validated instrument that evaluates the patient’s functional ability and quality of life, use of healthcare resources and medication, costs of care, and side effects. [6, 7] The study protocol includes following-up with nonresponders, contacting patients to fill in missing information, and the quality control of questionnaire coding and data entry to ensure accuracy. Follow-up data are available for 95.4% of patients.
The extensive data available in ARAMIS has provided an unparalleled opportunity to consider some of the outstanding issues relating to NSAID-induced adverse events:(1) what are the GI side effects of NSAID use;
(2) what is the extent of the problem;
(3) are there warning signs for serious GI complications;
(4) who is at greatest risk of serious GI complications;
(5) do some NSAIDs cause more GI toxicity than others; and
(6) do H2 antagonists and antacids help prevent serious GI complications?This article will discuss some of those issues using results from the literature and updated findings from the ARAMIS studies and will outline some areas of current and potential future research.
What Are The GI Side Effects Of NSAID Use?
NSAID-related GI side effects result from inhibition of prostaglandins, which play a key role in maintaining the integrity of the gastric mucosa. Although NSAID use is associated primarily with upper GI events and gastric ulcers, lower GI problems can also occur, including:(1) inflammation and permeability changes of the intestine and lower bowel;
(2) hemorrhage of the ileum, duodenum, and colon;
(3) perforation; and
(4) stricture formation. [8-11]The adverse GI events associated with NSAID use can be broadly categorized into 3 groups:
(1) “nuisance symptoms” (heartburn, nausea, dyspepsia, vomiting, abdominal pain);
(2) mucosal lesions as seen on endoscopy or x-rays; and
(3) serious GI complications (perforated ulcers, catastrophic bleeding that requires hospitalization).In any study of NSAID-induced GI complications, it is important to distinguish between the nuisance symptoms or endoscopic lesions and the serious GI complications. For this reason, endpoints for all ARAMIS studies have been defined as clinically significant events or lesions serious enough to result in a hospital stay of at least 24 hours. They include GI bleeding, clinically symptomatic gastritis, ulcers, or gastric outlet obstructions. [1] A definite diagnosis was not always possible despite severe GI symptoms requiring hospitalization. Gastrointestinal events were determined from the self-administered HAQ and confirmed by review of hospital records.
What Is The Magnitude Of The Problem?
Endoscopic lesions are common and may occur in up to 80% of NSAID users. [12] Approximately 15% of patients will have the nuisance symptoms. Using a liberal definition of an ulcer as a break of ≥3 mm in the gastric mucosa, about 25% of patients will have a gastric ulcer, and 40–50% will have either a gastric or duodenal ulcer. However, only 1–4% of patients will have clinically important GI complications each year. [10, 13, 14–17] Furthermore, reducing the incidence of endoscopic ulcers (e.g., with misoprostol) does not result in a comparable reduction in clinically significant GI complications. [18] Thus, there is little correlation between the occurrence of endoscopic ulcers and clinically significant events. For this reason, the ARAMIS studies reported here have limited the definition of GI adverse events to those requiring hospitalization.
Numerous studies have attempted to correlate NSAID use with serious GI complications by determining the ratio of NSAID-related events to non–NSAID-related events. Langman et al (1994) [19] found an odds ratio of 4.5 for NSAID-related peptic ulcer bleeding; Garcia-Rodriguez and Jick (1994) [20] reported a risk ratio of 3.9 for hospitalizations; and a meta-analysis of 40 studies published over 25 years found an overall odds ratio of 2.74 for serious GI complications and 7.75 for GI surgery. [16] The goal of current ARAMIS research is to better correlate GI events with NSAID use by determining adjusted odds ratios for specific causes of GI adverse events in patients taking NSAIDs versus control patients not taking NSAIDs. [21]
Incidence of GI Complications: ARAMIS Epidemiologic Study

Table 2

Table 3
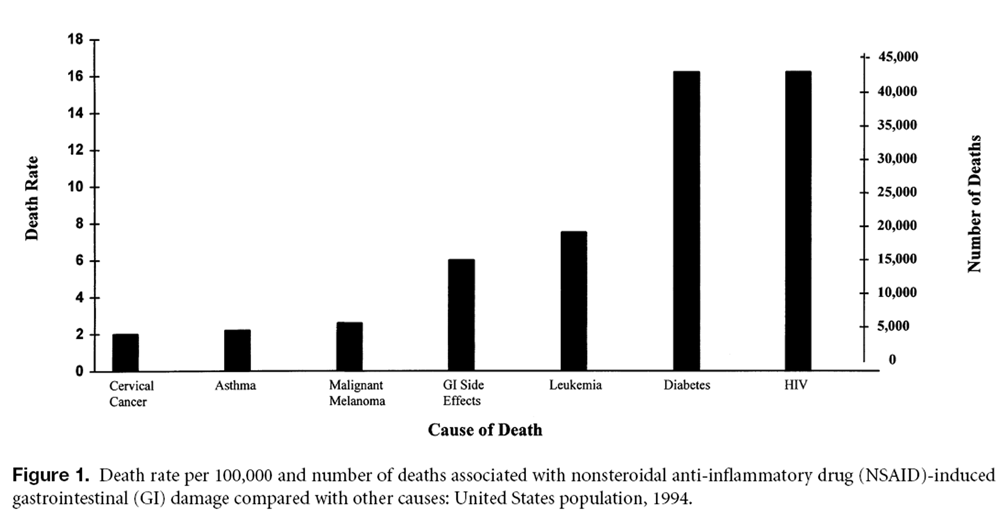
Figure 1 In this study, GI complications were defined as hospitalizations and deaths due to GI events. Complications were considered to be NSAID-related if the HAQ indicated NSAID use immediately before the event or if NSAIDs were noted on the hospital discharge summary. The patient population consisted of >4,000 arthritis patients with about 15,500 patient-years of observation (Table 2).
Hospitalizations. Of the hospitalizations for GI events in the RA patient group, 92.5% (124/134) were related to NSAID use, with an annual rate of 1.46% versus 0.27% for patients not taking NSAIDs. The relative risk of hospitalization for GI events for NSAID users was 5.49, only slightly less than the relative risk of lung cancer for smokers (7–8). The annual hospitalization rate for NSAID-related complications was significantly lower for OA patients than for RA patients and the relative risk about half that of RA patients. A likely explanation for the lower risk is that OA is a milder disease for which NSAID doses are generally lower, OA patients have fewer comorbid conditions, and prednisone (known to double the risk for NSAID-related GI events) is rarely used.
The Arthritis Foundation conservatively estimates that at least 13 million individuals in the United States with OA or RA regularly take NSAIDs (Table 3). Applying the ARAMIS data to these figures, the number of potential hospitalizations for serious GI complications is about 107,000 per year. These data correspond well to the figures obtained from the Tennessee Medicaid database. [22] At a conservative estimated cost of $10,000–$15,000 per hospitalization, the total annual cost exceeds $1 billion — a huge drain on national medical resources.
Deaths. Overall, approximately 10% of hospitalizations for upper GI bleeding result in death, and 80% of all ulcer- related deaths occurred in patients using an anti-inflammatory agent. [23] In the ARAMIS study, 26 deaths (all in RA patients) resulted from GI complications observed in the 12,224 patient-years of exposure to NSAIDs. Of these, 19 could be definitely attributed to NSAID use, for a raw annual GI death rate of 0.22% and a relative risk of 4.21. Although an incidence of 0.22% per year may sound trivial, over the 20–30 years that chronic arthritis patients may take NSAIDs, the incremental risk per patient and the total number of deaths add up very quickly.
Conservative calculations based on ARAMIS data estimate that the annual number of NSAID-related deaths (16,500) among patients with definite or probable RA or OA is comparable to the number of deaths from other common causes (Table 3; Figure 1). [24] NSAIDs cause more than one third as many deaths as human immunodeficiency virus (HIV) infection and almost as many deaths as asthma, cervical cancer, and malignant melanoma combined. If the estimates were to include all patients who take NSAIDs, the numbers would be overwhelming, yet the scope of this problem is generally underappreciated.
Are There Warning Signs For Serious GI Complications?
Dyspepsia is a common side effect of NSAID use, but is poorly correlated with endoscopic lesions or GI bleeding. [9, 10, 25] Of patients who develop ulcers or life-threatening GI complications, 50–60% will have had no previous warning signs or symptoms. [10, 23, 26, 27]
ARAMIS conducted a cohort study1 involving 1,921 patients on NSAIDs during their first 2.5 years of observation. Documented events included GI hospitalizations, upper GI endoscopies, NSAID-induced GI side effects not serious enough to warrant hospitalization (nausea, vomiting, upper or lower abdominal pain, heartburn, diarrhea, or blood in stools), and use of GI medications (H2 antagonists, sucralfate, or antacids). Patients who took misoprostol were excluded from analysis for 2 reasons:(1) the patient numbers were too small to allow independent analysis; and
(2) the mechanism of action differs from that of the other GI medications.During the 2.5-year observation period, the overall incidence of NSAIDrelated GI side effects was 15%, with a 2.2% incidence of serious GI complications requiring hospitalization. Patients who did have GI symptoms were slightly more likely to have a serious GI complication than those who had no warning symptoms (2.7% vs 2.0%), but the converse was not true: a staggering 81% (34/42) of patients who had serious GI complications had no prior GI symptoms.
Who Is At Risk For Serious GI Complications?
Since GI side effects are not a reliable warning sign, it is important to define risk factors for serious GI complications and to find ways to reduce that risk. A number of studies have addressed this issue with fairly consistent results, identifying as risk factorsadvanced age, [16, 22, 28–30]
higher NSAID dosages, [18, 19, 22, 31]
shorter duration of therapy, [16, 18, 19]
a history of GI problems, [18, 20, 31]
and
concomitant corticosteroid [20, 32, 33]
or
anticoagulant use. [20, 34]Most of these studies performed only univariate analyses.
ARAMIS evaluated risk factors for 114 RA patients who had GI events versus 1,921 RA patients who did not have GI events. Univariate analysis identified 11 variables associated with hospitalization or death at the predictive visit (before the event): age; disease duration; disability index (0–3); NSAID dose (% of maximum); number of categories of nonrheumatic drugs taken (0–8); NSAID GI side effects (ever); use of prednisone, antacids, or GI protective drugs; and comorbid conditions (ever).
Of note in this cohort study is the fact that female sex was not associated with an increased risk, whereas in casecontrol studies that is often the case, possibly due to the predominance of middle-aged women who are taking NSAIDs. Other categorical variables that univariate analysis did not correlate with an increased risk included white race, smoking (ever), and alcohol (ever). Continuous variables that were not significantly associated with an increased number of GI events included educational level, smoking (packs/day), alcohol (drinks/day), number of concurrent disease-modifying antirheumatic drugs (DMARDs), and 0–6 categories of comorbid conditions (ever).

Table 4 A multivariate model consisting of 5 variables — age, prednisone use, NSAID dose (% of maximum), disability index, and previous NSAID-induced GI side effect — was identified by stepwise logistic regression analysis (Table 4). The odds ratios (OR) for these variables can be used to estimate a patient’s risk of hospitalization for the following year.
Although “advanced age” is usually defined as ≥65 years, this is an arbitrary point. It is important to remember that individuals do not suddenly become “at risk” at the age of 65. Ongoing ARAMIS studies show that the risk increases steadily by roughly 4% per year increase in age.
The question of whether the risk for NSAID-related gastropathy is constant over time has been controversial. One school of thought — the mucosal adaptation theory — is that patients who are going to bleed will do so early in therapy and will discontinue taking NSAIDs or die, so that those who continue are “tolerant,” with a reduced risk of serious GI complications. The US Food and Drug Administration (FDA), in the process of deciding on revised labeling for NSAIDs, requested an ARAMIS study to look at this issue. The study followed approximately 1,600 new NSAID users or patients who restarted taking NSAIDs after a washout period of at least 6 months. The precise date and time of bleeding was obtained from hospital discharge summaries. A Kaplan-Meier survival analysis was performed to determine a continuous approximation of the hazard function, and the results indicate that throughout most of the 10-year follow-up period, the risk of GI bleed (the hazard function) remained constant. Patients on NSAIDs for 5 years have a 5-times greater risk of bleeding than do patients on NSAIDs for 1 year, whereas the risk after 3 months of NSAID therapy is approximately one-fourth the risk after 1 year. Thus, from the data, it appears that the body does not adapt to the insult that occurs with the use of NSAIDs.
The single most important factor that predicts GI bleeding is the duration of NSAID therapy. This factor was not included in the ARAMIS study because longitudinal data (e.g., duration of NSAIDs therapy) is not handled well by logistic regression methods. However, ongoing ARAMIS research is developing Cox proportional hazard models based on life-table analysis to incorporate this risk factor. The ultimate goal of this research is to develop point-based risk-factor algorithms — a simple one for patients themselves and a more complex and accurate one for physicians — to estimate individual risks from NSAID therapy.
Are Some NSAIDs More Toxic Than Others?

Table 5 All NSAIDs cause a full range of GI side effects, although they vary in frequency and severity. [10, 13, 19, 20, 35–40] Some of the ARAMIS studies have previously found substantial variations in the relative overall toxicity of NSAIDs using the Toxicity Index (TI). [41–45] A recent study utilized a subset of the TI to evaluate GI toxicity for 6,276 courses of 12 NSAIDs during 9,860 patient-years of observation. The GI-TI is a sum of GI symptoms per patient-year of exposure, weighted by severity and number of hospital days and adjusted for risk factors and other key variables (e.g., age, sex, center, drug initiation, disease duration, disability, educational level, duration of NSAID therapy, concurrent medications). Gastrointestinal toxicity, expressed as the GI-TI, varied from a low of 1 for salsalate to almost 4 for meclofenamate (Table 5). The differences in GI toxicity only become clinically or statistically significant between the NSAIDs at the upper and lower end of the range. The majority of the NSAIDs are in the middle range from 1.68–2.0.
As a result of the findings discussed above, the FDA has requested that NSAID manufacturers revise their labeling to include the following warnings:(1) serious GI toxicity can occur at any time, with or without warning symptoms;
(2) the absolute risk remains constant and the cumulative probability increases over time,
thus increasing the likelihood of developing a serious GI event with longterm NSAID use; and
(3) the lowest effective dose should be used for the shortest possible duration to minimize
the potential risk, and alternative therapies should be considered for high-risk patients.
Do H2 Antagonists and Antacids Help Prevent Serious GI Complications?
There are significant differences between the ulcers caused by Heliobacter pylori and those caused by NSAID use.The H. pylori ulcer is a predominantly duodenal ulcer, associated with low pH, and generally symptomatic.
NSAID-induced ulcers, on the other hand, are primarily gastric ulcers, associated with high pH, and usually asymptomatic.
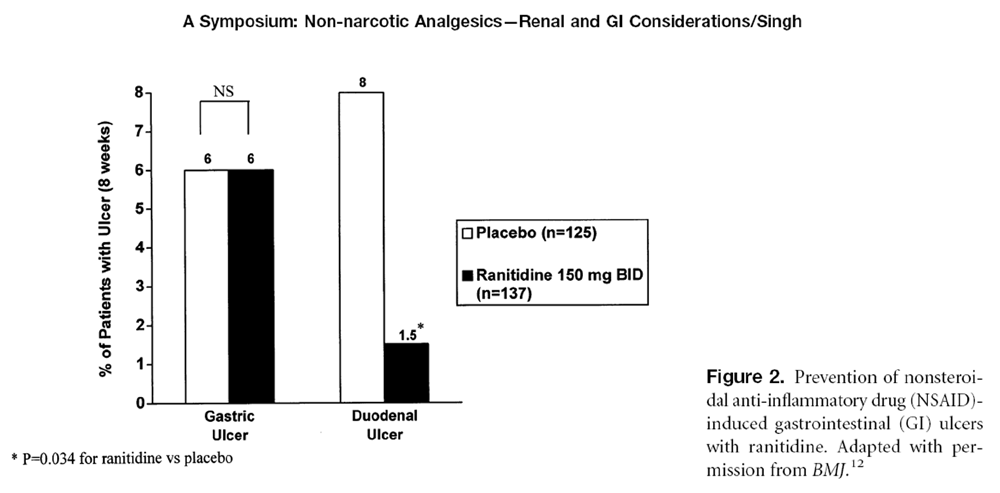
Figure 2 Acid suppression (e.g., with ranitidine) is very effective in preventing duodenal ulcers but has little effect on gastric ulcers (Figure 2). [12, 46, 47] Similarly, proton pump inhibitors reduce the incidence of duodenal but not gastric ulcers. Despite this well-established fact, approximately 34% of the 1,921 individuals in the ARAMIS study who took NSAIDs were taking concomitant acidreduction therapy. Of these 657 patients, 58.3% were taking antacids, 23.0% were taking cimetidine, 6.9% were taking ranitidine, 2.7% were taking sucralfate, and 2.2% were taking famotidine, while 8.5% were taking combinations of different GI medications. [1] Review of the ARAMIS database showed that 75% of GI medications co-prescribed with NSAIDS were for prophylaxis, and only 25% were for treatment. This is a dangerous practice, because these medications may suppress symptoms — potential warning signs — without reducing the risk of serious GI complications. They may provide a false sense of security to both physician and patient, encouraging long-term therapy with higher NSAID doses, which could eventually result in serious GI events.
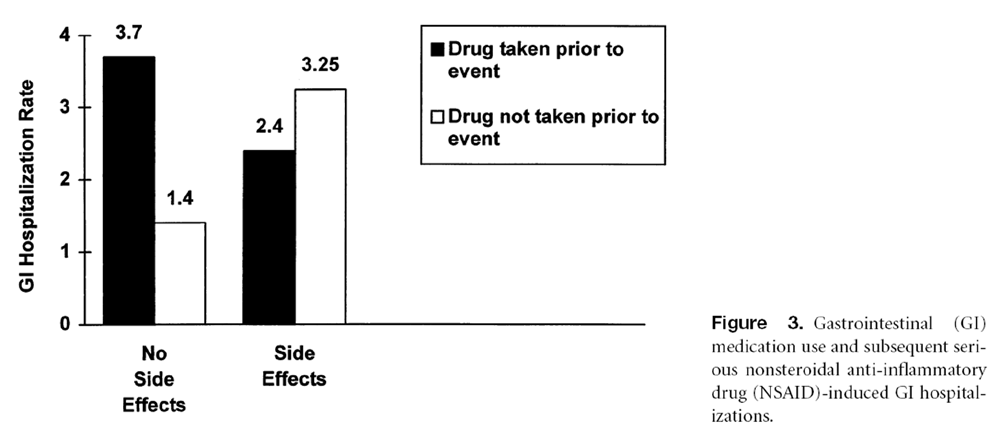
Figure 3

Figure 4 Indeed, the ARAMIS cohort study [1] showed that, of patients who had never had any GI side effects, those who were taking prophylactic GI medications had about 2.5 times more hospitalizations for NSAID-related GI complications than patients not taking GI medications (OR 2.69; 95% CI 5 1.36 –5.31, P ,0.05; Figure 3). For patients who had previous NSAID-related GI symptoms, the incidence of serious complications was similar for patients taking GI medications and patients not taking GI medications (OR 0.73; 95% CI 5 0.18 –2.96). The odds ratio was not significantly different (OR 0.57; 95% CI 5 0.13–2.45) after adjusting for differences in patient characteristics (i.e., age, sex, duration of RA, disability index, educational level, concurrent use of prednisone).
Using different methods (a case-control study design and a newly designed propensity score to measure the likelihood of having a gastroprotective drug prescribed), Avorn et al (1996) [48] reported similar results in a large group of New Jersey Medicaid patients (n = 3,524 with and 14,096 without hospitalizations). High-risk individuals taking histamine antagonist therapy, sucralfate, or omeprazole were about twice as likely (OR 1.7–2.1; P <0.05 each) to have serious GI bleeding as those not taking GI medications. The likelihood of hospitalization in the different groups compared with low-risk patients is shown in Figure 4 (Jerry Avorn, personal communication).
In clinical trials, the newer GI medications (e.g., misoprostol, omeprazole) have effectively reduced serious GI complications. Future ARAMIS studies will investigate their ability to similarly reduce serious GI events requiring hospitalization in clinical practice.
Currently, the accepted method for decreasing the risk of serious NSAID-related GI toxicity is to limit NSAID use. For OA, acetaminophen is the accepted first-line therapy when nonpharmacologic methods are insufficient; for RA, disease modifying antirheumatic drug (DMARD)-based strategies should be considered. Newer agents (e.g., tramadol and selective COX-2 inhibitors) may also be useful additions to the physician’s armamentarium for the treatment of chronic pain.
As patients in the ARAMIS database continue to be evaluated, research is currently underway to develop a simple point-score system based upon risk factors to estimate individual risks for developing serious NSAID-related GI complications. Once completed, this system should help the clinician quickly determine which patients are at risk for NSAID-associated adverse events and allow them to take the appropriate course of action.
References:
Singh G, Ramey DR, Morfeld D, et al.
Gastrointestinal tract complications of non-steroidal anti-inflammatory drug treatment in rheumatoid arthritis—
A prospective observational cohort study.
Arch Intern Med. 1996;156:1530–1536.Fries JF, McShane DJ.
ARAMIS (The American Rheumatism Association Medical Information System):
a prototypical national chronic-disease data bank.
West J Med. 1986; 145:798–804.Fries JF, Ramey DR, Singh G, et al.
A reevaluation of aspirin therapy in rheumatoid arthritis.
Arch Intern Med. 1993;153: 2465–2471.Singh G, Fries JF, Spitz PW, et al.
Toxic effects of azathioprine in rheumatoid arthritis: a national post-marketing perspective. (Abstr.)
Arthritis Rheum. 1989;32:837–843.Singh G, Fries JF, Williams CA, et al.
Toxicity profiles of disease modifying antirheumatic drugs in rheumatoid arthritis.
J Rheumatol. 1991;18:188–194.Fries JF, Spitz PW, Kraines RG, Holman HR.
Measurement of patient outcome in arthritis.
Arthritis Rheum. 1980;23: 137–145.Ramey DR, Singh GS, Fries JF.
The Health Assessment Questionnaire 1995: status and review.
In: Spilker B, ed.
Pharmacoeconomics and Quality of Life in Clinical Trials. 2nd ed.
Philadelphia: Lippincott-Raven; 1996:227–237.Buchanan WW, Brooks PM.
Prediction of organ system toxicity with anti-rheumatic drug therapy.
In: Bellamy N, ed.
Prognosis in the Rheumatic Diseases.
Boston: Kluwer Academic Publishers; 1991:403–450.Brooks PM, Day RO.
Nonsteroidal antiinflammatory drugs—differences and similarities.
N Engl J Med. 1991; 324:1716–1725.Hardin JG, Longenecker GL.
Handbook of Drug Therapy in Rheumatic Disease.
Pharmacology and Clinical Aspects.
Boston: Little, Brown and Company. 1992.Paulus HE.
Nonsteroidal Anti-inflammatory Drugs.
In: Kelly WN, Harris ED Jr, Ruddy S, Sledge CB, eds.
Textbook of Rheumatology.
Philadelphia: WB Saunders Company; 1989:765–791.Ehsanullah RS, Page MC, Tildesley G, Wood JR.
Prevention of gastroduodenal damage induced by non-steroidal anti-inflammatory drugs:
controlled trial of ranitidine.
BMJ. 1988;297:1017–1021.Caruso I, Bianchi Porro G.
Gastroscopic evaluation of antiinflammatory agents.
BMJ. 1980;280:75–78.McCarthy DM.
Nonsteroidal antiinflammatory drug induced ulcers: management by traditional therapies.
Gastroenterology. 1989;96:662–674.Gabriel SE, Jaakkimainen L, Bombardier C.
The cost-effectiveness of misoprostol for nonsteroidal antiinflammatory drug-associated adverse gastrointestinal events.
Arthritis Rheum. 1993;36:447–459.Gabriel SE, Jaakkimainen L, Bombardier C.
Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs.
A meta-analysis.
Ann Intern Med. 1991;115:787–796.Paulus HE.
Government affairs. FDA Arthritis Advisory Committee meeting:
risks of agranulocytosis/aplastic anemia, flank pain, and adverse gastrointestinal effects
with the use of nonsteroidal antiinflammatory drugs.
Arthritis Rheum. 1987;30:593–595.Silverstein FE, Graham DY, Senior JR, et al.
Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis
receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial.
Ann Intern Med. 1995;123:241–249.Langman MJ, Weil J, Wainwright P, et al.
Risks of bleeding peptic ulcer associated with individual non-steroidal antiinflammatory drugs.
Lancet. 1994;343:1075–1078.Garcia Rodriguez LA, Jick H.
Risk of upper gastrointestinal bleeding and perforation associated with
individual nonsteroidal anti-inflammatory drugs.
Lancet. 1994;343:769–772.Singh G, Ramey DR, Terry R, Triadafilopoulus G.
NSAID-related effects on the GI tract: an ever widening spectrum.(Abstr.)
Gastroenterology. 1997;112:A42.Griffin MR, Piper JM, Daugherty JR, et al.
Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons.
Ann Intern Med. 1991;114: 257–263.Armstrong CP, Blower AL.
Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulcerations.
Gut. 1987;28:527–532.National Center for Health Statistics.
GMWKI: total deaths for each cause by 5-year age groups, United States, 1994
(unpublished data from NCHS website data warehouse).
Hyattsville, MD: Public Health Service, 1997.Larkai EN, Smith JL, Lidsky MD.
Gastroduodenal mucosa and dyspeptic symptoms in arthritis patients during chronic nonsteroidal
anti-inflammatory drug use.
Am J Gastroenterol. 1987;82:1153–1158.Polisson R.
Nonsteroidal anti-inflammatory drugs: practical and theoretical considerations in their selection.
Am J Med. 1996;100(suppl 2A):31S–36S.Lichtenstein DR, Syngal S, Wolfe MM.
Nonsteroidal antiinflammatory drugs and the gastrointestinal tract. The double-edged sword.
Arthritis Rheum. 1995;38:5–18.Bjorkman DJ.
Nonsteroidal anti-inflammatory drug-induced gastrointestinal injury.
Am J Med. 1996;101(suppl 1A):25S– 32S.Longstreth GF.
Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage:
a population-based study.
Am J Gastroenterol. 1995;90:206–210.Greene JM, Winickoff RN.
Cost-conscious prescribing of nonsteroidal anti-inflammatory drugs for adults with arthritis.
A review and suggestions.
Arch Intern Med. 1992;152: 1995–2002.Hallas J, Lauritsen J, Villadsen HD, Gram LF.
Nonsteroidal anti-inflammatory drugs and upper gastrointestinal bleeding, identifying high-risk groups
by excess risk estimates.
Scand J Gastroenterol. 1995;30:438–444.Hochain P, Berkelmans I, Czernichow P, et al.
Which patients taking non-aspirin nonsteroidal anti-inflammatory drugs bleed?
A case-control study.
Eur J Gastroenterol Hepatol. 1995;7:419–426.Piper JM, Ray WA, Daugherty JR, et al.
Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs.
Ann Intern Med. 1991;114:735–740.Shorr RI, Ray WA, Daugherty JR, et al.
Concurrent use of non-steroidal anti-inflammatory drugs and oral anticoagulants places elderly persons
at high risk for hemorrhagic peptic ulcer disease.
Arch Intern Med. 1993;153:1665–1670.Lanza FL, Royer GL, Nelson RS, et al.
A comparative endoscopic evaluation of damaging effects of nonsteroidal antiinflammatory agents
on the gastric and duodenal mucosa.
Am J Gastroenterol. 1981;75:17–21.Lanza FL, Nelson RS, Greenberg BP.
Effects of fenbufen, indomethacin, naproxen and placebo on gastric mucosa of normal volunteers.
Am J Med. 1983;75:75–79.Lanza FL.
Endoscopic studies of gastric and duodenal injury after the use of ibuprofen, aspirin, and other
nonsteroidal antiinflammatory drugs.
Am J Med. 1984;77(suppl 1a):19 –24.Lanza F, Rack MF, Lyn M, et al.
An endoscopic comparison of the effects of etodolac, indomethacin, ibuprofen, naproxen, and placebo
on the gastrointestinal mucosa.
J Rheumatol. 1987;14:338–341.Lanza F, Rack MF, Doucette M, et al.
An endoscopic comparison of the gastroduodenal injury seen with salsalate and naproxen.
J Rheumatol. 1989;16:1570–1574.Carson JL, Strom BL, Morse ML, et al.
The relative gastrointestinal toxicity of the nonsteroidal anti-inflammatory drugs.
Arch Intern Med. 1987;147:1054–1059.Fries JF, Spitz PW, Williams CA, et al.
A toxicity index for comparison of side effects among different drugs.
Arthritis Rheum. 1990;33:121–130.Fries JF, Williams CA, Block DA.
The relative toxicity of non-steroidal anti-inflammatory drugs.
Arthritis Rheum. 1991;34:1353–1360.Fries JF, Williams CA, Ramey D, Bloch DA.
The relative toxicity of disease modifying antirheumatic drugs (DMARDs).
Arthritis Rheum. 1993;36:297–306.Singh G, Williams CA, Ramey Dr, Fries JF.
A toxicity index for comparison of gastrointestinal toxicity of non-steroidal anti-inflammatory drugs (NSAIDs). (Abstr.)
Arthritis Rheum. 1993;36(suppl 9):S178.Singh G, Ramey Dr, Morfeld D, Fries JF.
Comparative toxicity of non-steroidal anti-inflammatory agents.
Pharmacol Ther. 1994;62:1750–1791.Robinson MG, Griffin JW Jr, Bowers J, et al.
Effect of ranitidine on gastroduodenal mucosal damage induced by nonsteroidal anti-inflammatory drugs.
Dig Dis Sci. 1989;34: 424–428.Oddsson E, Gudjonsson H, Thjodleifsson B.
Comparison between ranitidine and omeprazole for protection against gastroduodenal damage caused by naproxen.
Scand J Gastroenterol. 1992;27:1045–1048.Avorn J, Solomon D, Levin R, Lo J.
Epidemiologic analysis of prophylactic drug use and NSAID gastropathy. (Abstr.)
Arthritis Rheum. 1996;39(suppl):S165.
Return to IATROGENIC INJURY
Return to SAFETY OF CHIROPRACTIC
Return to SPINAL PAIN MANAGEMENT
Since 8-01-1998


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |