

Deconstructing Chronic Low Back Pain in the Older Adult -
Step by Step Evidence and Expert-Based Recommendations
for Evaluation and Treatment.
Part III: Fibromyalgia SyndromeThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Pain Medicine 2015 (Sep); 16 (9): 1709–1719 ~ FULL TEXT
OPEN ACCESS Gita Fatemi, Meika A. Fang, Paula Breuer, Paul E. Cherniak, Angela Gentili,
Joseph T. Hanlon, Jordan F. Karp, Natalia E. Morone, Eric Rodriguez,
Michelle I. Rossi, Kenneth Schmader, Debra K. Weiner
VA Greater Los Angeles Healthcare System,
Los Angeles, California.OBJECTIVE: To present the third in a series of articles designed to deconstruct chronic low back pain (CLBP) in older adults. The series presents CLBP as a syndrome, a final common pathway for the expression of multiple contributors rather than a disease localized exclusively to the lumbosacral spine. Each article addresses one of 12 important contributors to pain and disability in older adults with CLBP. This article focuses on fibromyalgia syndrome (FMS).
METHODS: A modified Delphi approach was used to create the evaluation and treatment algorithm, the table discussing the rationale behind each of the algorithm components, and the stepped-care drug recommendations. The team involved in the creation of these materials consisted of a principal investigator, a 5-member content expert panel, and a 9-member primary care panel. The evaluation and treatment recommendations were based on availability of medications and other resources within the Veterans Health Administration (VHA) facilities. However, non-VHA panelists were also involved in the development of these materials, which can be applied to both VA and civilian settings. The illustrative clinical case was taken from the clinical practice of the principal investigator.
RESULTS: Following expert consultations and a review of the literature, we developed an evaluation and treatment algorithm with supporting materials to aid in the care of older adults with CLBP who have concomitant FMS. A case is presented that demonstrates the complexity of pain evaluation and management in older patients with CLBP and concomitant FMS.
CONCLUSIONS: Recognition of FMS as a common contributor to CLBP in older adults and initiating treatment targeting both FMS and CLBP may lead to improved outcomes in pain and disability.
KEYWORDS: Back Pain; Chronic Pain; Elderly; Fibromyalgia; Low Back Pain
From the FULL TEXT Article:
Introduction
Fibromyalgia syndrome (FMS) is a challenging diagnosis for many health care providers given the breadth of symptoms patients have on presentation and the paucity of specific objective findings. Twenty-five years ago, FMS was initially described as a syndrome characterized by widespread musculoskeletal pain that could not be explained by another diagnosis. [1] FMS has been increasingly recognized to encompass additional features such as fatigue and nonrestorative sleep, and these other symptoms are included in the updated 2010 American College of Rheumatology (ACR) criteria. [2] The prevalence of FMS increases with age, has a female preponderance, peaks in the seventh decade, and varies from <1% to 5%. [3]
Prior reports have shown a relationship between chronic low back pain (CLBP) and widespread pain among patients in a variety of settings. A cross-sectional postal questionnaire study of musculoskeletal symptoms in the community reported that 893 of the 2,893 respondents (31%) experienced low back pain in the previous week with 222 (24%) of these individuals having localized low back pain and 281 (31%) reporting widespread pain in at least four other areas. [4] Recently, a large cross-sectional comparative analysis of 647 patients who were seen for CLBP in a primary care setting revealed that approximately 25% of these individuals also experienced chronic widespread pain as defined by the 2010 ACR criteria for fibromyalgia. [5] Those patients with CLBP and chronic widespread pain were more likely to be female and have more somatic symptoms and comorbidities than patients with only CLBP. Over 40% of patients with a primary spine diagnosis who presented to an academic outpatient pain clinic met survey criteria for FMS. [6] In our clinical experience, one in five older adults with CLBP has evidence of FMS, a prevalence that has also been suggested by other investigators. [7]
Because of the high prevalence of chronic widespread pain among patients with CLBP, health care providers should perform a thorough history, physical examination, and limited laboratory testing to determine if FMS is a potential contributor to the pain and disability experienced by older adults with CLBP. Patients with FMS may experience pain in the low back, hips, and buttocks that could be mistaken for pain arising from disorders of the lumbar spine such as spinal stenosis or radiculopathy. FMS is under-recognized in older adults as Jacobson et al. found that in older adults, it took an average of 7 years for the diagnosis of FMS to be made from the time of symptom onset. [8] Moreover, many of these patients were treated with inappropriate medications such as opioids and had persistent, uncontrolled symptoms. [8]
Early recognition of FMS and other extra-spinal factors that may directly contribute to CLBP or independently cause pain and disability may affect management and optimize patient outcomes. We present an older patient with chronic upper and lower back pain who had co-existing FMS. This case demonstrates the multitude of symptoms that patients with CLBP and comorbid FMS often report and the success that can be achieved with a multifaceted approach to treatment.
Methods
A detailed description of the modified Delphi method consisting of a content expert panel and primary care review panel is provided in the series overview. [9] This technique was used to create the algorithm (Figure 1), the table detailing the rationale behind the algorithm components (Table 1), and the stepped-care medication table (Table 2).
Figure 1.
Algorithm for the evaluation and treatment of fibromyalgia syndrome in an older adult.
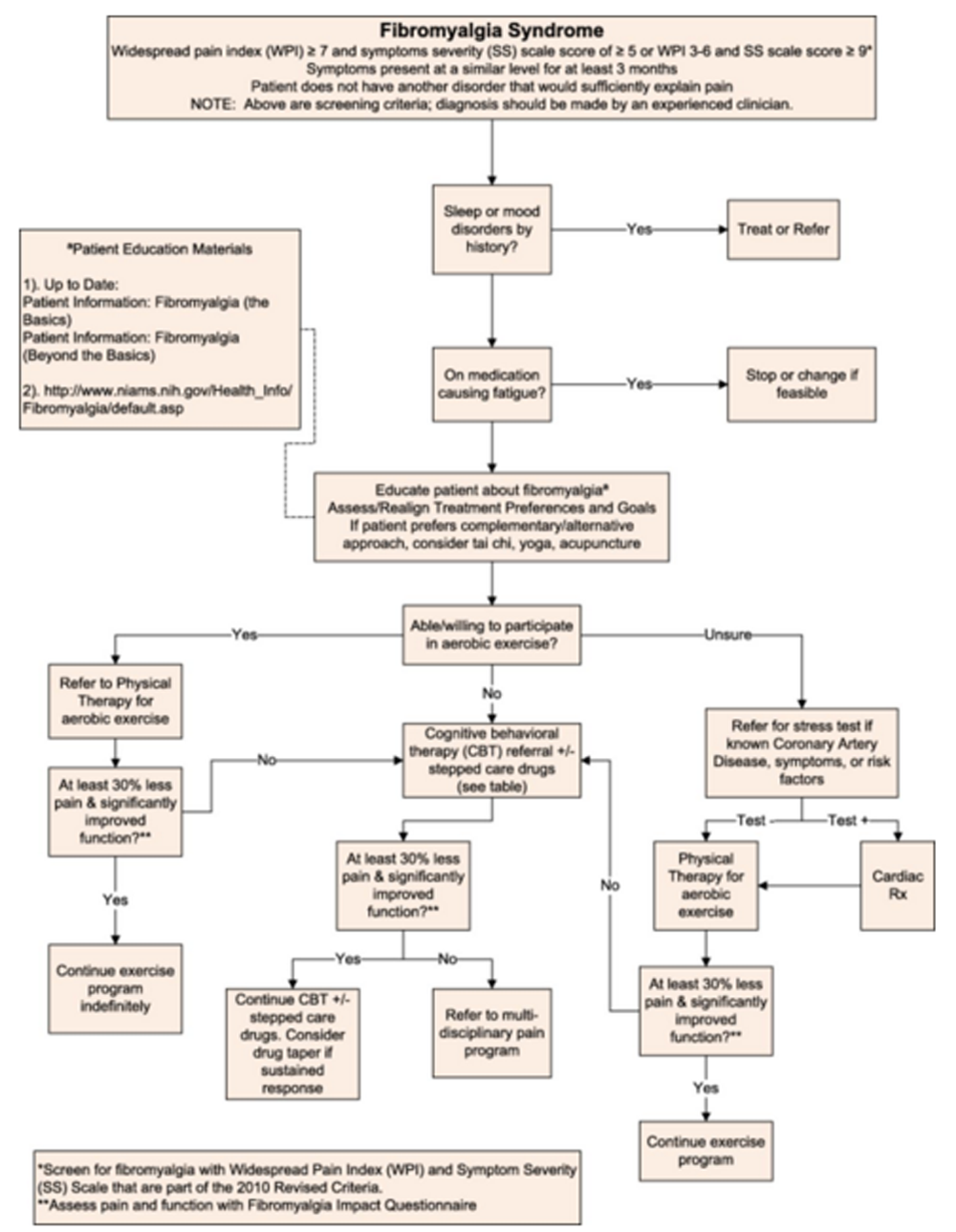
Table 1.
Fibromyalgia syndrome: theoretical and pragmatic underpinnings of algorithm recommendations
Algorithm component Comments 30% pain reduction as significant Data on 2,724 subjects from 10 placebo controlled trials of pregabalin in diabetic neuropathy, postherpetic neuralgia, chronic low back pain, fibromyalgia, and osteoarthritis. Aerobic exercise early in Rx Strong efficacy evidence in general population of patients with fibromyalgia; not tested explicitly in older adults.
Strong efficacy evidence of multiple exercise benefits in older adults in general (i.e., not specifically in those with fibromyalgia).Cognitive behavioral therapy (CBT) Strong efficacy evidence in general population of patients with fibromyalgia.
High quality evidence is lacking specifically in older adults. Arthritis pain self-management programs that contain some CBT elements demonstrate efficacy.Patient education There is little evidence-based data on the optimal patient education program. Experts have recommended individual and/or group education presented by health professionals knowledgeable about fibromyalgia. Education sessions would cover information about symptoms, course of fibromyalgia, comorbid conditions, potential etiologies for fibromyalgia, role of psychosocial factors in contributing to pain, pharmacologic and nonpharmacologic therapy, and self-management approaches. Education could also focus on strategies to prevent nocebos.
Suggested Patient Education materials:
Patient Information: Fibromyalgia (The Basics)
Patient Information: Fibromyalgia (Beyond the Basics)
National Institute of Arthritis and Musculoskeletal and Skin Diseases:
www.niams.nih.gov/Health_Info/Fibromyalgia/default.aspSleep disorder evaluation Sleep disorders are common in people with fibromyalgia and may be a risk factor for developing this condition. Mood disorder evaluation Fibromyalgia is frequently associated with psychiatric disorders such as anxiety and depression. Medications causing fatigue Chronic fatigue is a common problem in people with fibromyalgia. Minimizing the use of medications which cause fatigue may help alleviate symptoms of fatigue. Some medications that can cause fatigue include benzodiazepines, skeletal muscle relaxants, some antidepressants, antipsychotics, and anticonvulsants. GabapentinOL Not evaluated specifically in older adults with fibromyalgia.
Recommended as first line in veterans as it is on formulary.Duloxetine
and
VenlafaxineDuloxetine is FDA approved for the treatment of FMS. Milnacipran FDA approved for the treatment of fibromyalgia.
Not recommended as not available in VA, even nonformulary.Pregabalin FDA approved for treatment of fibromyalgia.
Non-formulary in VA.NortriptylineOL
and
DesipramineOLThere is strong efficacy evidence for amitriptyline in the treatment of fibromyalgia, but this tricyclic antidepressant has strong anticholinergic side effects in older adults and is not recommended (on Beers list).
Neither nortriptyline nor desipramine are on Beers list, thus if a tricyclic antidepressant is to be initiated, these are the preferred agents. Both are on formulary at the VA.Cyclobenzaprine is absent from the algorithm. There is strong efficacy evidence for cyclobenzaprine in the treatment of fibromyalgia, but because of strong anticholinergic side effects, it is on Beers list and not recommended for older adults.
Table 2.
Stepped care drug management of fibromyalgia*
Drug Dose/Titration Important Adverse Effects/Precautions Gabapentin Start 100 mg nightly. Increase by 100 mg weekly.
Renal dosing: CLcr ≥30 mg/min, titrate to 600 mg BID; CLcr 15–29 mL/min, titrate to 300 mg twice a day; CLcr <15 mL/min, titrate to 300 mg daily. Supplement dose after dialysis.Confusion, dizziness, somnolence, peripheral edema, weight gain. Withdrawal syndrome with abrupt discontinuation. Nortriptyline
and
DesipramineStart 10 mg at night. Increase by 10 mg weekly to max dose of 50 mg at night. Lower doses and slower titration recommended with hepatic impairment. Constipation, orthostatic hypotension, urine retention. Anticholinergic; may exacerbate narrow-angle glaucoma, BPH; falls, delirium, seizures, BBB. Can prolong QT and cause Torsades de Pointes. Get EKG before starting. Pregabalin Start 25–50 mg at night. Increase by 25–50 mg weekly up to 100 mg twice a day. Max dose 300 mg daily. Renal dosing: CLcr 30–60 mL/min, adjust dose to 150–300 mg daily; Clcr <15 mL/min, use no more than 75 mg/d. Confusion, dizziness, somnolence, peripheral edema, weight gain. Duloxetine Initiate 20–30 mg daily. Increase to 60 mg after 1–2 weeks. Max dose 60 mg daily. Not recommended in ESRD or with CLcr < 30 mL/min. Not recommended for use in hepatic impairment. Nausea, dry mouth, sedation/falls, urinary retention, and constipation. Abrupt d/c may cause withdrawal syndrome; contraindicated with hepatic disease and/or heavy EtOH. Venlafaxine ER Initiate 25 mg daily. Increase by 25 mg weekly up to 225 mg/d. ESRD: reduce dose by 50% and give after dialysis. Reduce dose by 50% in mild-moderate hepatic impairment. Nausea, dizziness, diaphoresis, dry mouth, insomnia, constipation 1. Prefer monotherapy starting with low doses.
• Initial trial of tricyclic antidepressant (nortriptyline or desipramine) OR initial trial of gabapentin depending on the patient's comorbidities, preference, cost, symptoms most concerning to patient.
• If pain or symptom reduction <30% with tricyclic antidepressant, try dual reuptake inhibitor (duloxetine or venlafaxine) and see #2 below.
• If pain or symptom reduction <30% with gabapentin, try pregabalin and see #2 below.
2. If pain or symptom reduction <30% with monotherapy, can try combination therapy using low doses of medications from different classes targeting the symptoms most concerning to the patient or referral to multidisciplinary pain treatment program.
3. If pain or symptom reduction >30% for an extended period of time, decrease to lowest effective dose of medication. Consider stopping medication to see if fibromyalgia can be controlled without pharmacologic therapy. Monitor for antidepressant discontinuation syndrome such as cholinergic rebound (nausea, sweating, urinary urgency) or serotonin/norepinephrine re-uptake inhibitor discontinuation syndrome (dizziness, weakness, nausea, headache, lethargy, insomnia, anxiety, poor concentration, and paresthesias). Monitor for symptoms of gabapentin or pregabalin withdrawal such as seizures.
CLcr = creatinine clearance;
BID = twice a day;
ESRD = end stage renal disease;
BPH = benign prostatic hypertrophy;
BBB = bundle branch block;
EtOH = ethanol.The five expert panel members for the FMS algorithm included a geriatrician, physical therapist, geriatric psychiatrist, pharmacist, and a rheumatologist.
Case Presentation
Relevant History: A 67–year-old female presents to the Pain Clinic in 2013 for evaluation of her chronic upper and lower back pain. During the 1980s, she injured her neck while playing volleyball. In 1999, she was involved in a car accident and experienced a whiplash injury to her neck. She has never experienced a spinal fracture and denies any history of back surgery. She continues to experience occasional neck pain. She also has shoulder pain that intermittently disrupts her sleep because she has to change positions in bed to feel better. For several years, she has suffered from bilateral right more than left sided upper back pain and lower back pain, as well as left-hand fourth and fifth digit numbness and tingling.
Last autumn, while she was working in her garden, she experienced worsening of her chronic upper back and lower back pain. The back pain is nonradiating and not associated with weakness in her upper or lower extremities. The pain is relieved with rest, exacerbated by activity, and does not awaken her at night. On occasion, she also notes right-sided abdominal pain and bilateral finger pain. She reports a history of chronic fatigue. She denies having any problems with her memory, fever, nightsweats, anorexia, weight loss, and changes in bowel or bladder habits. She tried tramadol without significant relief of her pain. Meloxicam relieves her back pain more effectively than tramadol. She also completed a course of physical therapy that alleviated her pain while she was participating in the program.
Relevant Physical Exam: The patient is awake, alert, fully oriented, cooperative, and in no acute distress. A physical exam is notable for several tender points of her neck and upper and lower back muscles after applying enough thumb pressure to cause the nailbed to blanch. The range of motion of her back and extremities is intact. Tenderness is noted over bilateral Heberden's and Bouchard's nodes.
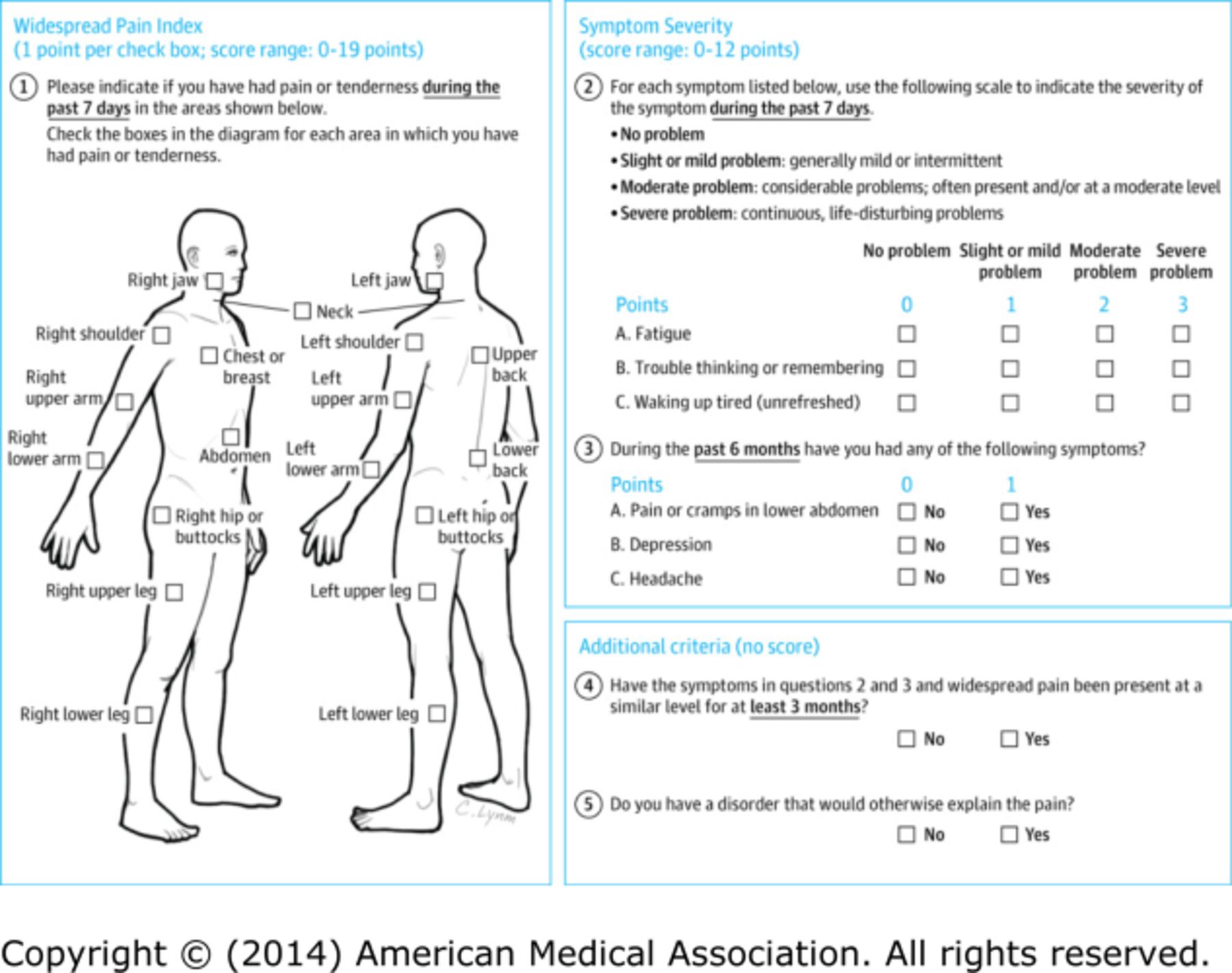
Relevant Tests: The patient completes a FMS patient self-report survey [10] that reveals a score of 9 on the Widespread Pain Index (WPI) and 6 on the Symptom Severity (SS) score (see Figure 2). Complete blood count, chemistries, liver function tests, erythrocyte sedimentation rate, C-reactive protein, creatine kinase, and thyroid stimulating hormone were all unremarkable. The diagnosis of FMS was confirmed by a rheumatologist.
Figure 2.
Patient self-report survey for the assessment of fibromyalgia based on criteria in the 2011 modification of the ACR preliminary diagnostic criteria for fibromyalgia. Scoring information is shown in blue. The possible score ranges from 0 to 31 points; a score ≥13 points is consistent with a diagnosis of fibromyalgia.
Clinical Course: We educated the patient regarding the diagnosis and treatment of FMS, her role in its management, and setting realistic treatment goals. We encouraged physical therapy specifically focused on aerobic exercise. She began gabapentin 100 mg by mouth every evening for a week that was titrated to 200 mg every evening. Gabapentin decreased her pain but this medication was eventually discontinued because of a 20 pound weight gain. Acupuncture, trigger point therapy, and aerobic exercise were recommended for the pain of her neck, upper back, and lower back. Topical lidocaine, transcutaneous electrical nerve stimulation, and acupuncture were ordered for the abdominal muscle pain. For her painful hand osteoarthritis, we prescribed acetaminophen, 1000 mg three times daily, and meloxicam as needed. She maintains realistic treatment expectations, has embraced self-management of her pain, and now focuses her energy and attitude on her ability to function despite the persistence of some pain.
Approach to Management
The patient presented came for evaluation of chronic upper and lower back pain and was ultimately diagnosed with FMS, a centralized pain disorder, that can contribute to pain and disability in people with CLBP. The finding of chronic widespread pain among patients with CLBP is common in the primary care setting. [4–7] Evaluating a patient for FMS should be performed in patients who have diffuse pain that is not entirely explained by injury or inflammation. Once the diagnosis of FMS has been made, a multidisciplinary approach to treatment is recommended. The expert panel that created the FMS algorithm (Figure 1) recommended assessing for the potential presence of a centralized pain disorder using ACR survey criteria for FMS. Unlike the original 1990 ACR survey criteria for FMS, the 2010 ACR criteria no longer require a tender point examination but do acknowledge that widespread musculoskeletal pain is accompanied by cognitive and somatic symptoms that are measured via physician assessment, the WPI, and SS Scale. [2, 3] A 2011 modification of the 2010 ACR survey criteria for use in clinical and epidemiological studies led to the development of a patient self-report questionnaire (Figure 2) [10] that can be easily administered in the clinic setting and determines whether or not a patient meets ACR survey criteria for FMS. In this patient, the history, physical examination, and basic laboratory testing were performed to exclude other conditions that may mimic FMS. [6] Evaluation with serologic tests such as the antinuclear antibody and rheumatoid factor is not indicated unless the patient has signs and symptoms suggestive of a systemic inflammatory rheumatic disease.
As shown in Figure 1, management of FMS in patients with CLBP starts with evaluating the patient for the presence of potentially modifiable risk factors, such as sleep and mood disorders, that are commonly seen in both CLBP and FMS. Over 80% of patients with FMS experience one or more sleep problems, such as difficulty falling asleep, staying asleep, or waking up too early and these sleep problems are associated with a decrease in health-related quality of life. [11] More than 60% of adults with CLBP have insomnia with pain intensity and fatigue as the main determinants associated with insomnia. [12] In general, insomnia is a common complaint in older adults with over 50% having difficulty going to sleep or maintaining sleep. [13] Recent studies have indicated that there is a reciprocal relationship between sleep disturbances and pain and that sleep problems are an important contributor to the initiation and persistence of chronic pain. [14] Older patients with CLBP and comorbid FMS are at significantly increased risk for a sleep disorder. If a sleep disorder is suspected, evaluation should be undertaken to determine if they may have insomnia, sleep-related breathing disorder, or sleep-related movement disorder. Medical history or medications should be reviewed to assess for potential contributors to insomnia. Minimizing use of medications aggravating insomnia as well as treating medical and psychiatric conditions contributing to sleep difficulties are recommended. Theophylline, oral decongestants such as pseudoephedrine, and stimulants such as methylphenidate are examples of medications that may cause or worsen insomnia, especially in older adults. An Insomnia algorithm will be published later in this CLBP article series.
Psychiatric conditions such as depression, anxiety, obsessive-compulsive disorder, and posttraumatic stress disorder (PTSD) are often associated with FMS. [6, 15] There is a higher prevalence of depression, anxiety, and PTSD in patients with FMS as compared with the general population. [16, 17] In approximately 66% of patients with FMS, an experience believed to be the most burdensome traumatic event and PTSD symptoms developed prior to the onset of FMS. [17] Physicians should, therefore, screen for psychiatric conditions in FMS patients. Treatment of the psychiatric disorders should be initiated or appropriate referrals made. Algorithms on Depression and Anxiety will be published later in this CLBP series.
Fatigue is a distressing symptom among patients with CLBP and FMS and is likely influenced by the presence of pain, mood disturbance, unrefreshing sleep, and use of certain medications. [18, 19] Because fatigue may occur as a result of medications that cause central or peripheral nervous system depression or lead to anemia, the patient's medication list should be thoroughly reviewed for offending drugs and stopped if feasible. [19] Examples of medications that can cause drug-related fatigue include benzodiazepines, skeletal muscle relaxants, anticholinergics, anticonvulsants, and sedating antidepressants.
The next steps in the management of CLBP complicated by FMS in older adults should focus on nonpharmacologic treatment (patient education, exercise, cognitive-behavioral therapy, complementary and alternative therapy). Patient education is aimed at helping patients set realistic treatment goals, determine treatment preferences, reduce disability, and increase self-management skills. The primary objective of multidisciplinary treatment is aimed at shared decision making with identification of goals of therapy from the patient's perspective. The focus is on optimizing function with the understanding that it is unrealistic to expect complete resolution of pain. [20] Patients should be instructed to expect, on average, 30% reduction in pain or 2 points on an 11 point (i.e., 0–10) scale. [21]
There is no consensus on an optimal patient education program. Experts have recommended individual or group education classes presented by health professionals knowledgeable about FMS. Patients with FMS have shown benefit from learning about its symptoms, the course of FMS, the role of psychosocial factors in contributing to pain, pain physiology, pharmacologic and nonpharmacologic therapy, and self-management approaches. [22, 23] Patient education material can be obtained from online resources (Figure 1). Health care providers and patients should be aware of the nocebo effect, the expectation that medical intervention will cause harm, as it is prevalent in patients with FMS. Nocebos contribute to treatment failure, and patients should, therefore, be educated to help prevent poor treatment outcomes. [24]
Various types of exercise (aerobic, resistance, and aquatic) are useful because they decrease pain and improve function in patients with FMS with successful exercise interventions usually involving 30–60 minutes of light to moderate intensity exercise occurring three times a week for 7 weeks. [25] Aerobic exercise is particularly recommended because it has both cognitive and physical benefit for older adults [26] and strong efficacy evidence for the treatment of FMS. [27] Depending on the older adult's experience with exercise and other comorbidities, it may be prudent to initiate such a program under the guidance of a physical therapist with the goals of self-maintained graded exercise and exercise pacing and to screen for cardiac and other medical conditions that would potentially contraindicate aerobic exercise. It is important that patients with FMS understand that the benefit of aerobic exercise diminishes if the exercise is discontinued. [27] Both land-based and pool exercises have been evaluated, and they appear to have equal efficacy and improve wellness and fitness. [28, 29]
For patients who are unable to participate in an exercise intervention, cognitive behavioral therapy (CBT) should be offered because it has some evidence for efficacy in the treatment of FMS with demonstrated improvement in pain, mood, and function. [30, 31] Reviews specifically focused on CBT for the treatment of chronic pain in the elderly have found CBT to significantly decrease self-reported pain intensity without an effect on medication use or depressive symptoms. [31]
Complementary and integrative nonpharmacologic modalities have also been evaluated in the treatment of FMS and are an attractive option for older adults because of the low risk of adverse effects. There is low to moderate evidence for acupuncture as a treatment to relieve pain and stiffness arising from FMS [32], and we have found it beneficial on a case by case basis. Tai chi, a Chinese martial art with slow meditative movements, has been promoted for its health benefits. Recent studies have shown the benefit of Tai Chi in FMS with reduced Fibromyalgia Impact Questionnaire scores and improvements in other patient-reported surveys of physical function and quality of life. [33, 34] Yoga has also shown significant benefit for reducing pain and fatigue in patients with FMS. [35]
If the older patient with CLBP complicated by FMS continues to have disabling widespread pain despite addressing sleep disturbances and mood disorders, providing patient education, and trying nonpharmacologic therapy, then pain medication would be the next step. The decision to prescribe medication for the treatment of FMS in older adults should be made judiciously and based on knowledge of potential drug–drug and drug–disease interactions as well as age-related changes in pharmacokinetics and pharmacodynamics. Guidelines for stepped care FMS pain management in older individuals is provided in Table 2 starting with low doses and titrating cautiously. This table includes starting dose and titration suggestions with adjustments for renal or hepatic dysfunction and highlights important adverse effects. [36, 37] There are few studies that evaluate the use of pharmacotherapy in the treatment of FMS in older adults. Fitzcharles et al. note that the potential therapeutic benefit of medications used to treat FMS in the elderly is often overshadowed by adverse effects. [38]
Only three medications (pregabalin, milnacipran, and duloxetine) are FDA-approved for the treatment of FMS. Despite its FDA approval for the treatment of FMS, milnacipran was not included in the stepped care drug management table because it is unavailable through the VHA. Amitriptyline and cyclobenzaprine have strong efficacy evidence in FMS, but are not included in the table because they meet Beers' criteria for potentially inappropriate medications in older adults due to their significant anticholinergic effects. [39] If prescribing a tricyclic antidepressant as step one in the treatment of FMS, nortriptyline, the active metabolite of amitriptyline, or desipramine are preferable options in older adults because they have less anticholinergic effects than amitriptyline and cyclobenzaprine.
Gabaergic analgesics such as gabapentin and pregabalin are second step agents. There is minimal evidence for gabapentin in reducing pain associated with FMS, and its use is limited because over 60% of people experience an adverse event. [40] If FMS does not respond to a step two agent, then a step three agent, a serotonin-norepinephrine reuptake inhibitor (SNRI), such as duloxetine or venlafaxine, could be tried. It is important to note that there are no studies that have evaluated formally SNRIs in older FMS patients. [22] Nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids should be avoided given their potential to do harm without clear benefit for the treatment of FMS. [39]
Resolution of Case
The patient has FMS which is a major contributor to pain and disability from her CLBP. She is representative of a subset of CLBP patients with a centralized pain phenotype whose pain is amplified by central nervous system factors and unlikely to be alleviated with only peripherally directed analgesics. [6] Successful management of these patients requires evaluation for any sleep problems, mood disorders, and psychosocial stressors as well as patient education regarding the treatment goal of optimizing function despite the persistence of some pain. She was educated about the inappropriateness of opioid analgesics and the importance of self-management with physical therapy and massage. The patient participated in several weeks of aerobic exercise with great improvement in her neck and upper back pain and planned to join a club where she would have access to aquatic therapy. She tried acupuncture for her myofascial pain with benefit, albeit lasting only several days postprocedure. She was started on gabapentin 100 mg at night time but had to stop this medication because of weight gain. She has maintained realistic treatment expectations, embraced self-management of her FMS, and focused her energy and attitude on her ability to function despite the persistence of some pain.
Summary
Older adults who present for evaluation of CLBP should be assessed for FMS, as chronic widespread pain is a common finding in those with CLBP. [4–6] Importantly, FMS is a diagnosis that is often overlooked in older adults, resulting in a delay in treatment. [6] When the diagnosis of FMS is suspected, the FMS patient self-report questionnaire should be administered as a screening tool. [2, 10] In the event that the patient screens positive, a specialist should be consulted to confirm FMS if doubt exists regarding the diagnosis. Important comorbidities such as depression, anxiety, sleep disorders, and PTSD should be addressed. [10–18] Medications should be reviewed to determine if they may be contributing to fatigue. [19]
As is true for all patients with chronic non-cancer pain, educating patients with FMS about realistic treatment goals is critical, focusing on optimizing function. [20] A multifaceted approach to treatment can achieve significant improvements in the health-related quality of life of older adults with CLBP and coexisting FMS.
Key Points
Older adults with CLBP should be evaluated for fibromyalgia as a contributor to their pain and functional impairment.
Older adults with widespread pain should not be automatically diagnosed with fibromyalgia. It is important to conduct a thorough history and physical exam as fatigue and widespread pain may indicate a serious underlying disorder such as malignancy or connective tissue disease.
Evaluation and treatment should include identification of conditions (e.g. mood disorders) and/or medications that may cause or exacerbate fibromyalgia symptoms.
Patients with fibromyalgia should be educated about their diagnosis to help create realistic treatment goals with an emphasis on reducing (not eliminating) pain and improving everyday function.
An array of treatment options is available for the treatment of FMS. Older adults with FMS should be educated and encouraged about the multiple treatment options available including aerobic exercise, cognitive behavioral therapy, judicious use of stepped-care medications, and interdisciplinary treatment.
Acknowledgments
This material is based on work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service. The contents of this report do not represent the views of the Department of Veterans Affairs or the US government.
The authors wish to thank Dave Newman for his overall assistance with this project and for his thoughtful review of the manuscript.
Conflict of interest:
The authors have indicated that they have no conflicts of interest regarding the content of this article.
References:
Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–72
Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–10
Jones GT, Atzeni F, Beasley M, et al. The prevalence of fibromyalgia in the general population—A comparison of the American College of Rheumatology 1990, 2010 and modified 2010 classification criteria. Arthritis Rheum 2015;67:568–75
Natvig B, Bruusgaard D, Eriksen W. Localized low back pain and low back pain as part of widespread musculoskeletal pain: Two different disorders? A cross-sectional population study. J Rehabil Med 2001;33:21–5
Viniol A, Jegan N, Leonhardt C, et al. Differences between patients with chronic widespread pain and local chronic low back pain in primary care — A comparative cross-sectional analysis. BMC Musculoskelet Disord 2013;14:351
Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum 2013;65:3285–92
Weiner DK, Sakamoto S, Perera S, Breuer P. Chronic low back pain in older adults: Prevalence, reliability, and validity of physical examination findings. J Am Geriatr Soc 2006;54:11–20
Jacobson SA, Simpson RG, Lubahn C, et al. Characterization of fibromyalgia symptoms in patients 55-95 years old: A longitudinal study showing symptom persistence with suboptimal treatment. Aging Clin Exp Res 2015;27:75–82
Weiner DK.
Deconstructing Chronic Low Back Pain in the Older Adult -
Shifting the Paradigm from the Spine to the Person
Pain Med 2015; 16 (5): 881–885Clauw DJ. Fibromyalgia: A clinical review. JAMA 2014;311:1547–55
Wagner JS, DiBonaventura MD, Chandran AB, Cappelleri JC. The association of sleep difficulties with health-related quality of life among patients with fibromyalgia. BMC Musculoskelet Disord 2012;13:199
Bahouq H, Allali F, Rkain H, Hmamouchi I, Hajjaj-Hassouni N. Prevalence and severity of insomnia in chronic low back pain patients. Rheum Int 2013;33:1277–81
Wennberg AM, Canham SL, Smith MT, Spira AP. Optimizing sleep in older adults: Treating insomnia. Maturitas 2013;76:247–52
Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain 2013;14:1539–52
Fietta P, Fietta P, Manganelli P. Fibromyalgia and psychiatric disorders. Acta Biomed 2007;78:88–95
Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: Relationship to somatic and psychosocial variables. Psychosom Med 2004;66:837–44
Häuser W, Galek A, Erbslöh-Möller B, et al. Posttraumatic stress disorder in fibromyalgia syndrome: Prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain 2013;154:1216–23
Vincent A, Benzo RP, Whipple MO, et al. Beyond pain in fibromyalgia: Insights into the symptom of fatigue. Arthritis Res Ther 2013;15:221
Zlott DA, Byrne M. Mechanisms by which pharmacologic agents may contribute to fatigue. PMR 2010;2:451–5. May;
Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep 2012;16:147–52
Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole MR.
Clinical Importance of Changes in Chronic Pain Intensity
Measured on an 11-point Numerical Pain Rating Scale
Pain 2001 (Nov); 94 (2): 149-158Hassett AL, Gevirtz RN. Nonpharmacologic treatment for fibromyalgia: Patient education, cognitive-behavioral therapy, relaxation techniques, and complementary and alternative medicine. Rheum Dis Clin North Am 2009;35:393–407
Rooks DS, Gautam S, Romeling M, et al. Group exercise, education, and combination self-management in women with fibromyalgia. Arch Intern Med 2007;167:2192–200
Mitsikostas DD, Chalarakis NG, Mantonakis LI, Delicha EM, Sfikakis PP. Nocebo in fibromyalgia: Meta-analysis of placebo-controlled clinical trials and implications for practice. Eur J Neurol 2012;19:672–80
Bidonde J, Busch AJ, Bath B, Milosavljevic S. Exercise for adults with fibromyalgia: An umbrella systematic review with synthesis of best evidence. Curr Rheum Rev 2014;10:45–79
Hindin SB, Zelinski EM. Extended practice and aerobic exercise interventions benefit untrained cognitive outcomes in older adults: A meta-analysis. J Am Geriatr Soc 2012;60:136–41
Busch AJ, Webber SC, Brachaniec M, et al. Exercise therapy for fibromyalgia. Curr Pain Headache Rep 2011;15:358–67
Häuser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 2010;12:R79
Bidonde J, Busch AJ, Webber SC, et al. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev 2014;10:CD011336
Bernardy K, Klose P, Busch AJ, Choy EH, Häuser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev 2013;9:CD009796–10
Lunde LH, Nordhus IH, Pallesen S. The effectiveness of cognitive and behavioural treatment of chronic pain in the elderly: A quantitative review. J Clin Psychol Med Sett 2009;16:254–62
Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev 2013;5:CD007070
Jones KD, Sherman CA, Mist SD, et al. A randomized controlled trial of 8-form Tai chi improves symptoms and functional mobility in fibromyalgia patients. Clin Rheum 2012;31:1205–14
Wang C, Schmid CH, Rones R, et al. A randomized trial of tai chi for fibromyalgia. N Engl J Med 2010;363:743–54
Langhorst J, Klose P, Dobos GJ, Bernardy K, Häuser W. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: A systematic review and meta-analysis of randomized controlled trials. Rheum Int 2013;33:193–207
Hanlon JT, Aspinall SL, Semla TP, et al. Consensus guidelines for oral dosing of primarily renally cleared medications in older adults. J Am Geriatr Soc. 2009;57:335–40
Maletta G, Agronin M, eds. Principles and Practice of Geriatric Psychiatry, 2nd edition. Philadelphia, PA: Lippincott Williams and Wilkins; 2011
Fitzcharles MA, Ste-Marie PA, Shir Y, Lussier D. Management of fibromyalgia in older adults. Drugs Aging 2014;31:711–9
American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–31
Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2014;4:CD007938
Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA 2004; 292(19):2388–95
Jones KD, Liptan GL. Exercise interventions in fibromyalgia: Clinical applications from the evidence. Rheum Dis Clin North Am 2009;35(2):373–91
Hauser W, et al.Efficacy of different types of aerobic exercise in fibromyalgia syndrome: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 2010;12(3):R79
Fleg JL. Aerobic exercise in the elderly: A key to successful aging. Discov Med 2012;13(70):223–8
Hadjistavropoulos T. Self-management of pain in older persons: Helping people help themselves. Pain Med 2012;13(Suppl 2):S67–71
Mork PJ, Nilsen TI. Sleep problems and risk of fibromyalgia: Longitudinal data on an adult female population in Norway. Arthritis Rheum 2012;64(1):281–4
Moldofsky H. Management of sleep disorders in fibromyalgia. Rheum Dis Clin North Am 2002;28(2):353–65
Arnold L, et al. Gabapentin in the treatment of fibromyalgia: A randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum 2007;56(4):1336–44
Arnold LM, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum 2004;50(9):2974–84
Derry S, et al. Milnacipran for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2012;3:CD008244
Arnold LM, et al. A 14-week randomized double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain 2008;9(9):792–805
Pauer L, et al. An international, randomized, double-blind, placebo-controlled, phase III trial of pregabalin monotherapy in treatment of patients with fibromyalgia. J Rheum 2011;38(12):2643–52
Pauer L, et al. Long-term maintenance of response across multiple fibromyalgia symptom domains in a randomized withdrawal study of pregabalin. Clin J Pain 2012;28(7):609–14

Return to LOW BACK PAIN
Since 1-22-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |