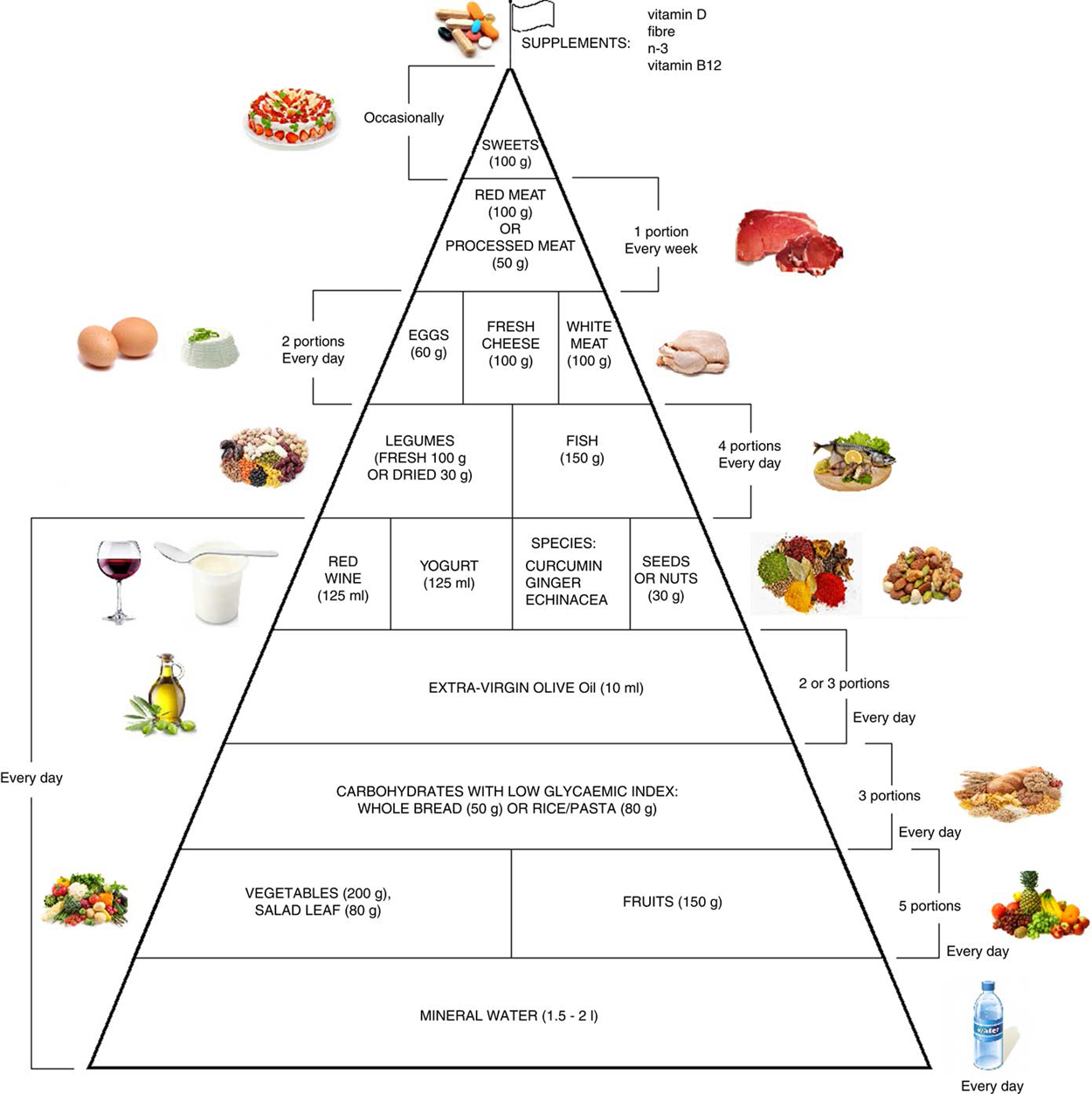
Food Pyramid for Subjects with Chronic Pain: Foods and
Dietary Constituents as Anti-inflammatory
and Antioxidant AgentsThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Nutr Res Rev 2018 (Jun); 31 (1): 131-151 ~ FULL TEXT
Mariangela Rondanelli, Milena Anna Faliva, Alessandra Miccono, Maurizio Naso,
Mara Nichetti, Antonella Riva, Fabio Guerriero, Manuela De Gregori,
Gabriella Peroni, Simone Perna
University of Pavia,Department of Public Health,
Experimental and Forensic Medicine,
Section of Human Nutrition,Endocrinology and Nutrition Unit,
Azienda di Servizi alla Persona,
Pavia,Italy.
Fig. 2 Food pyramid for the dietary management of chronic pain.
It is recommended to take whole grains daily (three portions of
lower-glycaemic index grains, for example whole rice or
Basmati rice or Doongara rice or rolled oats).Emerging literature suggests that diet constituents may play a modulatory role in chronic pain (CP) through management of inflammation/oxidative stress, resulting in attenuation of pain. We performed a narrative review to evaluate the existing evidence regarding the optimum diet for the management of CP, and we built a food pyramid on this topic. The present review also describes the activities of various natural compounds contained in foods (i.e. phenolic compounds in extra-virgin olive oil (EVO)) listed on our pyramid, which have comparable effects to drug management therapy. This review included 172 eligible studies.
The pyramid shows that carbohydrates with low glycaemic index should be consumed every day (three portions), together with fruits and vegetables (five portions), yogurt (125 ml), red wine (125 ml) and EVO;Consumed weekly:
legumes and fish (four portions);
white meat, eggs and fresh cheese (two portions);
red or processed meats (once per week);
sweets can be consumed occasionally.The food amounts are estimates based on nutritional and practical considerations. At the top of the pyramid there is a pennant: it means that CP subjects may need a specific customised supplementation (vitamin B12, vitamin D, n-3 fatty acids, fibre). The food pyramid proposal will serve to guide dietary intake with to the intent of alleviating pain in CP patients. Moreover, a targeted diet can also help to solve problems related to the drugs used to combat CP, i.e. constipation. However, this paper would be an early hypothetical proposal due to the limitations of the studies.
Keywords: ALA α-linolenic acid; COX cyclo-oxygenase; CRP C-reactive protein; EVO extra virgin olive oil; LAO long-acting specific opioid formulation; MCP-1 monocyte chemotactic protein-1; NMDA N-methyl-d-aspartate; NSAID non-steroidal anti-inflammatory drug; OA osteoarthrosis; OIC opioid-induced constipation; SIRT1 sirtuin-1; TLR Toll-like receptor; TRP transient receptor potential; Antioxidants; Chronic pain; Constipation; Food pyramid; Inflammation; Nutrients; Opioids.
From the FULL TEXT Article:
Introduction
A food-specific diet, possibly associated with dietary supplements, can provide helpful support for patients suffering from chronic pain. [1] A high number of chronic pain subjects have elevated levels of pro-inflammatory cytokines in blood and tissues. [2]
Regardless of underlying causes of pain, inflammation is the primary means of alert that calls into action the cells responsible for surveillance and protection, set in motion to limit tissue damage. [3] It is a normal biological process in response to tissue injury, to a microbial infection, and to a chemical irritation. [4]
Inflammation is caused by the migration of immune cells from the blood vessels and the release of mediators in the damage site. [5] This process is followed by the recruitment of inflammatory cells, release of reactive oxygen species and pro-inflammatory cytokines, in order to eliminate pathogens and repair damaged tissue. [6] In general, normal inflammation is rapid and self-limiting, but prolonged inflammation can cause various chronic painful disorders. [7]
If the acute inflammatory phase does not eliminate the pathogen resolving inflammation, the inflammatory process continues and develops into a chronic condition, associated with chronic pain. [8]
Inflammation represents a cause of nociceptive pain( Reference Zhang and An2 ) and osteoarthrosis (OA) is one of the most common syndromes [9] causing limited joint motion, pain and disability. In Europe osteoarthritis affects over 40 million adults, according to the European League Against Rheumatism (EULAR). [10] OA patients often cannot reach an adequate pain relief with drugs (non-steroidal anti-inflammatory drugs (NSAID) and weak opioids) and/or develop severe side effects. Risk of developing OA is largely related to lifestyle factors like diet and physical activity. Weight control and maintaining a BMI within the normal range (between 18·5 and 25 kg/m2) supports the analgesic and anti-inflammatory therapies. [11] Moreover, adipose tissue releases inflammatory molecules, such as leptin and cytokines, whose release can be reduced or even suppressed by the decrease of fat mass. [12]
Finally, a recent study by Emery et al. [13] showed that dietary intake of foods with anti-inflammatory effects (sea-food and plant proteins) mediates the relationship of body fat to body pain in men and women.
Adequate intake of specific foods and nutrients can effectively improve the inflammation state and oxidative stress [14] resulting also in chronic pain management. [15]
Although inflammation and oxidative stress are certainly the main factors determining chronic pain, other factors can also contribute significantly to chronic pain, such as psychosocial factors, biomechanical factors (processes of continuous muscle contraction favour microtrauma and the formation of trigger points), other causes of neural pain processing, etc. Various nutrients have been shown to produce an antinociceptive effect in animal models of neuropathic and inflammatory pain; in particular, it is known that Mg is a physiological antagonist of the N-methyl-d-aspartate (NMDA) receptor ion channel, and that the NMDA receptor plays a key role in central sensitisation, the primary mechanism through which Mg produces its analgesic effect, is believed to be a blockade of the NMDA receptor in the spinal cord. [16]
In addition, Mg blocks Ca channels and modulates K channels. The activation of the NO pathway could have an important role in the antinociceptive effects of systemic magnesium sulfate in the somatic (but not the visceral) model of inflammatory pain.
In clinical trials, most authors confirmed that Mg reduces opioid consumption and alleviates postoperative pain scores while not increasing the risk of side effects after opioids. [17]
Given this background, the aim of the present review was to evaluate the existing evidence regarding optimum diet therapy for the management of chronic pain, and to acknowledge the assumption that lower markers of inflammation and oxidative stress correspond with lower chronic pain.
Methods
This narrative review was performed following these steps [18]:
Configuration of a working group: three operators skilled in clinical nutrition (one acting as a methodological operator and two participating as clinical operators).
Formulation of the review question on the basis of considerations made in the abstract: ‘the state of the art on management of inflammation and oxidative stress through dietary approach in chronic pain’.
Identification of relevant studies: a research strategy was planned on PubMed (Public MedLine run by the National Center of Biotechnology Information (NCBI) of the National Library of Medicine of Bethesda, USA) as follows:
Definition of the keywords (chronic pain, foods, inflammation, oxidative stress, nutrients), allowing the definition of the interest field of the documents to be searched, grouped in inverted commas (‘…’) and used separately or in combination;
Use of: the Boolean (a data type with only two possible values: true or false) AND operator, that allows the establishment of logical relations among concepts;
Research modalities: advanced search;
Limits included: time limits: papers published in the last 20 years; languages: English;
Manual search performed by the senior researchers experienced in clinical nutrition through the reviewing of reviews and individual articles on the management of inflammation and oxidative stress by a dietary approach in chronic pain published in journals qualified in the Index Medicus.
Analysis and presentation of outcomes: the data extrapolated from the ‘reviewed studies’ were collated in tables; in particular, for each study we specified the author and year of publication, study characteristics and results.
The analysis was carried out in the form of a narrative review of the reports. We evaluated, as suitable for the narrative review, humans and in vitro or animal model studies of any design that considered:
the relevance of foods or nutrients for chronic pain management;
mechanism-based reasoning that showed a decrease of some inflammatory markers. The keywords considered and the kinds of studies chosen for specific foods are as below.

Water
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘water’ OR ‘hydratation’;three articles were sourced: two human in vivo studies and one cross-over study.

Fruits and vegetables
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘fruit’ OR ‘vegetables’;ten articles were sourced: one randomised controlled study, two narrative reviews, one cross-over study, two clinical trials, three cross-sectional studies and one placebo-controlled trial.

Carbohydrates with low glycaemic index/load
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘carbohydrates’;five articles were sourced: two randomised controlled studies, two narrative reviews and one cross-over study.

Olive oil and olives
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘olive oil’;twenty-four articles were sourced: four randomised controlled trial, twelve narrative reviews, one parallel study, one clinical trial, three in vitro studies and three clinical studies.

Red meat, white meat and fish
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘meat’ AND ‘fish’;five articles were sourced: one cohort study, one narrative review, one prospective study, one investigation and one animal model study.

Legumes
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘legumes’ AND ‘soybeans’;six articles were sourced: one meta-analysis, one narrative review, one cross-over study, one placebo-controlled trial, one in vitro study and one animal study.

Yogurt
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘yogurt’ AND ‘probiotics’ OR ‘fermented milk’;eleven articles were sourced: one randomised controlled trial, eight narrative reviews, one cross-over study and one animal model study.

Oil seeds
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘oil seeds’;fourteen articles were sourced: three meta analyses of other studies, one prospective study, one placebo-controlled trial, one systematic review, four cross-over studies and four animal model studies.

Spices
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘spices’;eight articles were sourced: one meta-analysis, one book chapter, two narrative reviews, one randomised controlled trial and three animal model studies.

Eggs
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘egg’;four articles were sourced: one randomised controlled trial, one narrative review and two in vitro studies.

Cheeses
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘cheeses’ AND ‘dairy product’;seven articles were sourced: three cross-over studies, one longitudinal study, two reviews and one comparative study.

Red wine
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘red wine’ AND ‘alcohol’;twenty-six articles were sourced. Among them, one survey, eight narrative reviews, one systematic review, three cross-over studies, two observational studies, two in vitro studies, eight animal model studies and one CONSORT study were selected and discussed.

Homemade sweets
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘sweets’ OR ‘sugar’;six articles were sourced: three cross-sectional studies, two narrative reviews and one randomised controlled trial.

Vitamin D
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘vitamin D’ OR ‘25 hydroxyvitamin D’ OR ‘25(OH)D’;seven articles were sourced. Among them, one prospective study, four narrative reviews and two longitudinal studies were selected and discussed.

Vitamin B12
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘vitamin B12’ OR ‘cobalamin’ OR ‘cyanocobalamin’;three articles were sourced, in particular one randomised controlled trial and two animal studies.

n-3 PUFA
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘omega 3’ OR ‘Ω3’ OR ‘polyunsaturated fatty acids’;six articles were sourced: one prospective study, two narrative reviews and three animal model studies.

Fibre
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘fiber’;two articles were sourced: one position paper and one narrative review.

Role of dietary fibre in opioid-induced constipation
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘constipation’ AND ‘fiber’ AND ‘opioids’;seven articles were sourced: two narrative reviews, one book chapter, two animal model studies, one position paper and one observational study.

Micronutrients: zinc and selenium
This research was carried out based on the keywords: ‘chronic pain’ AND ‘inflammation’ AND ‘oxidative stress’ AND ‘zinc’ AND ‘selenium’;eighteen articles were sourced: two clinical human studies, eleven reviews and position paper and five animal studies.

Levels of evidence
Moreover, levels of evidence, as defined in the EMBC Levels of Evidence Working Group (Oxford Centre for Evidence-Based Medicine), have been added in the tables, in order to better clarify the quality of the studies included. [19]
The key to the levels of evidence were:level 1 = systematic review of randomised trials or n-of-1 trial;
level 2 = randomised trial (of good methodological quality) or observational study with dramatic effect;
level 3 = non-randomised controlled cohort/follow-up study;
level 4 = case-series, case–control studies, or historically controlled studies;
level 5 = mechanism-based reasoning.The levels of evidence were considered from the viewpoint that reducing inflammation led to an improvement of chronic pain, which was the endpoint.
Results

Figure 1
page 5This review included 172 eligible studies and the dedicated flow-chart is shown in Figure 1.
In online Supplementary Tables S1–S18 the studies considered for the review are summarised.
Moreover, it is therefore thought to represent graphically, in a simple and intuitive way, what should be proper nutrition for the chronic pain patient, specifying the quality and amount of food, in order to counter the states of chronic inflammation and increased oxidative stress.
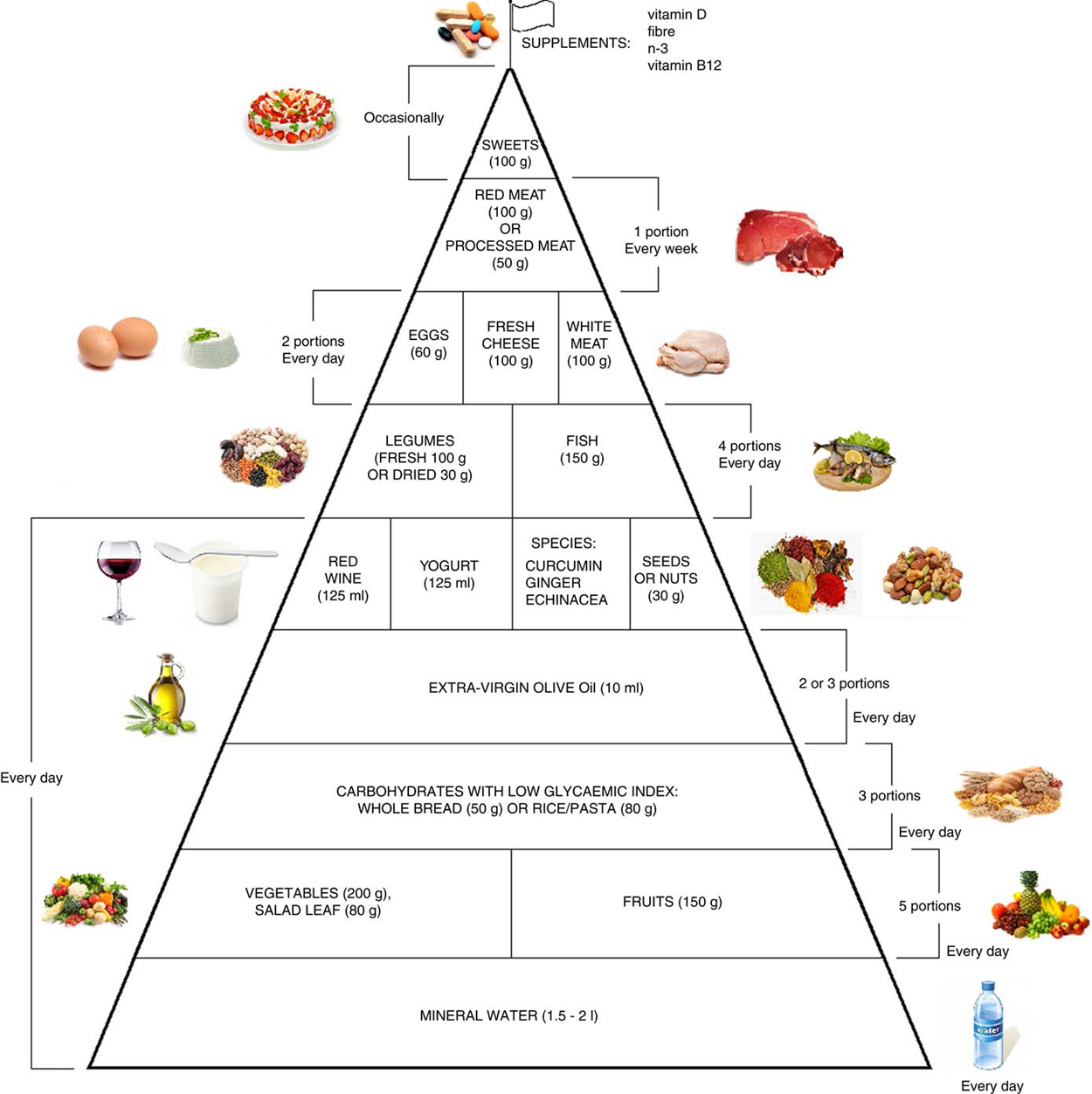
Figure 2 This pyramid (Figure 2) is divided into:
(a) foods that should be consumed daily ;
(b) foods that must be consumed 1, 2 or 4 times per week ;
(c) foods to be eaten occasionally .
The food amounts are estimates based on nutritional and practical considerations.
The pennant at the top of the pyramid means that chronic pain subjects need a specific customised dietary supplementation with vitamin B12, vitamin D, n-3 fatty acids and fibre.

Water
Water is the essential constituent of the human organism and is indispensable for the conduct of all physiological processes and biochemical reactions. Adequate water intake depends on many factors, such as age, sex, environmental conditions, activity level and level of sweating. It is helpful to drink about 1·5–2 litres daily, as specified in the latest revision of the Guidelines for Healthy Eating in 2003. [20]
Two recent studies have analysed the effects of dehydration on pain in healthy subjects by the ‘cold pressor test’ (CPT). Hypohydration can change the cerebrovascular response to CPT [21], resulting in increased pain sensitivity. [22] The mechanism underpinning the increase in pain perception due to hypohydration is unclear: the key factor causing the increase in pain centre activity is activated by the subjective feeling of thirst within a multimodal network designed to detect negative sensory inputs. An alternative, or possibly related, explanation of the increase in pain perception induced by hypohydration involves cortisol, as blood cortisol concentrations are increased by hypohydration. Pain perception is linked directly to cortisol and may be indirectly linked by cortisol’s involvement in key pain regulation processes such as regulation of the immune system and testosterone production, which may reduce pain by suppressing activity in the thalamus and middle frontal; therefore, cortisol may increase pain perception by lowering testosterone. [22]
Osteopathic treatment improves pain symptoms and the effect may be greater in euhydration than hypohydration. [23]
Online supplementary Table S1 summarises the studies investigating the role of water intake in chronic pain subjects.
In conclusion, hydration status is significantly correlated with pain; therefore, water is at the base of the pyramid.

Fruits and vegetables
Assmann et al. [24] underline that, during midlife, the adherence to a healthy diet that provides micronutrients, fibre and antioxidants while regulating energy intake may help to promote healthy ageing. Also the Dietary Approaches to Stop Hypertension (DASH) diet exerts an important role in preventing inflammatory disease, because of its low saturated fat content and emphasis on eating an abundance of fruits and vegetables.
Consumption of fruits and vegetables, which contain many vitamins, minerals and antioxidants, is inversely associated with inflammation and oxidative stress in adults: the higher the consumption of vegetables, the lower the occurrence of inflammation and oxidative stress [25–28]: Miller et al. [25] evaluated the difference between a typical American diet and a DASH diet (rich in fruit and vegetables) and they observed that the second one reduced oxidative stress (urinary isoprostanes) yet increased antibodies to oxidation of LDL.
Some researchers have focused their attention on the decrease in C-reactive protein (CRP) levels associated with diets rich in fruits and vegetables. [29–31] These three studies reached their conclusions based on FFQ.
Holt et al. [32] investigated this association in adolescents, confirming the results obtained for adults: the consumption of fruit and vegetables was inversely correlated with markers of inflammation (CRP, IL-6, TNF-α and 15-keto-dihydro-PGF2α metabolite) and oxidative stress (urinary 8-iso PG F2α, an F2-isoprostane) tested with a FFQ.
The inflammation reduction is secondary to the content in fruits and vegetables of high amounts of fibre, micronutrients (such as vitamins C and E, and folate) and phytochemicals (such as carotenoids, phenolics, isoflavones and indoles). In addition, intake of fruits and vegetables stimulates the production of butyrate, produced by bacterial fermentation of dietary fibre in the colon, which reduces inflammation of the mucosa by decreasing the activity of NF-κB in colonic cells in patients with ulcerative colitis. [33]
Flavonoids (in citrus fruits, apples, berries, tomatoes, fennel, cauliflower, etc.) appear to be involved in maintaining the sealing of intercellular junctions, which are part of the main determinants of the intestinal barrier function. [34]
Online Supplementary Table S2 summarises the studies that investigate the relationship between intake of fruits and vegetables and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers.
In conclusion, the literature is sufficient to demonstrate that fruit and vegetable consumption is inversely associated with pro-inflammatory cytokines and reactive oxygen species, secondary to inflammation and oxidative stress in adults. In order to reduce pro-inflammatory cytokines and reactive oxygen species, a daily intake of five portions of fruits and vegetables is recommended.

Carbohydrates with low glycaemic index/load
The consumption of high-glycaemic index food may contribute to oxidative stress and low-grade inflammation, both in acute and chronic pain, through changes in NF-κB. [35] An excessive postprandial glucose excursion causes the generation of NO, which in turn combines with superoxide to produce peroxynitrite, a potent and long-lived pro-oxidant molecule. [36]
Fardet [37] analysed the components of whole grains that have low glycaemic index, and concluded that these foods are rich in various bioactive compounds (polyphenols, phytic acid and lignin) with anti-inflammatory properties, including the reduction of free radicals and the activation of antioxidant enzymes, but the author underlined the necessity of other studies to determine the mechanisms involved.
These associations were further confirmed in a recent study, which compared a low-glycaemic index with a high-glycaemic index diet: in this study patients were randomised to different diets. [38] A dietary approach with foods with a low glycaemic index is more effective in regard to the reduction of chronic inflammation, taking as the first outcome high-sensitivity CRP, whereas glycaemic peaks are one of the main causes of oxidative stress. [38] In line with this, Ebbeling et al. [39] observed lower concentrations of CRP following a low-glycaemic index diet compared with a higher-glycaemic index diet.
Online Supplementary Table S3 summarises the studies investigating the relationship between carbohydrate intake with a low glycaemic index (<70) and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, all conditions present in chronic pain subjects.
In conclusion, it is recommended to take daily whole grains (three portions of whole grains, for example whole rice or Basmati rice or Doongara rice or rolled oats) in order to lower glycaemic index and load of the diet.

Olive oil and olives
The main source of lipid intake in the Mediterranean diet is represented by extra virgin olive oil (EVO). The intake of these lipids (rich in MUFA) has been recognised as an important factor contributing to the positive health profile characteristic of the Mediterranean population, because they have anti-inflammatory, antimicrobial and antioxidant activity. [40]
The high concentration of MUFA and many bioactive compounds (such as polyphenols) are mainly responsible for its anti-inflammatory properties; other seed oils do not have the same health benefits as EVO, as demonstrated by the study of Harper et al. [41] in which a supplementation of α-linolenic acid (ALA) of flaxseed oil did not decrease CVD risk, and by the study of Aguilera et al. [42] in which a supplementation of sunflower-seed oil did not protect as olive oil. [41, 42]
Lignans are the main components of the phenolic fraction of polyphenols in EVO [43] and the main representatives are acetoxipinoresinol and pinoresinol.
Despite the different concentrations among different EVO oils, lignans are present in greater quantities than other phenolic components, in a range between 0·65 and 99·97 mg/kg. [44] The anti-inflammatory properties of bioactive compounds of EVO could explain why the incidence of cancer and heart diseases is lower in the Mediterranean basin than in other geographic areas. [45]
Also, nutraceutical properties of EVO have been attributed to secoiridoids, such as oleuropein and its derivatives, the main alcohols 3,4-dihydroxyphenyl ethanol, also known as hydroxytyrosol and the p-hydroxyphenyl ethanol or tyrosol. [40, 46]
In addition, these phenolic components from EVO, in many preclinical models of disease, show a great variety of beneficial effects, mainly related to their antioxidant activity. [47–52] Oleuropein has an anti-inflammatory effect thanks to the increase in NO production, in macrophages and inhibiting lipoxygenase activity and the production of leukotriene B4. [47–51]
Moreover, oleuropein and hydroxytyrosol have vasodilator properties, anti-inflammatory activity and an anti-aggregating action on platelets. [47–50, 53, 54]
Decarboxymethyl-aglycone ligstroside or oleocanthal is a phenolic compound that mimics the inhibitory action of cyclo-oxygenase (COX) by the NSAID ibuprofen, as a natural NSAID. [55]
Oleocanthal was identified in the secoiridoid fraction of EVO phenolic compounds [54] and described as the compound responsible for oropharyngeal irritation associated with EVO intake [56], perceptually similar to the irritation caused by ibuprofen. [57, 58]
Subsequently, in a study by Beauchamp et al. [55], oleocanthal showed dose-dependent inhibition of the inflammatory enzymes COX-1 and COX-2 more powerful, in equimolar concentrations, as compared with ibuprofen.
Obviously the fruit olive (Olea europaea), from which EVO is derived by cold pressing, has significant anti-inflammatory and antinociceptive activities due to the presence of many phenolic compounds (among these the most important are hydroxytyrosol and tyrosol). [59] Takeda et al. [60] treated a group of twenty-five individuals with early-stage knee osteoarthritis: hydroxytyrosol was effective in improving pain in gonarthrosis, measuring with the Japanese Orthopedic Association score and a visual analogue scale score compared with the placebo group. Moreover, olive pits have cholinergic activity as demonstrated by the study of Cortés Castell et al. [61] who determined the effect of a polyphenolic extract from olive pits on the development of the nervous system as well as its effect on pain induced by the neurotoxin kainic acid, taking the zebrafish as the animal model. Olive fruits are rich in dietary fibre and, overall, one of the most important compound is ferulic acid that has anti-inflammatory activity. [62] Suntar et al. [63] have evaluated the anti-inflammatory and anti-nociceptive properties of leaves and fruits of Olea europaea L. (family Oleaceae) and the results revealed that ethanolic extract did not show a significant anti-inflammatory or analgesic activity, whereas the n-hexane extract displayed 12.7–27.8% inhibition on the carrageenan-induced hind paw oedema model at the 400 mg/kg dose. [63]
Online Supplementary Table S4 summarises the studies investigating the relationship between EVO intake and imbalance between antioxidant capacity, oxidative stress and pro/anti-inflammatory markers, which represent conditions present in chronic pain subjects.
In conclusion, the recommendation to all chronic pain subjects is daily EVO consumption, preferably raw, as a seasoning for food and a weekly consumption of olives (for example, to be added to salads).

Red meat, white meat and fish
An unbalanced diet that emphasises the consumption of food of animal origin at the expense of food of plant origin is associated with the development of chronic and degenerative diseases, which, in turn, are related to pain. [64]
Components of the Mediterranean diet associated with better cardiovascular health include low consumption of meat and meat products. [65]
These foods may produce inflammatory molecules; higher red meat consumption is associated with unfavourable plasma concentrations of inflammatory and glucose metabolic biomarkers (such as IL-6) in diabetes-free women, and the substitution of other protein food, such as poultry, is associated with a healthier biomarker profile of inflammatory and glucose metabolism. [66]
Moreover, there is the impact of beefsteak thermal processing on lipid oxidation and postprandial inflammation-related responses: Nuora et al. [67] evaluated the effect of the consumption of pan-fried and sous-vide steaks and it was shown that there were no postprandial differences in the plasma IL-6 and CRP levels. TNF-α levels after consuming the pan-fried meal remained at baseline throughout the monitoring period but there was a decrease in TNF-α levels after consuming the sous-vide meal.
Plasma monocyte chemotactic protein-1 (MCP-1) remained unaltered, relative to baseline values, after the pan-fried meal. In contrast, MCP-1 was reduced following the sous-vide meal. [67]
In order to obtain an adequate amount of protein if consuming animal products, we recommend consuming white meat twice per week, red/processed meat once per week, and fish four times per week.
Fish should contain larger amounts of n-3 PUFA, such as bluefish, mackerel, anchovies, sardines, tuna and swordfish, given the anti-inflammatory properties of these essential fatty acids: for example, the study of Maroon et al. [68], in which individuals took a supplementation of n-3 essential fatty acids (EPA and DHA), found in fish oil supplements, demonstrated a reduction of pain. [68, 69]
Online Supplementary Table S5 summarises the studies showing the relationship between intake of red or white meat or fish and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions present in chronic pain subjects.
In conclusion, excessive consumption of red meat causes an inflammatory state and alternative foods should be taken into consideration such as white meat and fish.

Legumes
Legumes are a pivotal component of the Mediterranean diet due to their beneficial effect on inflammatory markers.
In a recent cross-over study, subjects with type 2 diabetes were divided into two groups in order to analyse certain inflammatory markers as a result of two different types of 8–week diet intervention (identical isoenergetic diets, with the exception of two servings of meat replaced by two servings of vegetables). There was a significant decrease in CRP, IL-6 and TNF-α only during the period in which subjects took legumes compared with the period when they took meat, independent of weight change. [70] These results were confirmed in a systematic review that involved eight studies and 464 participants, showing that the consumption of legumes (excluding soyabeans) had a significant effect on the reduction of CRP. [71]
Regarding soyabeans, the literature reports that diets rich in soya create an anti-inflammatory effect through activation of the PPARγ receptor with consequent suppression of production of pro-inflammatory cytokines by immune cells. [72] Therefore, soya intake may be useful in controlling chronic pain, as reported by Shir et al. [73] in which rats were fed a soya or no-soya diet. With regard to studies on human subjects, Arjmandi et al. [74] showed a reduction in pain symptoms in patients with osteoarthritis taking a 3–month supplement of 40 g/d of soya proteins, as indicated by a significant increase in serum levels of insulin-like growth factor-1 and a significant decrease in serum levels of glycoprotein 39 (YKL-40) compared with a milk-based protein diet; specifically, the authors underline that major effects were observed in men rather than women, probably for higher levels of oestrogen in women as compared with men, because the effect of soya supplementation is related to its content of isoflavones. [74]
The main soya isoflavone is genistein. It has been shown in in vitro studies that genistein binds to oestrogen receptors and activates PPAR-γ, which has a prominent role in the suppression of inflammatory genes. In cultured chondrocyte cells, put in contact with lipopolysaccharide, genistein reduces IL-1β expression, COX and NO, indicating a clear anti-inflammatory effect. [75] The authors underline that there are no pharmaceutical agents that selectively can inhibit COX-2 production without having serious side effects. Therefore, genistein can be an attractive and viable alternative therapy for treatment or prevention of OA. [75]
Finally, legumes are rich in water-insoluble fibre, and this feature makes them suitable for subjects suffering from opioid-induced constipation (OIC).
Online Supplementary Table S6 summarises the studies investigating the relationship between intake of legumes and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions of chronic pain subjects.
In conclusion, it is recommended to consume legumes four times per week, either dried or fresh, and soyabean once per week for their anti-inflammatory activity and their high content of fibre.

Yogurt
Our body is an ideal habitat for the survival of bacterial strains. These micro-organisms are not always pathogenic; indeed, they often are an important defence and security barrier. The gut is the tract of the human body most colonised and overall resident bacteria form a complex and diverse ecosystem-defined microbiota. The alteration of the intestinal microbiota is a result of various conditions, such as prolonged use of antibiotics, changes in lifestyle, use of drugs, and diseases. [76]
Under normal conditions, the intestinal microbiota is made from different bacterial strains belonging mainly to the genera Bacteroides, Eubacterium, Bifidobacterium, Fusobacterium, Peptostreptococcus and to a lesser extent Escherichia coli, Enterobacter and Lactobacillus, which produce a series of molecules able to activate both the innate and acquired immune systems, which may be altered in pathological conditions or during drug treatment. [77]
It is therefore necessary that intestinal microbiota be adequately represented, in order to compete with the pathological bacteria that pass through the gastrointestinal tract, and in order to exert beneficial actions in different parts of the human body. [78]
Opioids, in particular morphine, are the main drugs that determine alteration of the innate and adaptive immunity of the patient, even though the mechanism of action is not fully known. [79] Specifically, opioids alter or suppress the function of pro-inflammatory cells (cytokines), through Toll-like receptors (TLR), while opioids in adaptive immunity act on B and T lymphocytes, respectively, through the hypothalamus–pituitary–adrenal axis, the molecules of histocompatibility class II (MHC II) and through the suppression of IL-2. [80] A recent study using animal models showed that chronic use of morphine induces a significant change in the composition of the intestinal microbiota and determines an increase in Gram-positive pathogenic bacteria and a reduction in deconjugating bile strains. Moreover, this study also demonstrated that the Firmiculates:Bacterioidetes ratio, one of the key markers of inflammation in the microbiome, promotes the pro-inflammatory phenotype, demonstrating how the immune status of the host involves the innate immune response. [81]
Probiotics are defined by the FAO/WHO as ‘living organisms which bring benefit to the health of the host, when administered in adequate amounts’ [82], while the literature defines prebiotics as a class of food products which constitute the necessary and optimum substrate for the growth and development of probiotics.
Among the prebiotics, inulin (contained in plants of the Lilliaceae family, such as leek, onion, garlic and asparagus, or of the Compositae family such as Jerusalem artichoke, dahlia and chicory), oligofructose and polyphenols have particular importance; they are metabolised by enteric bacteria. [83]
Yogurt is the best-known substrate to which to add probiotics. Yogurt may be also be added to prebiotics (for example, fructo-oligosaccharides) that are useful in preventing alterations of the microbiota and to counteract constipation [84], both critical situations induced by opioid therapy.
Yogurt intake is recommended in all pathologies characterised by an inflammatory state. [85] The intake of Bifidobacterium animalis subsp. lactis (BB-12) interacts with the peripheral myeloid cells via TLR-2, a receptor capable of recognising certain typical structures of pathogens and microbes, and it is implicated in the defence of the organism, in particular, of innate immunity. [86] Subjects who ingested yogurt with BB-12 probiotics had a lower expression of TLR-2 and thus a decrease in the triggering of the immune response as a result of inflammation. These results demonstrated a potential anti-inflammatory effect of BB-12 in healthy adults, and also indicated that the matrix to which the probiotics are added may influence the immunomodulatory properties.
Other authors conducted studies on the activity of probiotics on the immune system. Klein et al. [87] integrated yogurt with Lactobacillus acidophilus 74–2 and Bifidobacterium animalis subsp. lactis DGCC 420 (B. lactis 420) and demonstrated that the percentage of granulocytes and monocytes with phagocytic activity increased by 92–95%, with a concomitant increase in L. acidophilus and B. lactis concentrations in faeces.
Probiotics (Lactobacillus bulgaricus and Streptococcus thermophilus, as well as other strains that may be added such as Lactobacillus acidophilus 74–2, Bifidobacterium animalis subsp. lactis DGCC 420, Bifidobacterium animalis subsp. lactis), and the protein content in yogurt, have an anti-inflammatory and immunomodulatory activity in human subjects, demonstrated by numerous intervention studies. [88]
Online Supplementary Table S7 summarises the studies investigating the relationship between intake of yogurt or fermented milk and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions present in chronic pain subjects.
In conclusion, the daily intake of yogurt can prevent the alteration of the microbiota, a consequence of opioid therapy. It is also an excellent way to counteract the inflammatory state that characterises chronic pain patients.

Nuts and seeds and their oils
The use of seeds (nuts, such as walnuts, hazelnuts, almonds, and pistachios, pumpkin seeds, flax seeds, grapeseed) and oils derived from them for chronic pain treatment has been widely discussed in the literature. In animal models an extract of grape seeds may be useful as a natural treatment option for pain by suppressing the development of peripheral and central sensitisation, probably due to the high content of proanthocyanidins. [89, 90]
Walnuts are complex food matrices containing different macro- and micronutrients and other components that can influence inflammation and endothelial function. Examples of these components are essential fatty acids, n-3 PUFA, Mg, l-arginine and some antioxidants. Several intervention studies support the anti-inflammatory effect of ALA in human subjects. For example, there was a decrease in serum concentrations of inflammatory markers (CRP and vascular cell adhesion molecule-1 (VCAM-1)) after dietary supplementation with ALA. [91] The increased consumption of ALA from nuts is able to improve anti-inflammatory effects by inhibiting the peripheral production of IL-6, IL-1β and TNF-α. [92]
Moreover, walnuts are one of the main sources of Mg in the diet. Cross-sectional studies have suggested an inverse association between Mg intake and the concentrations of CRP. [93] Some lines of experimental evidence have also suggested that Mg intake may have beneficial effects on endothelial function [94], probably mediated by the effects of this mineral on systemic inflammation. As regards arginine, a conditionally essential amino acid contained in high amounts in walnuts (3·62 g in 100 g), it could have a positive effect on endothelium-dependent vasodilatation [95]; however, endothelium-independent vasodilation and levels of intercellular adhesion molecule-1, CRP, homocysteine and oxidation biomarkers were similar after each diet (a Mediterranean diet and a diet in which walnuts replaced about 32% of the energy from monounsaturated fat). [95] A cross-sectional study evaluated the association between the intake of arginine and CRP, demonstrating that individuals with higher intakes of arginine had 30% less probability of having a CRP of more than 3·0 mg/l. All studies led to the conclusion that the consumption of foods rich in arginine, such as nuts, can reduce the risk of diseases characterised by the presence of inflammation. [96]
Pistachio is another seed with significant anti-inflammatory activity. It is particularly rich in γ-tocopherol, vitamin K, phytosterols, carotenoids, some minerals (Cu, Fe and Mg) and B vitamins. [97] A diet rich in pistachios (pistachio was added for 4 weeks by replacing the monounsaturated fat content constituting about 20% of daily energy intake) significantly improves endothelium-dependent vasodilatation (+30%), contributes to a decrease in serum IL-6, total oxidant status, lipid hydroperoxide, and malondialdehyde, and leads to an increase in superoxide dismutase, with a significant change in TNF-α levels and CRP. [98]
Flax seeds, rich in ALA, are also known for their anti-inflammatory effect: Faintuch et al. [99] showed a decrease in CRP values, serum amyloid A, leucocytes and fibronectin in a group of obese patients, supplemented with 30 g/d of flax flour.
Pumpkin seeds have several beneficial effects on health, including antioxidant and anti-inflammatory properties. They have a high content of vitamin E (tocopherol). [100] Chang [101] found that the administration of pumpkin extract can significantly increase the blood and hepatic activity of superoxide dismutase and glutathione peroxidase in mice, as well as the concentration of malonaldehyde.
Pumpkin seeds are considered a good source of anti-inflammatory substances, which can help in many diseases, such as arthritis. Pumpkin seed oil can significantly inhibit arthritis in mice, with an action similar to indomethacin. [102]
Online Supplementary Table S8 summarises the studies about the relationship between intake of oilseeds and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions present in chronic pain subjects.
In conclusion, the intake of 30 g/d of oilseeds (particularly walnuts and pistachios) can be a suitable nutritional option to counterbalance the pro-inflammatory activity in chronic pain patients.

Spices
It is clear that the Mediterranean diet has an important anti-inflammatory role, probably affecting the arachidonic acid cascade, the expression of some proinflammatory genes, and the activity of immune cells. [103] The adoption of this dietary pattern could counter the effects of several inflammatory markers, decreasing, for example, the secretion of circulating and cellular biomarkers involved in the atherosclerotic process. [104]
Daily consumption of spices is often recommended because they are useful for flavouring dishes without using excessive amounts of salt, but spices have many other positive health properties. The extracts of plants belonging to the Zingiberaceae family (for example, turmeric, ginger, Javanese and galangal ginger) are powerful ipoalgesic agents, useful in chronic pain treatment. [105] Specifically, curcumin (obtained from Curcuma longa) attenuates hyperalgesia induced by opioids, one of the main chronic pain treatments, possibly by inhibiting CaMKIIα (Ca/calmodulin-dependent protein kinase type II alpha chain) and its downstream signalling. [106] In addition, curcumin has antinociceptive and anti-inflammatory effects. [107]
Treatments with herbal preparations (including Zingiber officinale) are useful in reducing chronic joint pain. [108] Gingerols and shogaols, found in Zingiber officinale, have an analgesic effect as they are agonists of the transient receptor potential (TRP) cation channel subfamily V1 (TRPV1), showing inhibitory activity on the metabolism of arachidonic acid through COX2 (PG and thromboxanes). [109, 110]
In addition, gingerols also exert analgesic effects through the activation of vanilloid receptor subtype-1 (VR1); the potency of gingerols increases with increasing size of the side chain and with the overall hydrophobicity in the series. [111] Finally, a particularly volatile compound present in many spices (such as oregano, cinnamon, rosemary, thyme and black pepper), named β-caryophyllene, is a selective agonist of the peripheral cannabinoid receptor type 2 (CB2), involved in modulating inflammation, especially in neuropathic pain, by reducing pain and neuro-inflammation in animal models. [112]
Online Supplementary Table S9 summarises studies investigating the relationship between spice intake and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions of chronic pain subjects.
In conclusion, the daily use of spices, especially turmeric and ginger, could have a significant positive activity on chronic pain control due to their antinociceptive and anti-inflammatory actions.

Eggs
Eggs contain particularly beneficial substances, such as fat-soluble and B vitamins, minerals, choline and carotenoids, high-quality proteins, and bioactive components with both pro- and anti-inflammatory roles that contribute to fighting inflammation in human populations.
Phospholipids (for example, phosphatidylcholine, phosphatidylethanolamine, lysophosphatidylcholine, phosphatidylinositol and sphingomyelin), mostly found in the yolk, are an example of this dual role: although the evidence suggests that phosphatidylcholine has anti-inflammatory effects. [113, 114] Through the role of TNF-α, phospholipids are also involved in inflammatory and atherosclerosis processes through the formation of trimethylamine-N-oxide, which promotes the formation of atherosclerotic plaques. [115]
For this reason, in addition to the known higher cholesterol content, it is recommended to limit egg intake to twice per week. Eggs also contain lutein and zeaxanthin (belonging to the family of carotenoids) with antioxidant and anti-inflammatory properties, whose bioavailability is greater than in vegetable foods. Notably, normal-weight individuals have greater pro-inflammatory responses to egg intake than overweight individuals with the metabolic syndrome, or type 2 diabetes. [112] A recent study highlighted the ability of eggs (one egg per d) to decrease TNF-α in populations with a low degree of chronic inflammation. [116]
Online Supplementary Table S10 summarises studies about the relationship between intake of eggs and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions of chronic pain subjects.

Cheeses
It is known that dairy products (milk, yogurt and cheese) exert important functions in the human body thanks to the content of high-quality proteins, Ca and vitamins, in particular vitamin D, and for this reason they should be taken daily. Cheeses are divided into fresh and mature: the first include ricotta, mozzarella, first salt, and cottage cheese. Among the latter, examples are Parmesan, fontina, provolone and scamorza. Experimental and epidemiological studies have shown that regular intake of dairy products has inhibitory effects against the development of some chronic degenerative diseases, characterised by low levels of inflammation, such as the metabolic syndrome. [117]
Moreover, some cheeses contain whey protein, quickly absorbed by the human body, with antioxidant properties. [118] Whey protein may exert its effect by enhancing glutathione concentration. [118]
Schmidt et al. [119] compared the effect of a high-fat dairy meal, a high-fat non-dairy meal supplemented with milk and a high-fat non-dairy control meal on postprandial inflammatory and metabolic responses in healthy men: they suggested that full-fat milk and dairy products have no significant impact on the inflammatory response to a high-fat meal. [119]
Demmer et al. [120] performed a randomised controlled cross-over trial and compared the intake of saturated fat in the form of cheese compared with plant sources of saturated fat; the results of this study demonstrated that postprandial CRP was significantly lower in response to the cheese compared with the vegan test meal. [120]
The 6–week cross-over study by Dugan et al. [121], which compared inflammatory responses (IL-6, IL-1β, TNF-α and MCP-1) as a result of diet with low-fat dairy (10 oz (283 g) 1% milk, 6 oz (170 g) non-fat yogurt, 4 oz (113 g) 2% cheese) or with low-fat non-dairy intake (carbohydrate-based control: 1·5 oz (43 g) granola bar and 12 oz (340 g) 100% juice), demonstrated in patients with the metabolic syndrome that three dairy product servings per d of low-fat dairy food improved systemic inflammation. [121]
The same positive results have been demonstrated by Bordoni et al. [122] who carried out a systematic review of fifty-two clinical trials investigating inflammatory markers in relation to the consumption of dairy products. However, in this review there are important limitations; in particular no distinction is made between cheeses with a lower content of saturated fat and cheeses with a high content of saturated fat and cholesterol; in fact, it is well known that excessive intake of saturated fat and cholesterol can cause increased levels of inflammation. Recently, Rocha et al. [123] provided a new perspective on the potential mechanism by which SFA could modulate TLR4-induced inflammatory responses. However, now even this topic is heavily debated in the literature because the results do not all agree. Van Meji & Mensink [124] carried out a comparative study on the inflammation response in which thirty-five overweight subjects consumed, in a random order, low-fat dairy products (500 ml low-fat milk and 150 g low-fat yogurt) or carbohydrate-rich control products (600 ml fruit juice and three fruit biscuits) daily for 8 weeks. The results of this study indicated that in overweight subjects, low-fat dairy product consumption, compared with carbohydrate-rich product consumption, may modulate TNF-α signalling by increasing soluble TNF receptor-2, but that it does not affect other markers of low-grade systemic inflammation and endothelial function in overweight subjects. [124]
Online Supplementary Table 11 summarises studies that investigate the relationship between intake of cheeses and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, all conditions present in chronic pain subjects.
In conclusion, the intake of cheeses, in particular with a high content of whey protein, is recommended at least twice per week.

Red wine
The consumption of moderate doses of wine (one glass of 125 ml/d for women and two glasses for men) is useful in the prevention of many inflammatory diseases, probably by activating sirtuin-1 (SIRT1). [125, 126]
Red wine is rich in numerous active molecules against inflammation and oxidative stress (in particular flavonoids, such as quercetin and myricetin, catechin and epicatechin, proanthocyanidins, anthocyanidins and the stilbene resveratrol). These molecules determine an increased expression of nicotinamide adenine dinucleotide (NAD)-dependent deacetylase SIRT1, a protein capable of deacetyl nuclear and cytoplasmic proteins controlling critical cellular processes, such as apoptosis and metabolism. SIRT1 regulates the production of insulin and glucose, lipids and cell survival. [127]
Flavonoids have a significant positive effect on immune system functions and on inflammatory cells, as demonstrated in numerous in vitro and animal model studies. [128, 129] Flavonoids are able to inhibit the expression of isoforms of NO synthase, COX and lipoxygenase, which are responsible for the production of a large amount of NO, prostanoids, leukotrienes, and other mediators of the inflammation process such as cytokines, chemokines and adhesion molecules. [130] The flavonoids also inhibit phosphodiesterase involved in cell activation. Many anti-inflammatory effects of the flavonoids affect the biosynthesis of cytokines that mediate adhesion of circulating leucocytes to sites of injury. Some flavonoids are potent inhibitors of the production of PG, a group of powerful pro-inflammatory signalling molecules. [131]
As regards resveratrol, recent studies have shown that it modulates neuronal excitability in the nervous system, both central and peripheral channels through the TRP ion channels and voltage-dependent channels. [132–136]
For example, TRP ankyrin 1 (TRPA1) modulates the mechanical transduction in primary sensory neurons [137], and it has been shown that resveratrol is a potent inhibitor of TRPV1 in vitro and in vivo [136], suggesting that resveratrol attenuates the generator potential via the mechanical transduction process. In addition, resveratrol modulates the channels of Na and K in the dorsal root ganglia neurons that are associated with the generation of action potentials. [132, 133] On the contrary, in the hippocampus, resveratrol significantly suppresses glutamate-induced currents in CA1 pyramidal postsynaptic neurons [138] that contribute to excitatory synaptic transmission. In addition, literature data show that resveratrol decreases the duration of the action potential and L-type Ca2+ currents in excitable tissues. [134] Together, these results suggest that systemic administration of resveratrol may suppress sensory transmission, including nociception, in both the central and peripheral nervous system, as demonstrated in a recent animal model study, in which the acute intravenous administration of resveratrol suppressed trigeminal nociceptive transmission. [139]
Online Supplementary Table S12 summarises the studies about the relationship between red wine intake and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, present in chronic pain subjects.
In conclusion, the numerous molecules active against inflammation and oxidation of red wine determine increased expression levels of SIRT1. Resveratrol modulates neuronal excitability. It is recommended to consume one 125 ml glass/d for women and two glasses for men to control pain in chronic pain patients without continuous opioid and/or benzodiazepine drug treatments; a greater amount should be avoided because of the risk of addiction/abuse.

Alcohol consumption and opioids
The association between alcohol and opioid analgesics may result in ‘dose dumping’. This is defined as the unintended quick release (in a short period of time) of the total amount (or a significant fraction) of the drug in a dosage form with modified release.
Alcohol is related to dose-dumping in all long-acting specific opioid formulations (LAO), and it significantly increases their side effects. [140] Therefore, it has been recommended that patients not drink alcohol during treatment, because they may have ‘the rapid release and absorption of a potential fatal dose of opioids’. [141]
The mechanism by which alcohol alters the pharmacokinetic properties of LAO are little known. Several studies have shown that the concomitant use of alcohol increases the maximum plasma concentration (Cmax) of some opioids and decreases the time to Cmax (Tmax), despite no evidence of dose dumping. [142–144] Specifically, Fiske et al. [142] demonstrated that increased systemic exposure to oxymorphone was greatest in the presence of excessive levels of ethanol (40%), which models serious ethanol abuse, but its clinical importance is not known.
These observations underscore the importance of educating patients not to consume alcoholic beverages while taking LAO therapies. Fatal poisonings involving pharmacological opiates are often associated with the use of alcohol and are probably due to a combination of effects on the central nervous system and of the respiratory tract. [145, 146]
Ali et al. [147] showed that opioids have a significantly decreased ventilatory response to hypercapnia when administered together with ethanol. About 12% of patients under chronic opioid treatment consume ethanol [148], and therefore physicians must take into consideration the possible development of adverse events. [149]
In conclusion, given the prevalence of concomitant use of opioids and ethanol and the potential risks to health (respiratory depression and sedation) associated with simultaneous administration [150], it is important to implement information on safety, pharmacokinetics and pharmacodynamics following concurrent administration of alcohol and opioids.

Homemade sweets
The consumption of sweets is placed at the top of the pyramid, signifying that their intake should be occasional (maximum once per week). As described in the chapter on cereal consumption, the intake of high-glycaemic index foods (and sweets are foods with a high glycaemic index) can contribute to greater oxidative stress and create a state of low-grade chronic inflammation. [35]
The intake of a Western-style diet (with high amounts of simple sugars and sweets) causes an increase in inflammatory markers [151], such as CRP, IL-6, E-selectin, soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1), after adjustment for all confounding factors. [152, 153]
Nevertheless, we should not underestimate the positive effect of dark chocolate (minimum 70% of cocoa solids) that is able to reduce NO production and then oxidative stress thanks to its flavonoid content. [154] Regular consumption of dark chocolate determines the reduction in inflammation, evaluated by CRP. [155]
Online Supplementary Table S13 summarises studies investigating the relationship between intake of sweets and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions present in chronic pain subjects.
In conclusion, it is right to enjoy the satisfaction of consuming a sweet, but homemade preparations are preferred, based on wholemeal flour with a lower glycaemic index than a refined one, and dark chocolate (70% cocoa).

Dietary supplements
On top of the food pyramid for the dietary management of chronic pain there is a pennant, to remind us that chronic pain subjects require special dietary supplements: vitamin D, vitamin B12, micronutrients (Zn and Se), fibre and n-3 fatty acids.Vitamin D
Vitamin D is classified as a fat-soluble vitamin and it is activated in the skin by exposure to sunlight, while only 20% is taken with food (especially through food of animal origin such as eggs, salmon, herring and liver).
If the diet is not sufficient to satisfy the Recommended Daily Allowance (RDA) of vitamin D, its supplementation is necessary because hypovitaminosis D is accompanied by numerous diseases, namely osteoporosis and musculoskeletal problems, but the literature also suggests an association between vitamin D deficiency and chronic pain. [156] Vitamin D deficiency correlates with a greater likelihood of developing chronic widespread pain. [157] In accordance with these results, Costan et al. [158] demonstrated that supplementation with vitamin D (daily bread fortified with 125 µg vitamin D3) in patients with osteoporosis and residing in a nursing home not only improves bone health and quality of life, but also locomotion, daily activities and pain.
The literature data have explained this link between vitamin D and chronic pain, demonstrating that low levels of vitamin D are associated with increased central hypersensitivity, such as increased sensitivity to mechanical pain and severity of somatic symptoms in chronic pain patients. [159] It also points out that vitamin D plays a crucial role in diseases related to the immune system and in autoimmune disease, because the receptors for the vitamin are not only present in cells related to Ca homeostasis, but also in cell lines involved in immune regulation (for example, dendritic cells). [160]
Moreover, treatment with vitamin D improves the quality of sleep, mood, and the level of pain. For example, Shipton & Shipton [161] and Straube et al. [162] evaluated the effect of supplementation of vitamin D; however, in both studies it could not be stated conclusively that vitamin D deficiency was directly linked to the aetiology or maintenance of chronic pain states.
Online Suplementary Table S14 summarises the studies about the relationship between intake or supplementation of vitamin D and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions present in chronic pain subjects.
In conclusion, in chronic pain patients, the assessment of blood vitamin D is recommended.

Vitamin B12
Vitamin B12 (cobalamin) is a water-soluble vitamin and is found in foods of animal origin (meat, eggs, fish), although some micro-organisms and certain algae are able to produce it. In many studies, the anti-nociceptive effects of the vitamin have been highlighted. [151, 152] In a recent study on mice, vitamin B12 was co-administered with morphine and the results showed a reduction in the development of morphine tolerance, probably through the indirect inhibition of NMDA receptors, and, consequently inhibition of NO synthesis. [163] Other similar experiments on animal models have highlighted the anti-inflammatory effects of cobalamin supplementation in enhancing its potential use for the treatment of acute and chronic neuropathic pain. [164]
Recently, human studies have shown that the intramuscular injection of vitamin B12 is significantly important for the treatment of localised pain in the spine. [165] In particular, Chiu et al. [165] demonstrated that intramuscular methylcobalamin is both an effective and safe method of treatment for patients with non-specific low back pain. Online Supplementary Table S15 summarises the studies investigating the relationship between intake or supplementation of vitamin B12 and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions present in subjects with chronic pain. In conclusion, if the diet is not sufficient to satisfy the Recommended Daily Allowance (RDA) of vitamin B12, in chronic pain subjects, it would be suitable to recommend the evaluation of blood vitamin B12 and a specific supplementation.

n-3 PUFA
Linolenic acid and its metabolites (n-3 PUFA) are well known for their anti-inflammatory properties, as well as analgesic and anti-nociceptive activities: Freitas et al. [66] evaluated a supplementation of fish oil in mice via modulation of circulating lymphocytes, besides GPR40/FFAR1 expression at the spinal level. [66, 67]
The main derivatives are n-3 EPA (20 : 5) and DHA (22 : 6) and it is interesting to remember how the enzyme Δ-6 desaturase, the key enzyme for the transformation from linolenic acid to EPA and DHA, its active metabolites, may be deficient in some situations, for example, ageing. Thus, it appears to be advantageous to administer EPA and DHA and not the precursor linolenic acid.
n-3 Fatty acids are found in fatty fish (salmon, tuna, etc.) and some plant sources, such as flaxseed and algae. In general, PUFA are able to activate the hypothalamic GPR40 protein, which in turn plays an important role in chronic pain control. [69] Some studies have shown that the regular consumption of PUFA (as supplementation) is equivalent to some drugs for the treatment of joint pain and constitutes an available alternative to NSAIDs. [68] The effectiveness of n-3 essential fatty acids in rheumatoid arthritis and some cases of osteoarthritis has been demonstrated in the study of Maroon et al. [68]; the conversion of EPA into anti-inflammatory PG of the PGE3 series is by the same COX enzyme used by arachidonic acid to convert to the proinflammatory PG PGE2 series. [68]
Moreover, an animal model study suggested a role of n-3 supplementation as an adjunct to opioids in pain therapy, showing that they might contribute to the reduction of the occurrence of morphine side-effects. [168]
However, a review by Boe & Vangsness [169] concluded that despite the overwhelming popularity of fish oil supplements and the assumption of benefit for patients with chronic pain in arthritis, there is insufficient clinical evidence at this moment to justify the use of fish oil in the treatment or prevention of osteoarthritis; for this reason there remains a significant need for trials to evaluate the efficacy and safety of n-3 fatty acids in a standardised form. [169]
Online Supplementary Table S16 summarises studies regarding the relationship between intake or supplementation of n-3 PUFA and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions in chronic pain subjects.
In conclusion, if chronic pain subjects are unable to consume four portions of fish/week, it is helpful to take a daily dietary supplementation of 1800 mg of EPA and 1200 mg of DHA.

Fibre
According to the Guidelines for Italian healthy eating, fibre consumption is optimal in the order of 30 g/d. [20] Normally, proper nutrition involves the introduction of fruit and vegetables as well as whole grains that are naturally rich in fibre and that meet the daily requirement. Fibres are classified into two major groups: soluble and insoluble. [20] Insoluble fibre (cellulose, lignin and hemicellulose) resists the action of digestive enzymes and is mixed with faeces; it increases the volume of stool, making it softer and homogeneous. Insoluble fibre is present mainly in whole grains. The water-soluble fibres (pectins, gums, mucilages, guar gum, oligosaccharides) absorb water, swell, and become a gelatinous mass. Soluble fibre is present in fruits and vegetables.
In the correct diet, it is necessary to associate the two types of fibre, in order to alleviate constipation. [170, 071]
While increasing the consumption of fibre, it is also important to increase and ensure an adequate intake of fluids, particularly in the summer, when fluid loss is higher.
Dehydration is a potential cause of the increased risk of constipation, which could also be solved by maintaining a proper distribution of meals during the day, in order to properly activate the gastro-ileocaecal reflection, favouring the progression of the food to the rectum.
Online Supplementary Table S17 summarises the studies about the relationship between intake of supplementation of fibre and constipation, conditions present in chronic pain subjects, in particular in patients with opioid-related adverse drug events.
In conclusion, a diet rich in fibre (30 g/d, in a ratio of 3:1 between insoluble and soluble fibre with an adequate intake of water) can be helpful in reducing the glycaemic response of the diet and in preventing and solving problems related to the consumption of drugs used to combat chronic pain, such as OIC.
Role of dietary fibre in opioid-induced constipation
The most current diagnostic criteria of constipation have been developed during the Rome III Consensus Conference [172]:(1) At least two or more of the following characteristics in at least 25% of the discharges: effort in defecation of hard stools; sensation of incomplete evacuation; feeling of occlusion; less than three defecations per week; need for manual manoeuvre to evacuate.
(2) Rare evacuations without resorting to laxatives.
(3) Exclusion from the diagnosis of irritable bowel syndrome.Presence of other symptoms, such as bloating or abdominal distension, may be present, but they are not the symptomatic elements. The pathological mechanism, which causes the OIC, is due to the effect of these drugs on peripheral µ-receptors in the gut wall. [173] The µ-opioid receptors are present in the neurons of the myenteric plexus and submucosal and immune cells of the lamina propria. [174] The activation of µ-opioid receptor inhibits neuronal excitatory and inhibitory pathways in the enteric nervous system, which coordinates motility.
The inhibition of excitatory neuronal pathways decreases peristaltic contractions; the blockade of inhibitory neuronal processes increases the muscular activity of the gastrointestinal tract and elevates the resting muscle tone with the consequence of inducing spasm. These mechanisms lead to a delay in gastric emptying and slow intestinal transit. [175]
The opioid drugs in cancer patients increase the use of treatment for constipation, with a predominance of monotherapy. [176]Patients using opioids, suffering from OIC, incur a greater number of adverse effects, which result in increased use of medical care, thereby raising the cost of health care. Patients with opioid-related adverse drug events have a great risk for hospitalisation, ranging from 10·3 to 55%.
Estimates of OIC have been found to vary widely, with investigations identifying rates of OIC among opioid users ranging from 15 to 90% [18, 177–179], resulting in an increase in care costs from 7·4 to 47%. [180]
Improving the management of constipation can lead to an optimisation of drug therapy and prevents the interruption of opioid drugs already in use. This interruption would lead to deterioration in the quality of life, which in turn would lead the patient to new access to medical care, with recovery therapy with multiple drugs and associated increased costs. [181]
About 10% of emergency room visits have a diagnosis of constipation. Furthermore, constipation was reported to be one of four factors that significantly and independently predict hospitalisation in hospice care. [182] Several studies in patients with advanced cancer receiving hospice care in fact showed that the prevalence of constipation is between 23 and 84%. [183–189] In addition, a series of studies conducted in a large hospice in Florida evaluated OIC, demonstrating that it occurs in 40–64% of hospitalised cancer patients. However, the number was found to be lower when the data were obtained from a screening of staff (40%) and higher when the researchers interviewed patients about their symptoms (63–64%). [189–191]
Moreover, it is important to note that possible associations have been hypothesised between the impairment of intestinal integrity and musculoskeletal chronic pain, like fibromyalgia and chronic myofascial pain, emphasising the synthesis and release of proinflammatory cytokines in these clinical situations. [192]
In conclusion, counteracting constipation is an important point in the management of patients with opioid-related adverse drug events, as various studies have shown that the presence of constipation is significantly negatively correlated to the overall quality of life. [193–196]
Multidisciplinary patient management, including analysis of life-style, should be performed at the beginning of opioid therapy and continue for the duration of the treatment; typical measures should include consumption of dietary fibre, fluid and increased physical activity. [197]
In ambulatory cancer patients with drug-induced constipation, an increase in fibre consumption should be recommended up to 30–35 g/d [198], according to specific protocols. [199, 200] Animal model studies confirm that fibre can be a preventive factor for OIC. Moreover, the presence of SCFA products from the microbiota improve intestinal transit, promoting colon motility. [201] The use of prebiotics could further help, improving immunity in the intestine, increasing IgA and acting on oxidative stress, positively affecting intestinal peristalsis. [202]
OIC improvement may also occur thanks to non-pharmacological management. Cancer patients should be encouraged to eat more high-fibre foods, such as fruits (plums, peaches and apples), vegetables (squash, broccoli, carrots and celery) and whole grains, and to increase fluid intake. [203]
Online Supplementary Table S18 summarises the studies about the relationship between intake of supplementation of fibre and constipation, conditions present in patients with opioid-related adverse drug events.
In conclusion, a targeted diet, rich in fibre (35 g/d, in a ratio of 3:1 between insoluble and soluble fibre with an adequate intake of water) and with five meals daily, can be helpful in preventing and solving problems related to the assumption of drugs used to combat chronic pain, such as OIC.

Micronutrients: zinc and selenium
Zn, the second most abundant trace element in the human body (after Fe) [204], plays mainly antioxidant and anti-inflammatory roles [205]; as a cofactor of Cu-Zn superoxide dismutase, it is important for antioxidant defence [206] and Zn depletion increases the inflammatory status, impairs immune system functioning, antioxidant defence, and affects DNA structure. [204–209] Moreover, in animals, Zn formulations determine, at non-toxic systemic or local doses, indubitable analgesic effects in different models of pain: acute visceral, mechanical and thermal pain, under regular, inflammatory and neuropathic conditions. [210, 213]
Se is also an essential trace mineral important to human health. [214, 215] This mineral constitutes a key component of various selenoproteins involved in enzymic activities and particularly in redox homeostasis and in thyroid hormone metabolism. [216, 217]
Moreover, optimal Se intake can mitigate dysfunctional inflammatory responses, in part, through the regulation of eicosanoid metabolism. [218] A poor Se status has been associated with an increased risk of several chronic diseases [214] and cancer. [219]
In humans, an inadequate intake of both Zn and Se and their low intracellular stores are correlated with pain in patients with chronic myofascial pain. [220]
Finally, a recent review by Ciubotariu et al. [221] showed that both human and animal studies revealed decreased serum Zn under opioid-administration conditions, attributed mainly to increased urinary elimination (humans) or redistribution (animals).
For all these reasons, Ciubotariu et al. [221] concluded that Zn dietary supplementation in patients treated with opioids for cancer-related chronic pain should be considered, due to the high incidence of Zn deficiency, also well-documented in opioid consumers.
Online Supplementary Table S19 summarises studies regarding the relationship between intake or supplementation of Zn and Se and imbalance between antioxidant capacity and oxidative stress and pro/anti-inflammatory markers, conditions in chronic pain subjects.
In conclusion, the assessment of the intake of Zn and Se by dietary diary, possibly associated with Zn and plasma Se evaluation, are important assessments to be made in a chronic pain patient, particularly in patients who undergo opioid treatment, in order to consider dietary supplementation with Zn and Se.
Conclusion and limitations
A specific diet can provide helpful support in patients suffering from chronic pain. Moreover, a targeted diet can also help to solve problems related to the consumption of drugs used to combat chronic pain, such as constipation. Chronic pain is strictly associated with inflammation and oxidative stress, though other factors can also contribute significantly to chronic pain, such as psychosocial factors, biomechanical factors, other causes of neural pain processing, etc. For this reason we have performed a review in which we have considered scientific papers basing on the assumption that lower inflammation and oxidative stress are related to lower pain.
However, it is important to note that the causal relationship between the higher inflammatory biomarkers found in chronic pain patients is largely unknown, because various vicious circles have been involved concerning, for example, stress. The stress of being in pain chronically may increase inflammatory markers themselves, so it is possible that chronic pain causes inflammation, not the other way around. Dysfunctional bidirectional pathways between stress, inflammation, the brain and the immune, endocrine and neurotransmitter systems have been extensively described and implicated in pain. [200]
Thus, the nutrition pyramid described in our review is hypothetical, even in the light of several limitations of the present review. The main limitation is the fact that to date there are no randomised controlled trials in the literature clearly showing that improved nutrition, via a lowering of general inflammation, improves chronic pain. Further, to build the pyramid, we could only make a narrative review of the literature, not a meta-analysis.
A further limitation concerns in particular the section dedicated to supplementation; as a matter of fact the anti-inflammatory activity demonstrated by in vitro and in vivo studies for giving an isolated nutrient is not equivalent to getting the same nutrients in real whole foods. There are numerous examples in the literature where this presumption does not hold up.
In conclusion, even if this nutritional pyramid is hypothetical, we hope that it can serve the valuable purpose of helping researchers focus on the often–ignored possible connections between nutrition and chronic pain. Further investigation is needed in the future and, specifically, more randomised clinical trials should be conducted that directly study nutrition and chronic pain, in order to understand the specific mechanisms that interconnect inflammation, chronic pain and nutrition.
Supplementary Material
Online Supplementary Tables S1–S18 NOT available on publisher's website
Acknowledgements
The authors declare no commercial or financial conflict of interests.
References:

Return to NUTRITION
Return to PAIN AND NUTRITION
Return to SPINAL PAIN MANAGEMENT
Since 7-26-2023


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |