

Effect of Intensive Patient Education vs Placebo
Patient Education on Outcomes in Patients With
Acute Low Back Pain: A Randomized Clinical TrialThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: JAMA Neurol 2019 (Feb 1); 76 (2): 161–169 ~ FULL TEXT
OPEN ACCESS Adrian C. Traeger, PhD; Hopin Lee, PhD; Markus Hübscher, PhD; Ian W. Skinner, PhD;
G. Lorimer Moseley, PhD; Michael K. Nicholas, PhD; Nicholas Henschke, PhD;
Kathryn M. Refshauge, PhD; Fiona M. Blyth, PhD; Chris J. Main, PhD;
Julia M. Hush, PhD; Serigne Lo, PhD; James H. McAuley, PhD
Neuroscience Research Australia,
Sydney, New South Wales, Australia.
Sydney School of Public Health,
Faculty of Medicine and Health,
The University of Sydney,
Sydney, New South Wales, Australia.
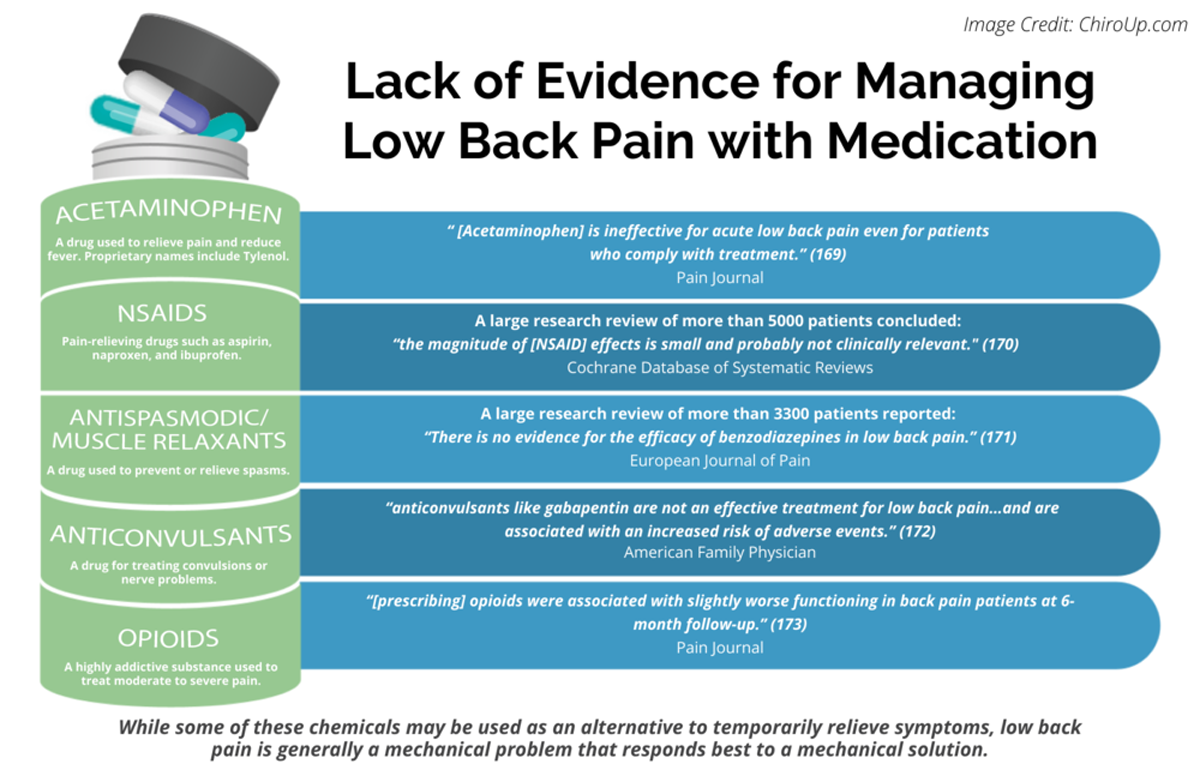
FROM: Pain 2019 (Dec)
FROM: Cochrane Database 2020 (Apr)
FROM: European Journal of Pain 2017 (Feb)
FROM: American Family Physician 2019 (Mar 15)
FROM: Pain 2013 (Jul)Importance: Many patients with acute low back pain do not recover with basic first-line care (advice, reassurance, and simple analgesia, if necessary). It is unclear whether intensive patient education improves clinical outcomes for those patients already receiving first-line care.
Objective: To determine the effectiveness of intensive patient education for patients with acute low back pain.
Design, setting, and participants: This randomized, placebo-controlled clinical trial recruited patients from general practices, physiotherapy clinics, and a research center in Sydney, Australia, between September 10, 2013, and December 2, 2015. Trial follow-up was completed in December 17, 2016. Primary care practitioners invited 618 patients presenting with acute low back pain to participate. Researchers excluded 416 potential participants. All of the 202 eligible participants had low back pain of fewer than 6 weeks' duration and a high risk of developing chronic low back pain according to Predicting the Inception of Chronic Pain (PICKUP) Tool, a validated prognostic model. Participants were randomized in a 1:1 ratio to either patient education or placebo patient education.
Interventions: All participants received recommended first-line care for acute low back pain from their usual practitioner. Participants received additional 2 × 1-hour sessions of patient education (information on pain and biopsychosocial contributors plus self-management techniques, such as remaining active and pacing) or placebo patient education (active listening, without information or advice).
Main outcomes and measures: The primary outcome was pain intensity (11–point numeric rating scale) at 3 months. Secondary outcomes included disability (24–point Roland Morris Disability Questionnaire) at 1 week, and at 3, 6, and 12 months.
Results: Of 202 participants randomized for the trial, the mean (SD) age of participants was 45 (14.5) years and 103 (51.0%) were female. Retention rates were greater than 90% at all time points. Intensive patient education was not more effective than placebo patient education at reducing pain intensity (3–month mean [SD] pain intensity: 2.1 [2.4] vs 2.4 [2.2]; mean difference at 3 months, –0.3 [95% CI, –1.0 to 0.3]). There was a small effect of intensive patient education on the secondary outcome of disability at 1 week (mean difference, –1.6 points on a 24–point scale [95% CI, –3.1 to –0.1]) and 3 months (mean difference, –1.7 points, [95% CI, –3.2 to –0.2]) but not at 6 or 12 months.
Conclusions and relevance: Adding 2 hours of patient education to recommended first-line care for patients with acute low back pain did not improve pain outcomes. Clinical guideline recommendations to provide complex and intensive support to high-risk patients with acute low back pain may have been premature.
Trial registration: Australian Clinical Trial Registration Number: 12612001180808.
From the FULL TEXT Article:
Introduction
For the past 5 years, the Global Burden of Disease Study [1] has consistently ranked low back pain as the leading cause of disability worldwide. Low back pain is second only to the common cold as a reason for consulting a general practitioner. [2] A recent international review highlighted a global crisis in the mismanagement of low back pain, with high rates of guideline-discordant care in both high- and low-middle income countries. [3–5] In their call to action, the Lancet Low Back Pain Series Working Group authors recommended that researchers and policy makers: “Develop and implement strategies to ensure early identification and adequate education of patients with low back pain at risk for persistence of pain and disability.” [3–5]
To manage uncomplicated acute low back pain (fewer than 6 weeks of pain duration), international guidelines recommend that general practitioners provide advice, education, reassurance, and simple analgesics, if necessary. [6] Although many patients receiving this care improve rapidly, 33% experience a recurrence in the next 12 months [7] and 20% to 30% develop chronic pain (defined as pain duration of 3 months or more). [8]
Patients who are at high risk of pain chronicity may require additional care, including second-line options such as physical (eg, spinal manipulation) and/or psychological therapies (eg, psychologically informed physiotherapy). [6] However, most trials that have evaluated adding second-line treatment options to standard guideline care for patients with acute low back pain have failed to demonstrate effectiveness compared with placebo (eg, addition of spinal manipulation, nonsteroidal anti-inflammatory drugs, or both [9]; addition of structured exercises [10]; and addition of acupuncture, massage, or chiropractic care [11]). Patient education, a treatment that authors of a 2008 Cochrane review [12] concluded was effective for acute low back pain when applied in an intensive format and that every major clinical guideline recommends (but with little instruction on intensity), [13] has never been tested in a placebo-controlled trial. Any benefits observed in previous trials of patient education for acute low back pain could be explained by nonspecific effects of the clinical encounter or the characteristics of the usual care comparison.
Pain education, a form of intensive patient education that is often included in pain management programs, requires up to 2 hours during several encounters with a trained health practitioner. It involves detailed discussion of pain, including psychosocial contributors and advice about pacing and activity. Trials have found clinically meaningful effects of pain education on pain and disability in samples of patients with chronic pain. [14]
It is unknown whether intensive patient education, in addition to recommended first-line care, can improve outcomes for patients with acute low back pain. To address this gap in the literature, we conducted, to our knowledge, the first randomized, placebo-controlled trial of patient education for acute low back pain (Preventing Chronic Low Back Pain [PREVENT] Trial). [15]
Methods
Study Design
This was an assessor-blinded, 1:1 parallel group, randomized, placebo-controlled trial. We published a study protocol prior to enrolling participants15 (the original trial protocol is available in Supplement 1). The trial was prospectively registered. The University of New South Wales Human Research Ethics Committee, Sydney, New South Wales, Australia, approved the study on February 5, 2013 (reference number: HC12664). We obtained written, informed consent from all participants before they enrolled in the trial.
Treatments took place at physiotherapy clinics, general practices, or clinic rooms at a research institute (Neuroscience Research Australia) in Sydney, Australia. One of 2 trial clinicians (A.C.T. and I.W.S.) provided the treatment at participating centers. We recruited participants between September 10, 2013, and December 2, 2015. Trial follow-up was completed on December 17, 2016.
Participants
We sought to recruit participants aged 18 to 75 years who were seeking care for acute low back pain with or without referred leg pain. Participants with signs of radiculopathy (spinal nerve root compromise) were included. All participants were referred from general practitioners or physiotherapists.
We excluded potential participants if they had the following:(1) chronic low back pain (more than 1 on a 11–point pain intensity numeric rating scale for more than 3 months),
(2) less than 3 of 10 on the pain intensity numeric rating scale over the past week,
(3) low risk of pain chronicity (less than 30% absolute risk of chronic pain according to the Predicting Inception of Chronic Pain (PICKUP) Tool [8] [eMethods 1 in Supplement 2]),
(4) clinical features of serious spinal pathology (eg, cauda equina syndrome, infection, fracture, or cancer) assessed by a clinician,
(5) poor command of the English language,
(6) previous spinal surgery, or
(7) a mental health condition that would preclude study participation.Referring clinicians were trained to provide all recruited participants with guideline-based care (advice to stay active, avoid bed rest, option of spinal manipulation, and/or simple analgesics). Staff were reimbursed per participant recruited for time spent on the study.
Randomization and Masking
We randomized participants in a 1:1 ratio to either intensive patient education or placebo patient education. The allocation schedule was generated by a researcher not involved in any other aspect of the study. That researcher used a computerized random number table to generate the allocation sequence in random block sizes of 4, 6, 8, and 10. The same researcher who generated the allocation sequence placed allocation codes into sequentially numbered, sealed, opaque envelopes.
Before randomization, all participants completed baseline data collection and received a standardized short history and physical examination (approximately 10–minute length) with the trial clinicians (A.C.T. and I.W.S.). The short history and physical examination were standardized using pro forma documents (eMethods 2 in Supplement 2). The trial clinicians opened the envelope containing the group allocation. The allocation was concealed from participants, referring clinicians, other trial staff, and outcome assessors.
All treatment was provided during the acute phase of low back pain within 6 weeks of pain onset. Each participant received 2 × 1–hour individual, face-to-face sessions of either patient education or placebo patient education. The trial clinicians (A.C.T. and I.W.S.) who provided the patient education sessions were the same clinicians who provided the placebo patient education. An expert in pain education (G.L.M.) trained both trial clinicians to deliver the patient education intervention. An expert clinical psychologist in pain management (M.K.N.) trained both trial clinicians in the placebo patient education intervention. Training for the patient education intervention took approximately 16 hours, with 6 to 8 hours allocated for practicing role-play scenarios. Training for the placebo patient education took approximately 4 hours and was supplemented with 4 online 45–minute videos demonstrating techniques for providing a credible consultation that did not include advice or education.
InterventionsIntensive Patient Education We adapted the information and advice provided in the patient education group from the book Explain Pain, [16] a text typically used for people with chronic pain. The intervention is described in full and according to the template for intervention description and replication (TIDieR) checklist in eMethods 3 in Supplement 2. In short, participants in the patient education group were provided with a detailed explanation about the biopsychosocial nature of pain in the format of diagrams, metaphors, and stories.
The patient education intervention involved 3 main components:(1) reframing unhelpful beliefs about low back pain,
(2) presenting information about the biologic basis and protective nature of both acute and chronic low back pain, and
(3) evaluating understanding of new concepts and discussing techniques to promote recovery.Content was tailored to the individual according to specific concerns (eg, “I am worried I will have this back problem forever”) and misconceptions (eg, “I can’t work because my back is permanently damaged”) that participants expressed during the consultation. Trial clinicians encouraged all participants to self-manage their low back pain by remaining active and avoiding bed rest. Trial clinicians also instructed participants on behavioral therapy techniques such as pacing.
Placebo Patient Education We designed the placebo patient education sessions to control for time with an expert clinician. The sessions mimicked all aspects of the patient education sessions (listening, showing interest, and attention of the clinician) but without the education component. Participants in the placebo patient education group received no information, advice, or education about low back pain from the trial clinician. Participants were encouraged to talk about any topic that they desired. Trial clinician responses were aimed to maintain the discussion for the duration of the session. We included additional detail on the placebo intervention in eMethods 4 in Supplement 2.Outcomes and Measurements
We collected self-reported data from participants at baseline (the first intervention session); 1 week after the 2 intervention sessions were complete; and 3, 6, and 12 months after the date of low back pain onset. Participants used online forms to complete outcome assessments. Baseline data included age, sex, duration of episode, number of previous episodes, other painful areas, and work status. An assessor who was masked to treatment allocation arranged the collection of outcome data using online forms. Participants completed the credibility and expectancy questionnaire [17] in paper format immediately after the trial clinician explained the rationale for the study and before randomization. Trial staff monitored adherence to the 2 intervention sessions using a study calendar. The trial clinician audio recorded all intervention sessions, with the participants’ verbal consent, to monitor treatment fidelity. Treatment fidelity was evaluated by 2 researchers (G.L.M. and M.K.N.), who listened to the first and second sessions from 10 randomly selected participants and judged whether the sessions were patient education or placebo patient education. We used κ to determine agreement.
The primary outcome was mean pain intensity during the past week (reported on an 11–point pain intensity numeric rating scale), assessed 3 months after the onset of low back pain. Secondary outcomes and process measures are described in eMethods 5 of Supplement 2.
Statistical Analysis
We published our statistical analysis plan before analyzing our results. [18] A sample of 202 participants was required to ensure 80% power to detect a mean difference of 1 point on an 11–point numeric rating scale for pain intensity. Our power calculation assumed an SD of 2.3 and a 2–sided α of .05 and was adjusted with 15% loss to follow-up. We estimated the effect of the intervention on the primary outcome using a mixed model for repeated measures. We treated time as a categorical variable (1 week and 3, 6, or 12 months) and included group × time interactions to determine treatment effects at each time point. As an exploratory sensitivity analysis, we calculated P values from mixed models for repeated measures comparing between-group difference during the full 12–month trial, controlling for baseline and including all time points as categorical. We determined statistical significance to be P < .05 for a 2–sided test. We did not include study site (physiotherapy practice, general practice, or research institute) in the model because there was no evidence of site differences between groups (χ2 test, P = .14). Details of the analysis of secondary outcomes is provided in eMethods 5 of Supplement 2 and the complete mediation analysis [19] in eResults 1 of Supplement 2. Two authors (S.L. and H.L.) performed the statistical analyses.
Results
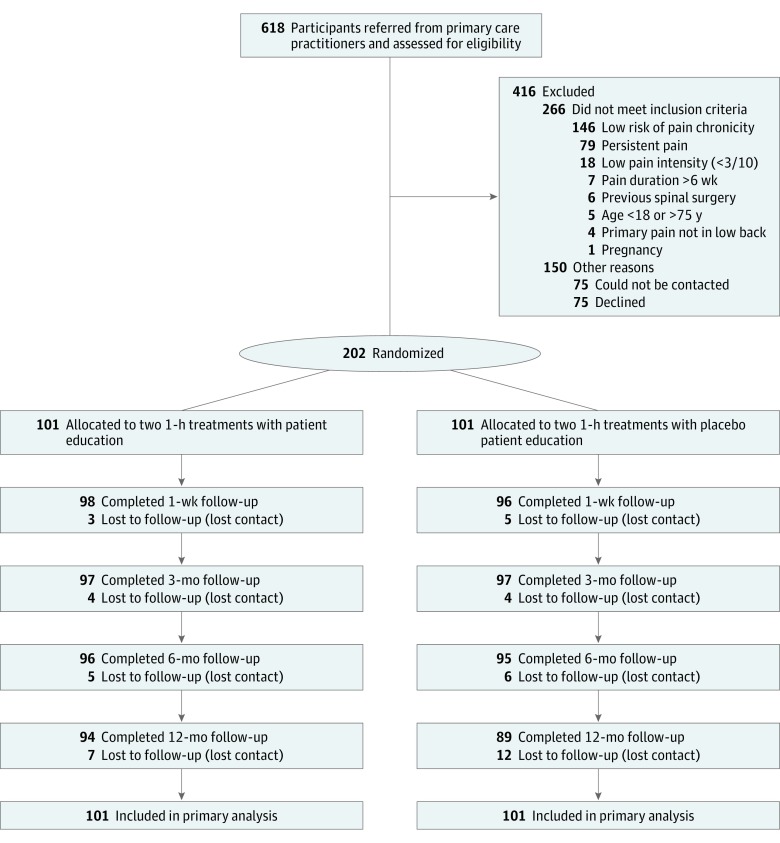
Figure 1

Table 1

Table 2
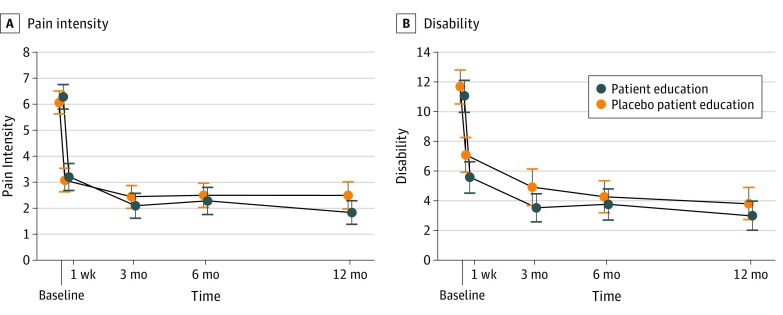
Figure 2

Table 3 Between September 10, 2013, and December 2, 2015, we screened 618 potential participants. Figure 1 shows the flow of participants through the trial. The main reasons for participant exclusion included low risk of pain chronicity (n = 146), chronic pain (n = 79), declined participation (n = 75), or could not be contacted after initial referral from the primary care practitioner (n = 75). Other reasons for exclusion are shown in Figure 1. One potential participant was excluded in error because of pregnancy.
The 2 groups had similar demographic and clinical characteristics at baseline (Table 1). Of 202 participants randomized for the trial, 103 (51.0%) were female. Participants were middle-aged (mean [SD] age, 45.1 [14.5] years), had fewer than 2 weeks of low back pain, and had experienced 3 previous episodes of low back pain. Physiotherapists referred most participants (83%). Half of the sample (52%) felt there was a need for further investigation of their symptoms. Psychological characteristics were similar between groups; scores for depression and catastrophizing scales were lower and scores for self-efficacy were higher than those seen in samples from patients with chronic pain who attended tertiary care. [20]
All participants completed both trial sessions. Treatment credibility scores were not different between groups (mean [SD] credibility and expectancy questionnaire score for patient education vs placebo patient education: 36.6 [8.8] vs 35.3 [10.5]; mean difference, –1.3; 95% CI, –4.0 to 1.4). For our treatment fidelity check, raters correctly categorized all recordings as patient education or placebo patient education. There was perfect agreement between raters (κ = 1).
The primary analysis (Table 2) showed that patient education was not more effective than placebo patient education at reducing pain intensity at our primary end point (3–month follow-up mean difference, –0.3 points on an 11–point scale; 95% CI, –1.0 to 0.3; P = .31). Mean (SD) pain intensity decreased from 6.3 [2.4] at baseline to 2.1 [2.4] at 3 months in the patient education group and from 6.1 [2.2] at baseline to 2.4 [2.2] at 3 months in the placebo patient education group. (Figure 2).
There was a small effect of treatment group on disability, with patient education lower than placebo patient education at 1 week (mean difference, –1.6 points on a 24–point scale; 95% CI, –3.1 to –0.1; P = .03) and at 3 months (mean difference, –1.7 points; 95% CI, –3.2 to –0.2; P = .03) (Table 3). There were no between-group differences in disability at 6– or 12–month follow-up.
There were some significant between-group differences in secondary outcomes (Table 3). The odds of having a recurrence of low back pain at 12 months were lower in the patient education group than in the placebo patient education group (odds ratio, 0.44; 95% CI, 0.24–0.82). Pain interference and the odds of seeking health care were also lower in the patient education group at 3 months (pain interference: mean difference, –0.8; 95% CI, –1.5 to –0.1; P = .02; health care seeking: odds ratio, 0.43; 95% CI, 0.19–0.93), but results for these variables were not lower at 6 or 12 months. Pain attitudes and reassurance at 1 week were higher in the patient education group (pain attitudes: mean difference, –0.9; 95% CI, –1.2 to –0.5; P < .001; reassurance [“How reassured do you feel that there is no serious condition causing your back pain?”]: mean difference, 1.2; 95% CI, 0.4–2.0; P = .003), and the effect on pain attitudes persisted at 12 months.
Patient education was not more effective than placebo patient education for reducing depressive symptoms, the incidence of chronic low back pain, or global perceived change (Table 3). The causal mediation analysis confirmed that patient education reduced catastrophizing and unhelpful beliefs (primary treatment targets), but these psychologic mechanisms did not reduce pain intensity (full results of mediation analysis reported in eResults 1, eTables 1 and 2, and eFigures 1–3 in Supplement 2). There were no reported adverse events in either treatment group. There was no evidence that out-of-trial therapy confounded treatment effects (eResults 2 and eTable 2 in Supplement 2).
Discussion
Our study provides evidence that intensive patient education is not effective compared with placebo for patients with acute low back pain. Two 1–hour sessions of patient education were no more effective than a placebo intervention for improving pain at our primary end point of 3 months or at 1 week, 6 months, or 12 months after the onset of acute low back pain. Disability was significantly lower in the intervention group compared with the placebo group at 1 week and 3 months but not at 6 months or 12 months. The short-term effects on disability, although consistent with those from similar trials, [21] were below published guidance on clinically meaningful effects (2 points on a 24–point Roland Morris Disability Questionnaire and 1 point on a 10–point numeric rating scale). [22] Our results suggest that offering more intensive patient education to patients with acute low back pain than that provided as part of standard practice does not reduce pain intensity or lead to meaningful reductions in disability.
Our results challenge a widespread belief that patient education is an effective strategy for treatment of acute low back pain. For example, every clinical guideline recommends patient education to manage acute low back pain. [13] These recommendations are, however, often unaccompanied by an evidence statement (eg, neither US [23] nor UK [22] guidelines cite evidence for patient education) or instruction on how patient education interventions should be conducted. [24] Two systematic reviews have concluded that primary care–based patient education is effective for acute low back pain. [12, 25] The available Cochrane review [12] of individual patient education included 6 trials of patient education compared with usual care: 3 trials of brief interventions (<20 minutes) and 3 trials of intensive interventions (>2 hours). The authors concluded that intensive patient education may be more effective at increasing return-to-work rates compared with usual care based on 2 trials (n = 1,432). However, those trials did not include pain or disability outcomes. Although a more recent review of 14 trials found that brief patient education could reduce back pain–related distress (n = 4,872), [25] it was unclear whether these interventions could improve other clinical outcomes such as pain. [26] Of importance, our mediation analysis (eResults 1 in Supplement 2) suggests that interventions aimed at reducing pain-related distress (eg, catastrophization) are unlikely to influence the pain experience as much as previously thought.
Strengths and Limitations
This trial [15] had several strengths. It was the first trial, to our knowledge, to test a patient education intervention against a credible placebo (ie, a professional consultation without any information or advice) in patients with acute low back pain. This strategy allowed us to determine the specific effects of patient education and control for effects produced by a clinical encounter, for example, those from the attention of a health professional or from the credibility of an impending treatment. We trained 2 trial clinicians to ensure treatment fidelity. Retention rates were high (>90% at all time points). We followed a published trial protocol [15] and statistical analysis plan. [18] Data were collected and analyzed by researchers who were masked to group allocation.
We used PICKUP, a validated prognosis model [8] to exclude people with acute low back pain who were at lower than average risk of pain chronicity. Approximately 40% of included participants developed chronic low back pain, a rate twice that of other trials on acute low back pain conducted in the same geographical area of Sydney (approximately 15%–20%). [9, 27] We are therefore confident that we included participants who were at high risk of pain chronicity.
This study also has limitations. First, trial clinicians could not be blinded to treatment allocation. However, results of our audit suggested that there were no systematic differences in treatment credibility or treatment fidelity. Second, interventions in the PREVENT trial [15] were provided by trial physiotherapists, and it is unclear whether our results would have been the same if the participant’s health practitioner provided the intervention. Third, we performed a number of statistical comparisons, which although planned, increased the risk of Type I error. Interpretation of the statistically significant effects of intensive patient education on some secondary outcomes, such as pain interference and recurrence and odds of seeking health care (Table 3), must consider this potential limitation. Finally, because both groups received basic patient education as part of recommended first-line care and many recovered despite being classified as being high risk, the potential for between-group differences may have been reduced.
Conclusions
For patients with acute low back pain who received first-line care, intensive patient education was no more effective than a placebo intervention. Adding complex, time-consuming treatments to primary-care based advice and reassurance is likely to be unnecessary for most patients with acute low back pain.
Supplementary Material
Clinical Trial Protocol (476K, pdf)
Supplement 2 (1.3M, pdf)
Supplement 3 (21K, pdf)
References:
Disease GBD;
Global, Regional, and National Incidence, Prevalence, and Years Lived With
Disability for 328 Diseases and Injuries for 195 Countries, 1990-2016:
A Systematic Analysis for the Global Burden of Disease Study 2016
Lancet. 2017 (Sep 16); 390 (10100): 1211–1259Deyo RA, Weinstein JN.
Low back pain.
N Engl J Med. 2001;344(5):363-370.
doi: 10.1056/NEJM200102013440508Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J et al.
What Low Back Pain Is and Why We Need to Pay Attention
Lancet. 2018 (Jun 9); 391 (10137): 2356–2367
This is the second of 4 articles in the remarkable Lancet Series on Low Back PainFoster NE, Anema JR, Cherkin D, Chou R, Cohen SP, et al.
Prevention and Treatment of Low Back Pain:
Evidence, Challenges, and Promising Directions
Lancet. 2018 (Jun 9); 391 (10137): 2368–2383
This is the third of 4 articles in the remarkable Lancet Series on Low Back PainBuchbinder R, van Tulder M, Öberg B, Costa LM, Woolf A, at al.
Low Back Pain: A Call For Action
Lancet. 2018 (Jun 9); 391 (10137): 2384–2388
This is the fourth of 4 articles in the remarkable Lancet Series on Low Back PainTraeger A, Buchbinder R, Harris I, Maher C. Diagnosis and management of low-back pain in primary care. CMAJ. 2017;189(45):E1386-E1395. doi: 10.1503/cmaj.170527
da Silva T, Mills K, Brown BT, Herbert RD, Maher CG, Hancock MJ.
Risk of recurrence of low back pain: a systematic review.
J Orthop Sports Phys Ther. 2017;47(5):305-313.
doi: 10.2519/jospt.2017.7415Traeger AC, Henschke N, Hübscher M, et al.
Estimating the Risk of Chronic Pain: Development and
Validation of a Prognostic Model (PICKUP) for
Patients with Acute Low Back Pain
PLoS Med. 2016 (May 17); 13 (5): e1002019Hancock MJ, Maher CG, Latimer J, et al..
Assessment of diclofenac or spinal manipulative therapy, or both, in addition
to recommended first-line treatment for acute low back pain: a randomised controlled trial.
Lancet. 2007;370(9599):1638-1643.
doi: 10.1016/S0140-6736(07)61686-9Machado LA, Maher CG, Herbert RD, Clare H, McAuley JH.
The effectiveness of the McKenzie method in addition to first-line care
for acute low back pain: a randomized controlled trial.
BMC Med. 2010;8:10.
doi: 10.1186/1741-7015-8-10Eisenberg DM, Post DE, Davis RB, et al..
Addition of choice of complementary therapies to usual care
for acute low back pain: a randomized controlled trial.
Spine (Phila Pa 1976). 2007;32(2):151-158.
doi: 10.1097/01.brs.0000252697.07214.65Engers A, Jellema P, Wensing M, van der Windt DA, Grol R, van Tulder MW.
Individual patient education for low back pain.
Cochrane Database Syst Rev. 2008;(1):CD004057.
doi: 10.1002/14651858.CD004057.pub3Koes BW, van Tulder M, Lin CW, Macedo LG, McAuley J, Maher C.
An Updated Overview of Clinical Guidelines for the Management
of Non-specific Low Back Pain in Primary Care
European Spine Journal 2010 (Dec); 19 (12): 2075–2094Louw A, Zimney K, Puentedura EJ, Diener I.
The efficacy of pain neuroscience education on musculoskeletal pain:
a systematic review of the literature.
Physiother Theory Pract. 2016;32(5):332-355.
doi: 10.1080/09593985.2016.1194646Traeger AC, Moseley GL, Hübscher M, et al..
Pain education to prevent chronic low back pain:
a study protocol for a randomised controlled trial.
BMJ Open. 2014;4(6):e005505.
doi: 10.1136/bmjopen-2014-005505Butler DS, Moseley GL.
Explain Pain. 2nd ed.
Adelaide, South Australia: Noigroup Publications; 2013.Devilly GJ, Borkovec TD.
Psychometric properties of the credibility/expectancy questionnaire.
J Behav Ther Exp Psychiatry. 2000;31(2):73-86.
doi: 10.1016/S0005-7916(00)00012-4Traeger AC, Skinner IW, Hübscher M, et al..
A randomized, placebo-controlled trial of patient education for
acute low back pain (PREVENT Trial): statistical analysis plan.
Braz J Phys Ther. 2017;21(3):219-223.
doi: 10.1016/j.bjpt.2017.04.005Lee H, Moseley GL, Hübscher M, et al..
Understanding how pain education causes changes in pain and disability:
protocol for a causal mediation analysis of the PREVENT trial.
J Physiother. 2015;61(3):156.
doi: 10.1016/j.jphys.2015.03.004Nicholas MK, Asghari A, Blyth FM.
What do the numbers mean? normative data in chronic pain measures.
Pain. 2008;134(1-2):158-173.
doi: 10.1016/j.pain.2007.04.007Hill JC, Whitehurst DG, Lewis M, Bryan S, Dunn KM, Foster NE,
K onstantinou K, Main CJ, Mason E, Somerville S, et al:
Comparison of Stratified Primary Care Management For Low Back Pain
With Current Best Practice (STarT Back): A Randomised Controlled Trial
Lancet. 2011 (Oct 29); 378 (9802): 1560–1571National Institute for Health and Care Excellence (NICE):
Low Back Pain and Sciatica in Over 16s: Assessment and Management (PDF)
NICE Guideline, No. 59 2016 (Nov): 1–1067Qaseem A, Wilt TJ, McLean RM, Forciea MA;
Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain:
A Clinical Practice Guideline From the American College of Physicians
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 514–530Stevens ML, Lin CC, de Carvalho FA, Phan K, Koes B, Maher CG.
Advice for Acute Low Back Pain: A Comparison of What
Research Supports and What Guidelines Recommend
Spine J. 2017 (Oct); 17 (10): 1537–1546Traeger AC, Hübscher M, Henschke N, Moseley GL, Lee H, McAuley JH.
Effect of primary care-based education on reassurance in patients
with acute low back pain: systematic review and meta-analysis.
JAMA Intern Med. 2015;175(5):733-743.
doi: 10.1001/jamainternmed.2015.0217Chou R.
Reassuring patients about low back pain.
JAMA Intern Med. 2015;175(5):743-744.
doi: 10.1001/jamainternmed.2015.0252Williams CM, Maher CG, Latimer J, et al..
Efficacy of paracetamol for acute low-back pain:
a double-blind, randomised controlled trial.
Lancet. 2014;384(9954):1586-1596.
doi: 10.1016/S0140-6736(14)60805-9
Return to LOW BACK PAIN
Return to BEST PRACTICES
Since 7-07-2023


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |