

A Structured Protocol of Evidence-based Conservative Care
Compared with Usual Care for Acute Nonspecific
Low Back Pain: A Randomized Clinical TrialThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Arch Phys Med Rehabil. 2012 (Jan); 93 (1): 11–20 ~ FULL TEXT
Gregory F. Parkin-Smith, MTech(Chiro), MSc, DrHC, Ian J. Norman, BSc, MSc, PhD,
Emma Briggs, BSc, PhD, RN, Elizabeth Angier, BSc, MSc(Chiro),
Timothy G. Wood, BSc, MTech(Chiro), James W. Brantingham, DC, PhD
School of Chiropractic & Sports Science,
Murdoch University,
Perth, Australia.
g.parkin-smith@murdoch.edu.au
OBJECTIVE: To compare a protocol of evidence-based conservative care with usual care for acute nonspecific low back pain (LBP) of less than 6 weeks' duration.
DESIGN: Parallel-group randomized trial.
SETTING: Three practices in the United Kingdom.
PARTICIPANTS: Convenience sample of 149 eligible patients were invited to participate in the study, with 118 volunteers being consented and randomly allocated to a treatment group.
INTERVENTIONS: The experimental group received evidence-based treatments for acute nonspecific LBP as prescribed in a structured protocol of care developed for this study. The control group received usual conservative care. Participants in both groups could receive up to 7 treatments over a 4-week period.
MAIN OUTCOME MEASURES: Oswestry Low Back Disability Index (ODI), visual analog scale (VAS), and Patient Satisfaction Questionnaire, alongside estimation of clinically meaningful outcomes.
RESULTS: Total dropout rate was 14% (n=16), with 13% of data missing. Missing data were replaced using a multiple imputation method. Participants in both groups received an average of 6 treatments. There was no statistically significant difference in disability (ODI) scores at the end of week 4 (P=.33), but there was for pain (VAS) scores (P<.001). Interestingly, there were statistically significant differences between the 2 groups for both disability and pain measures at the midpoint of the treatment period (P<.001). Patient satisfaction with care was equally high (85%) in both groups. Minimally clinically important differences in scores and number needed to treat scores (NNT<6) indicated that the experimental treatment (protocol of care) offered a clinically meaningful benefit over the control treatment (usual care), particularly at the midpoint of the treatment period.
CONCLUSIONS: Overall, the 2 treatment groups were similar based on primary or secondary outcome measure scores for the full treatment period (4 weeks, with up to 7 treatments). However, there were statistically significant and clinically meaningful differences in both disability and pain scores at week 2 (midpoint) with 4 treatments, suggesting that the protocol of care (spinal manipulation and reassurance) had a more rapid effect than usual care.
From the FULL TEXT Article:
Background
Low Back Pain (LBP) is an extremely common problem throughout the world, particularly in western countries. [1, 2] Although most cases of LBP resolve, up to 25% of persons with LBP have significant persistent pain or disability, and seek care. [3] Understandably, the purpose of intervention would be to reduce symptoms as rapidly as possible, curb costs, and reduce the risk of chronicity. [4, 5]
Conservative treatment, such as manual and manipulative therapy, is emerging as a promising approach to managing nonspecific LBP, [6] be it in conjunction with drug treatment or as an alternative when drug treatment is contraindicated or surgery premature. Currently, the selection of conservative treatments by a musculoskeletal practitioner to treat acute LBP is influenced by a mixture of the best available research evidence, practitioner preferences, and clinical traditions. [7]
Many conservative treatments have not yet been tested in clinical trials or have been shown to be no better than placebo, [8, 9] but are still used in clinical practice. For example, interventions are used in combination in musculoskeletal practice for nonspecific LBP (nonclassified), yet only slightly improve pain and disability in the short-term, and there is currently no evidence that supports or refutes that these interventions provide clinically meaningful improvements. [10] Therefore, a positive, predictable outcome for LBP with standard care approaches, leading to a reduction in disability and pain scores, seems to remain elusive. [11]
Synthesis of the literature indicates that the combination of treatments commonly encountered in clinical practice for nonspecific LBP (ie, usual care) may be partial in reflecting evidence-based treatment, [12] may not represent the application of clinical guideline recommendations, and may not be targeted toward specific subgroups of LBP patients. [13]
Therefore, successful outcomes for acute nonspecific LBP are likely to be enhanced by(1) matching treatment to the characteristics of the patient, and
(2) selecting treatments that are supported by both the best available research evidence and clinical expertise.For this reason, the array of conservative treatments available to musculoskeletal practitioners needs to be distilled into a package of care that is targeted toward acute nonspecific LBP, with a view to attain better and more rapid outcomes.
Consequently, the purpose of this study was to compare an evidence-based protocol of conservative care, acceptable to an advisory group of practicing clinicians, with usual conservative care for acute nonspecific LBP of less than 6 weeks’ duration. The primary objective was to compare the outcomes of the experimental treatment (protocol of care) with the control treatment (usual care), and to explore numerical results for clinically meaningful outcomes. The secondary objective was to determine patient satisfaction with care. The hypothesis was that the experimental treatment (protocol of care) would result in a greater change in low back–related disability, pain, and patient satisfaction with care over the control treatment (usual care).
METHODS
Setting and Practitioners
This study was implemented across 3 private chiropractic practices in separate locations in the United Kingdom. Each practice offered both the experimental treatment (protocol of care) and the control treatment (usual care), delivered by randomly allocated practitioners.
The average clinical experience for the control group (usual care) practitioners was 14 years and for the experimental group (protocol of care) practitioners, 16 years. It was not feasible for practitioners to be blind to the treatments provided to their patients, but they were masked from the treatment provided by practitioners in the other group and did not collect any data.
Recruitment and Patient Participants
Patients were recruited through convenience sampling from those presenting with LBP through a process of screening and assessment by the participating chiropractors. After assessment, eligible patients were invited to participate in the study, but were allowed a “cooling-off” period of up to 3 days. The cooling-off period provided the eligible patient the opportunity to consider volunteering for the study without feeling pressured to do so, and also acted as a quantification period. The concept of the “qualification period” was considered so that participant selection should aim to ensure that patients entering the study truly had the condition for which the treatment was intended.
Eligible participants who volunteered were then allocated to a treatment group using concealed randomization, after providing written informed consent.
Inclusion Criteria
Inclusion criteria were as follows:(1) a diagnosis of acute nonspecific LBP, without neurologic or vascular deficit, established by the participating chiropractor who initially examined the patient;
(2) experienced LBP for a period of less than 6 weeks (42d);
(3) did not participate in activities or have an occupation that included vigorous activity that may perpetuate or aggravate the existing problem;
(4) had an initial pain score of at least 35/100 (35%) on a pain scale (visual analog scale [VAS]); and (5) had not received manipulative therapy of the spine before.
Potential participants were excluded if(1) they were older than 60 years, because the spinal manipulation prescribed in this trial could be more hazardous;
(2) there was a possibility of a serious spinal, pathologic, or psychiatric disorder;
(3) there were any contraindications to the treatment(s) proposed in this trial (eg, the patient could not tolerate spinal manipulation);
(4) they had previously had spinal surgery, as the clinical outcome was likely to be very different;
(5) they could not walk 100m when free of back pain or get up and down from the floor, because keeping active could be difficult; and
(6) they had received treatment from another health care provider in the previous 3 months for spinal pain, although eligible participants could have had prior episodes of spinal pain but not in the 3 months before the current episode.This project received approval from the King’s College London Research Ethics Committee (PNM/07/08-56).
InterventionsControl group: usual care. Usual care was a combination of treatments in which the frequency of use and timing of delivery were chosen at the discretion of the chiropractor. Usual care might have included, although not exclusively, joint mobilization, spinal manipulation, traditional manual and manipulative techniques, soft tissue treatments, health promotion, exercise or rehabilitation therapy, and patient education or advice.
Experimental group: structured protocol of evidence-based conservative care. The structured protocol of care comprised a package of treatments supported by the best available research evidence, which was found acceptable to an advisory group of practicing chiropractors and judged by them as feasible to deliver in clinical practice (See Appendix 1). The timing and frequency of treatments were prescribed in the protocol, which was monitored and matched to a protocol checklist.The method used to decide on the treatments for the structured protocol of care was the amalgamation of the best available scientific evidence and clinical expertise, as encountered in other pragmatic clinical trials. [14, 15] The theoretic basis for the development of the protocol was the evidence-based practice approach, [16] where, first, a combination of treatments supported by the best available research evidence was selected and, second, this combination of treatments was approved by an advisory group of practicing chiropractors (representing clinical expertise).
The key features of the protocol of care delivered in this trial were as follows:
Patient advice and education, using The Back Book [17, 18]
Encouragement of the participant to remain active and return to work, if possible [19–22]
Regular use of spinal manipulation (grade V; manual, high-velocity, low-amplitude, thrust-type manipulation only) [23, 24]
The discretionary use of premanipulative joint mobilization and gentle soft tissue work only. [25, 26] These premanipulative procedures would be very brief with the aim of having the patient relax and to “warm-up” the area to be treated. Premanipulative procedures were not indeed as specific treatment with therapeutic intent, such as grade IV joint mobilization or myofascial trigger point therapy.
Exercise therapy, [20, 27] back school, [28, 29] joint mobilization (with therapeutic intent), [26] massage (with therapeutic intent), [25] electrotherapy/physical agents (heat, cold), [30–32] and traction/ lumbar supports [33–35] were considered, but excluded from the protocol because the evidence for their use was either insufficient, equivocal, or negative.
Although clinical guidelines offer mixed support for spinal manipulation, a recent practice guideline recommends its use, [36] and specific trials [37, 38] support the effectiveness of spinal manipulation in the subgroup of patients with acute LBP of short duration. Six clinical practice guidelines, [8, 9, 39–42] chosen on the basis of rigor of development [43] and recency of publication, showed general agreement between the treatments chosen for the structured protocol of care (experimental group) in this trial.
Treatment Period
Participants in both intervention groups could receive up to 7 treatments over a 4–week treatment period. Data were collected at the baseline, week 2, and week 4. This number of treatments over a 4–week period is the average number commonly encountered in an acute LBP trial, and was the number of treatments considered to be reasonable by the advisory group of practitioners.
Outcome Measures
The primary outcomes were(1) the Oswestry Low Back Disability Index (ODI) [44, 45] and
(2) the VAS. [46, 47] The ODI measures 10 domains of function to provide a score out of 50.In turn, a disability score may be calculated where 0% to 20% indicates minimal disability; 21% to 40%, moderate disability; 41% to 60%, severe disability; 61% to 80%, extreme disability and 81% to 100%, bed bound or exaggerating symptoms. The VAS is a vertical scale (in millimeters) between 0 and 100, where 0 (bottom anchor) represents no pain and 100 (top anchor) represents the worst imaginable pain. Changes of 27% in the ODI score (14/50) and 25% in the VAS (25/100) are reported as being clinically meaningful. [48, 49]
The secondary outcome was patient satisfaction with care, measured using the Patient Satisfaction Questionnaire (PSQ). [50, 51] The PSQ is a multi-item scale covering information, emotional support and assurance, and the effectiveness of prescribed treatment. Each item is rated on a 5–category response scale from 1 (strongly agree) to 5 (strongly disagree). The categorical data collected in (or with) the PSQ were converted to continuous data, in the form of a proportion (%) for analysis. The PSQ was completed at the end of the treatment period by each participant.
Data Management
Completed questionnaires were collected from participating patients by the clinic manager of each practice at specific time points before each treatment session. The collected data were entered as coded data and stored in coded research files, separate from the clinical records.
Sample Size Calculation
The priori sample size was determined using the combination of(1) data from published trials, and
(2) prospective data from the first 40 participants (20 per group) in this trial, to yield 80% power of detecting a difference at a 5% level of significance (1–tailed test; G*Power 3.0 softwarea).The combination of these 2 methods allowed the researchers to select a reasonable sample size while preserving statistical power.
A 25% or greater between-group difference in either low back–related pain or disability was considered clinically meaningful in this study, [48, 49] requiring a trial to have 47 participants per group (N = 94). With the use of the data from the first 40 randomized participants in this study, a total of 51 per group (N = 102) were calculated (effect size index, .48). Allowing for a patient dropout rate of about 20%, the chosen sample size per group was 60 (N = 120).
Randomization and Masking
Randomization was conducted independently, using a random numbers process, thereby conferring allocation concealment. Each random treatment assignment was placed in a sealed opaque envelope, producing a series of sequentially numbered envelopes that were shared equally between the 3 practice sites. As each eligible patient was consented to participate, the practice manager then opened an envelope and identified the treatment group to which the participant was assigned.
Statistical Methods
Intention-to-treat analysis was performed by replacing missing data using a multiple imputations method by means of a Gibbs sampler (WinMICE version 0.1b) and statistically comparing the original data sets to the data sets with missing data inserted. This imputation method was applied after the data sets were adjusted statistically to account for covariates and for any potential difference between the 2 groups at baseline.
Several approaches for dealing with missing data are used in practice. Many of them, however, give biased results because they fail to take all available information into account. Multiple imputation is a method to draw valid inferences from incomplete data. The calculations were performed using a multilevel regression model that generated 5 multilevel imputations — the 5 imputed values being generated by the software and an acceptable number of values from which to calculate a mean value. The mean values were then used to replace missing scores, and the data were then analyzed using standard statistical tests.
The data sets were amenable to the F test using weighted analysis of covariance (ANCOVA). [52] Differences in baseline data and regression toward the mean were considered potential confounding factors and were accounted for statistically using ANCOVA analysis. [53] Statistical analysis was conducted using the weighted 1–way ANCOVA for between-group analyses, using data from baseline, midpoint (week 2), and final (week 4) consultations. The data were also adjusted using ANCOVA to account for the covariates of duration of symptoms, smoking, and exercise. If significant differences between data sets were discovered, Tukey’s honestly significant difference analyses were performed to establish where the differences lay. All analyses were conducted at a 95% confidence level ( = = .05), using 2–tailed tests (XLStat softwarec).
The estimated differences in between-group means were explored to identify any clinically meaningful change as a result of the interventions. The minimally clinically important difference (MCID) benchmark was 25%.
The data obtained using the ODI and VAS outcome measures were amenable to dichotomization, producing 2 categories: “improved” and “not improved,” respectively. The decision-making rule was that if a participant’s score changed by 25% or greater for either outcome measure, this would be classified as “improved,” whereas a change of less than 25% would be classified as “not improved.” The frequency of dichotomized data was then represented as a ratio (in percent) of cases. Based on these dichotomized data, the number needed to treat (NNT) was calculated to provide insight into the number of patients that would need to be treated with the experimental treatment for 1 patient to benefit over the control treatment. A NNT of 6 or greater would imply that neither intervention was likely to be better over the other, [54] while a NNT of less than 6 would imply that the experimental treatment (protocol of care) would be of clinical benefit over the control treatment (usual care).
RESULTS
Patient Characteristics and Baseline Data

Figure 1 The study is summarized in figure 1 (Consolidated Standards for Reporting Trials [CONSORT]). [55] Of the 288 patients screened, 149 were eligible for the study, with 118 suitable participants providing consent. Fifty-nine participants were allocated to each of the treatment groups. Twelve participants dropped out of the study (did not return for further treatment), and the data from 4 participants were excluded because they were incomplete (total dropout, n = 16), making data from 102 participants available for analysis (control, 53; experimental, 49).
No adverse events or complications were reported (defined as death, hospitalization, or persistent severe stiffness, pain, or disability), and no patients were known to have left the trial because of side effects, but rather dropped out by choice. Although there were isolated cases of transient, benign symptoms of stiffness or pain reported in both intervention groups, these benign side effects subsequently resolved in all cases and were not reported as adverse effects.
Examination of all the data sets in this trial revealed that 13% of the data were missing (n = 118), which were accounted for by imputing missing values. [56] A stand-alone software tool was used to create multiple imputed (completed) data sets. These data sets could then be analyzed using standard statistical packages. Statistical analysis comparing the original data (n = 102) to the data with missing data inserted (n = 118) showed no significant differences across all data sets (P > 05); hence, the data sets with missing data replaced were used in analysis.

Table 1
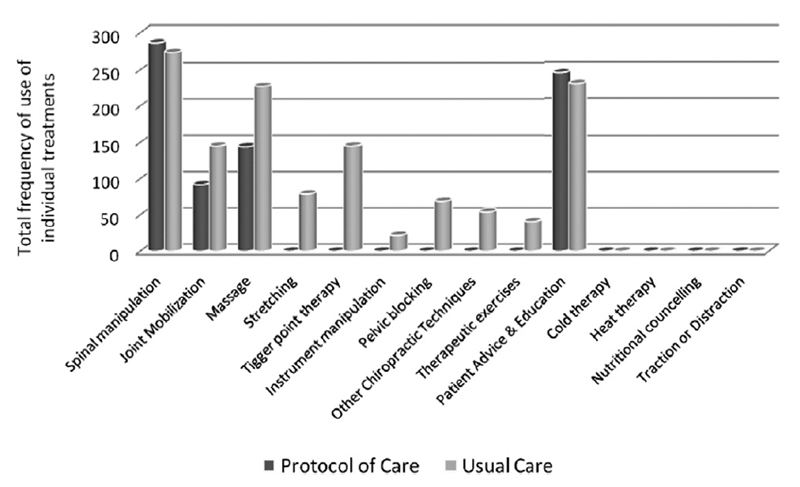
Figure 2

Table 2

Table 3 The patient characteristics and initial scores appeared to be similar at baseline (table 1). The frequency of individual treatments used in both groups is presented in figure 2.
Between-Group Analysis
Statistical analysis of week 4 data (after up to 7 treatments; mean, 6) did not show any significant differences (weighted ANCOVA; P = .33) for ODI, but there was a significant difference for VAS (weighted ANCOVA; P < 001), suggesting that the experimental group was better than the control group in terms of pain, but not disability (table 2). The overall outcome was equivocal based on the results at week 4.
Statistical analysis showed a significant difference (weighted ANCOVA; P < .001) between the 2 treatment groups when comparing data from week 2 (after 4 treatments) for both ODI and VAS, with a greater reduction in scores in the experimental group (protocol of care) (see table 2). Comparison of the week 2 (midpoint) data of the experimental group (protocol of care) with that of the week 4 (final) data of the control group (usual care) showed that the means were very similar for the ODI at 15.5% and 11.4%, respectively. This shows that a similar change in scores occurred in the experimental group within 2 weeks as opposed to 4 weeks in the control group, implying that the 2 treatment groups achieved the same outcome, although this occurred more quickly in the experimental group (protocol of care).
Comparison of the difference in between-group means with the preselected MCID value (MCID = 25%) showed that both the ODI and VAS scores were all well below this threshold value at week 2 and at week 4 (see table 2). NNT scores for ODI at week 2 and week 4 were both below the preselected reference value of NNT = 6 (table 3), but were above this threshold value for the VAS (see table 3). Although the difference in between-group means was low, the overall trend in the NNT scores suggests that there was a clinically meaningful difference between the 2 groups, particularly at week 2, signifying that the experimental treatment (protocol of care) may provide more rapid clinical outcomes than the control treatment (usual care) at this specific time point.
Participants in both groups were very satisfied with care, with the mean score being 85% (95% confidence interval, 84%–87%) for both groups. There was no statistically significant difference between the 2 groups (t test; P = .64).
Within-Group Analysis
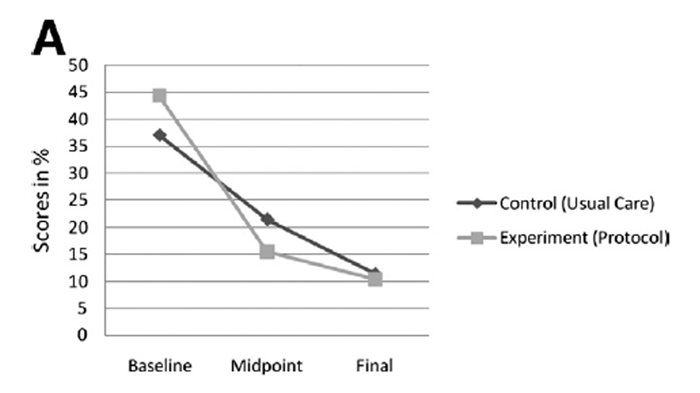
Figure 3 Although the purpose of this trial was not to explore the effectiveness of the individual treatment groups, supplementary analysis provides insight into the possible trends in outcomes. Statistical analysis of the baseline, week 2, and week 4 data for both ODI and VAS found statistically significant differences (weighted repeated-measures analysis of variance; P < .001), indicating that the treatments in both groups resulted in a significant change in disability and pain scores over the treatment period (Figure 3).
The experimental treatment (protocol of care) generated 28.9% and 36.5% change in scores from baseline to midpoint (week 2) for the ODI and VAS, respectively, while the control treatment (usual care) produced a 15.6% and 26.2% change in scores, respectively. The overall change (baseline to week 4) for the ODI and VAS was 34% and 49.1% in the experimental group, respectively, and 25.7% and 39% in the control group, respectively, which are comparatively similar. These scores imply that the experimental treatment imparted most of its effect within 2 weeks with 4 treatments, while the control treatment needed the full 4–week treatment period (see figure 3).
DISCUSSION
The most important finding is that for the full treatment period (4wk; up to 7 treatments; mean, 6 treatments), the research hypothesis for this study should be rejected, implying that the treatments in both groups are likely to generate similar outcomes. However, the experimental treatment (protocol of care) appears to generate a significant change in primary outcome measures within 2 weeks with 4 treatments compared with the control treatment (usual care). Therefore, the results of this study may act as a guide to musculoskeletal practitioners who regularly use manual and manipulative therapy for acute LBP, where a structured protocol may yield comparable results to usual care in a shorter period with less treatment.
Indeed, the outcomes in the experimental group based on disability (ODI) scores are supported by Fritz et al, [37] where 2 sessions of spinal manipulation were reported to provide at least a 50% improvement in disability (ODI) in participants with symptoms of less than 16 days’ duration and no symptoms extending below the knee. As the experimental group demonstrated improvements in pain and disability more quickly (eg, within 4 treatments compared with 6 in the usual-care control group), the outcomes of this study are consistent with a “lessis- more” approach. Further, based on a less-is-more approach, this study’s results support use of an evidence-based protocol of care.
A strength of the current study lies in the examination of NNT and the comparison of the differences in between-group means with the preselected MCID value. These clinical indicators provided insight into whether one treatment group had any clinical benefit over the other, which in turn would be reflected in real-life clinical practice. The clinical indicator of NNT suggested that the experimental treatment had a greater clinical effect than the control treatment at week 2, but had an equivocal effect at week 4 (after the full treatment period).
Although differences in between-group mean scores were lower than the MCID value of 25% or greater, there was indeed a greater change in scores in the experimental group when compared with the control group. It is likely that an MCID value of 25% or greater is too high for between-group comparison, particularly if there is an overlap in some of the treatments used in both intervention groups, as in this study. Within-group differences in means from baseline to the final consultation show a clinically meaningful change in both groups for both outcome measures (see table 2), implying that the treatments in both groups were clinically meaningful, despite low between-group scores.
Patient satisfaction for both treatment groups was very high (> 80%), suggesting that the use of a structured protocol of care does not alter the patient-perceived satisfaction with care over usual care.
Although it was not feasible for practitioners to be blind to the treatments provided to their patients, a positive attribute of the research design was that practitioners were masked from the treatment provided by practitioners in the other group, with a view to reduce treatment contamination. Practitioners also did not collect data, perform any data analysis, or have access to the research files containing the data, thus reducing practitioner bias.
Study Limitations
This study was designed as a pragmatic trial and, therefore, does not permit conclusions to be drawn regarding the effectiveness of specific treatments. Because the participants in this trial were fee-paying patients seen in chiropractic practice, and thus a specific population of patients with LBP, the results may not be wholly generalizable to other clinical contexts. This trial did not include a placebo or no-treatment group. Therefore, no specific inferences can be made regarding the efficacy of the individual treatment groups, only insights into possible outcomes.
The concept of MCID can be very useful in clinical trials where clinical meaning is explored alongside traditional statistical testing. In this trial, within-group differences in means compared with the MCID value showed a clinically important change in both treatment groups, but betweengroup scores were not useful, as the MCID value was set too high. The MCID value for between-group comparison is recommended to be set at approximately 10% to 15%, particularly if there is an overlap in some treatments provided in groups.
The authors admit that there were some mixed results related to clinical significance, particularly the effect size calculation (not reported in this article), which is likely attributed to the sample size used in this trial. The sample size calculation was done using continuous data for only the primary outcome measure of the ODI. This implies that in future studies, sample sizes should take into consideration all the outcome measures and the nature of the data. Hence, sample sizes for future similar studies would be in the order of 120 subjects per group.
Another limitation of the study is that the nature of the missing data could not be explored, since the reasons for patient dropout were not collected. Because the nature of the data may have an influence on outcomes, the reasons for patient dropout should be examined in future studies.
CONCLUSIONS
Overall, the 2 treatment groups were similar based on primary or secondary outcome measure scores for the full treatment period (4 weeks, with up to 7 treatments). However, there were statistically significant and clinically meaningful differences in both disability and pain scores at week 2 (midpoint) with 4 treatments, suggesting that the protocol of care had a more rapid effect than usual care. The results of this study offer guidance to musculoskeletal practitioners, who regularly use manual and manipulative therapy (MMT) for acute LBP, that an evidence-based, structured protocol of care may yield comparable results to usual care in a shorter period with less treatment.
APPENDIX 1: PROTOCOL OF EVIDENCE-BASED CONSERVATIVE CARE
Initial Consultation
The patient received a thorough assessment (case history, physical examination, and orthopedic examination), thereby screening the patient for eligibility for the study. The eligible patient was then introduced to the study, provided an information sheet, and invited to participate.
The initial consultation was followed by the cooling-off period of up to 3 days to allow the patient the opportunity to consider joining the study without feeling pressured to do so. Also, the cooling-off period acted as a quantification period, to ensure that the patient indeed had acute LBP and not just brief, transient symptoms.
Second Consultation
The eligible patient who volunteered for the study then provided written informed consent, after which the participant was randomly allocated to an intervention group, at which time baseline data were also collected. Thereafter, the participant was then given a report of assessment findings, and the cause of the back pain, the prognosis, the treatment plan, and the likely care outcomes were discussed.
Advice and reassurance were provided to the participant, with the clinician explaining to the patient that radiographs and other investigations are normally neither routine nor always useful. The patient was reassured that LBP is usually not a serious problem and that it generally improves rapidly. The core reassurance was as follows:
Acute nonspecific LBP usually has a good prognosis (“should respond well to treatment”; “recovery in a few weeks”).
Positive messages are given (“no permanent damage or disease”; “back pain is very common”).
There is usually no need for x-ray examinations.
There is usually no underlying serious pathology (“there is nothing to worry about”).
The following advice was also to be given at the second consultation:
Advise the patient to stay active as much as possible and that activity is helpful (“not too much sedentary rest”; “no prescriptive bed rest”; “avoid bed rest if possible, only rest if necessary”; “but no specific exercise”), and attempt to return to normal activities, including work, as soon as possible. Activity is the best treatment option for acute LBP.
Unless symptoms and clinical findings indicated some serious spinal disease, the report of findings to the patient was “de-dramatized” by the clinician (“the prognosis is good”, “normally not a permanent problem”, “responds well to treatment”).
Patient was to avoid taking any analgesics or anti-inflammatory drugs, unless necessary.
Comment: The content and timing of treatment were crucial in that advice and reassurance were provided to participants at the onset of their treatment program.
The educational tool (The Back Book) was provided to the participant at the second consultation.
The Back Book was described to the patient and the contents explained, using a 2–phased approach:
Information phase: The clinician described the cause, course, and (im)possibilities of treatment of LBP. This was to ensure that the participant would understand the information and would receive more reassurance than if just told: “you don’t need to worry.”
Self-care phase: The clinician emphasized that the participant was to set specific goals for resuming activities or work and preventing future episodes. The clinician addressed any patient questions related to goal setting and encouraged the patient to read The Back Book. The content of the booklet reinforced the informational phase.
The patient was advised not to use analgesics or nonsteroidal anti-inflammatory drugs (prescribed or over-the-counter) unless absolutely necessary.
The manual and manipulative therapy was then delivered as follows:
Brief premanipulative soft tissue techniques, applied at the discretion of the clinician, as needed (not intended to have a specific therapeutic effect)
Brief premanipulative joint mobilization techniques, applied at the discretion of the practitioner, as needed (not intended to have a specific therapeutic effect)
Regular (unless contraindicated) spinal manipulation using manually applied, high-velocity, low-amplitude, thrust-type manipulation
Comment: Each participant could receive up to a maximum of 7 treatments over a 4–week period. Usually this would be a treatment session every 2 to 4 days, or about twice per week. The exact number of treatments would depend on the patient’s response to treatment, at the discretion of the clinician. Appointment scheduling was such that participants could be treated ideally with approximately 2 treatments per week, although scheduling was flexible to cater to the participant’s needs.
Third Consultation
The participant was briefly assessed, and the prescribed treatment was provided, as long as there were no contraindications to further treatment or adverse events. The advice and reassurance provided at the second consultation were reiterated, and the patient was reminded to read The Back Book.
Fourth and Fifth Consultations
The participant was briefly assessed, and the prescribed treatment was provided, as long as there were no contraindications to further treatment or adverse events. Advice and reassurance did not have to be repeated.
Sixth Consultation
Data were collected from the participant using the study’s outcome measures at the beginning of the visit, before treatment. The participant was briefly assessed, and the prescribed treatment was provided, as long as there were no contraindications to further treatment or adverse events. Advice and reassurance were reiterated, and the patient was reminded to read The Back Book.
Seventh and Eighth Consultations
The participant was briefly assessed, and the prescribed treatment was provided, as long as there were no contraindications to further treatment or adverse events. Advice and reassurance did not need to be repeated.
Ninth Consultation
Data were collected from the participant using the study’s outcome measures at the beginning of the visit. No treatment was provided, and the participant exited the study. The participant was reassessed and, if still symptomatic, was offered continued care or a referral to another health care provider for assessment. If the back pain had resolved (< 25% on a pain and disability scale), then the participant was discharged from care.
References:
Cassidy JD, Cote P, Carroll LJ.
Incidence and course of low back pain episodes in the general population.
Spine 2005;30:2817-23.Loney PL, Stratford PW.
The prevalence of low back pain in adults: a methodological review of the literature.
Phys Ther 1999; 79:384-96.Walker BF, Muller R, Grant W.
Low back pain in Australian adults. Health provider utilization and care seeking.
J Manipulative Physiol Ther 2004;27:327-35.Wand BM, Bird C, McAuley JH.
Early intervention for the management of acute low back pain.
Spine 2004;29:2350-6.Gatchel R, Polatin P, Noe C, Gardea M, Pulliam C, Thompson J.
Treatment- and cost-effectiveness of early intervention for acute low-back pain
patients: a one-year prospective study.
J Occup Rehabil 2003;13:1-9.van Tulder MW, Becker A, Breen A, Koes BW.
European Guidelines for the Management of Acute Nonspecific Low Back Pain in Primary Care
European Spine Jou 2006 (Mar); 15 Suppl 2: S169–191Seferlis T, Nemeth G, Carlsson G.
Conservative treatment in patients sick-listed for acute low back pain.
Eur Spine J 1998;7: 461-70.Bekkering G, Hendricks HJM, Koes BW, Osstendorp RAB.
Dutch physiotherapy guidelines for low back pain.
Physiotherapy 2003; 89:82-96.Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr., Shekelle P, Owens DK:
Diagnosis and Treatment of Low Back Pain: A Joint Clinical Practice Guideline
from the American College of Physicians and the American Pain Society
Annals of Internal Medicine 2007 (Oct 2); 147 (7): 478–491Walker B, French S, Grant W, Green S.
Combined chiropractic interventions for low-back pain.
Cochrane Database Syst Rev 2010;(4):CD005427.Kinkade S.
Evaluation and treatment of acute low back pain.
Am Fam Physician 2007;75:1181-8.Assendelft WJJ, Morton SC, Yu EI, Suttorp MJ, Shekelle PG.
Spinal manipulative therapy for low back pain: a meta-analysis of effectiveness
relative to other therapies.
Ann Intern Med 2003;138:871-81.Armstrong MP, McDonough S, Baxter GD.
Clinical guidelines versus clinical practice in the management of low back pain.
Int J Clin Pract 2003;57:9-13.Underwood M, UK BEAM Trial Team.
United Kingdom Back Pain Exercise and Manipulation (UK BEAM) Randomized Trial:
Effectiveness of Physical Treatments for Back Pain in Primary Care
British Medical Journal 2004 (Dec 11); 329 (7479): 1377–1384Globe GA, Morris CE, Whalen WM, et al.
Chiropractic Management of Low Back Disorders: Report From a Consensus Process
J Manipulative Physiol Ther 2008 (Nov); 31 (9): 651–658Sackett D, Rosenberg W, Gray J, Haynes R, Richardson W.
Evidence-Based Medicine: What It Is and What It Isn't
British Medical Journal 1996 (Jan 13); 312 (7023): 71–72Cherkin DC, Deyo RA, Battie M, et al.
A Comparison of Physical Therapy, Chiropractic Manipulation, and Provision
of an Educational Booklet for the Treatment of Patients with Low Back Pain
New England Journal of Medicine 1998 (Oct 8); 339 (15): 1021-1029Burton AK, Waddell G.
Information and advice to patients with back pain can have a positive effect.
A randomized controlled trial of a novel educational booklet in primary care.
Spine 1999;24:2484-91.Waddell G, Feder G, Lewis M.
Systematic reviews of bed rest and advice to stay active for acute low back pain.
Br J Gen Pract 1997;47:647-52.McLain K, Powers C, Thayer P, Seymour R.
Effectiveness of exercise versus normal activity on acute low back pain:
an integrative synthesis and meta-analysis.
Online J Knowl Synth Nurs 1999;95-102.Henrotin Y, Cedraschi C, Duplan B, Bazin T, Duquesnoy B.
Information and low back pain management.
Spine 2006;31: E326-E334.Engers A, Jellema P, Wensing M, Van der Windt DA, Grol R, Van Tulder MW.
Individual patient education for low back pain.
Cochrane Database Syst Rev 2008;(1):CD004057.Ferreira ML, Ferreira PH, Latimer J.
Efficacy of spinal manipulative therapy for low back pain of less than
three months’ duration.
J Manipulative Physiol Ther 2003;26:593-601.Bronfort, G, Haas, M, Evans, RL, and Bouter, LM.
Efficacy of Spinal Manipulation and Mobilization for Low Back Pain and Neck Pain:
A Systematic Review and Best Evidence Synthesis
Spine J (N American Spine Soc) 2004 (May); 4 (3): 335–356Furlan A, Imamura M, Dryden T, Irvin E.
Massage for low back pain.
Cochrane Database Syst Rev 2008;(4):CD001929.Hanrahan S, Van Lunen B, Tamburello M, Walker M.
The shortterm effects of joint mobilizations on acute mechanical low back dysfunction
in collegiate athletes.
J Athl Train 2005;40:88-93.Hayden J, Van Tulder MW, Malmivaara A, Koes BW.
Exercise therapy for treatment of non-specific low back pain.
Cochrane Database Syst Rev 2005:(3):CD000335.Maier-Riehle B, Harter M.
The effects of back schools: a metaanalysis.
Int J Rehabil Res 2001;24:199-206.Heymans MW, Van Tulder MW, Esmail R, Bombadier C, Koes BW.
Back schools for non-specific low back pain.
Cochrane Database Syst Rev 2004;(4):CD000261.French S, Cameron M, Walker BF, Reggars J, Esterman A.
Superficial heat and cold for low back pain.
Cochrane Database Syst Rev 2006;(1):CD004750.Hurley DA, McDonough SM, Dempster M, Moore AP, Baxter GD.
A Randomized clinical trial of manipulative therapy and interferential therapy for
acute low back pain.
Spine 2004;29: 2207-16.Thompson J, Bower S, Tyrer S.
A double-blind randomised controlled trial on the effect of transcutaneous spinal
elecroanalgesia (TSE) on low back pain.
Eur J Pain 2008;12:371-77.Harte A, Baxter GD, Gracey J.
The efficacy of traction for back pain: a systematic review of randomised controlled trials.
Arch Phys Med Rehabil 2003;84:1542-53.Clarke J, Van Tulder MW, Blomberg S, et al.
Traction for low back pain with or without sciatica.
Cochrane Database Syst Rev 2007;(2):CD003010.van Duijvenbode I, Jellema P, van Poppel M, Van Tulder MW.
Lumbar supports for the prevention and treatment of low back pain.
Cochrane Database Syst Rev 2008;(2):CD001823.National Institute for Health and Clinical Excellence (NICE).
Low Back Pain: Early Management of Persistent Nonspecific Low Back Pain
London: National Institute for Health and Care Excellence; 2009.
[Report No.: Clinical guideline 88].Fritz JM, Childs JD, Flynn TW.
Pragmatic application of a clinical predicition rule in primary care to identify patients
with low back pain with a good prognosis following a brief spinal manipulation intervention.
BMC Family Practice 2005;6:29.Fritz JM, Delitto A, Erhard RE.
Comparison of classificationbased physical therapy with therapy based on clinical
practice guidelines for patients with acute low back pain.
Spine 2003;28: 1363-72.Stanley J. Bigos, MD, Rev. O. Richard Bowyer, G. Richard Braen, MD, et al.
Acute Lower Back Problems in Adults. Clinical Practice Guideline No. 14.
Rockville, MD: Agency for Health Care Policy and Research,
Public Health Service, U.S. Department of Health and Human Services; 1994Royal College of General Practitioners.
Clinical Guidelines for the Management of Acute Low Back Pain
London: Royal College of General Practitioners; (1999).[UK]Philadelphia Panel.
Philadelphia Panel evidence-based clinical practice guidelines on selected
rehabilitation interventions for low back pain.
Phys Ther 2001;81:1641-62.European Cooperation in Science and Technology (COST).
COST action B13. European guidelines for the management of low back pain.
Eur Spine J 2006;15(Suppl 2):S125-7.Arnau J, Vallano A, Lopez A, Pellise F.
A critical review of guidelines for low back pain treatment.
Eur Spine J 2006;15:543-53.Davidson M, Keating JL (2002)
A Comparison of Five Low Back Disability Questionnaires: Reliability and Responsiveness
Physical Therapy 2002 (Jan); 82 (1): 8–24Roland M, Fairbank JC.
The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire.
Spine 2000; 25.3115-24.Langley G, Sheppeard H.
The visual analogue scale: its use in pain measurement.
Rheumatol Int 1985;5:145-48.Wewers M, Lowe N.
A critical review of visual analogue scales in the measurement of clinical phenomena.
Res Nurs Health 1990; 13(4):227-36.Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet- Nilsson N.
Responsiveness and minimally clinically important differences for pain and disability
instruments in low back pain patients.
BMC Musculoskelet Disord 2006;25:82.Ostelo R, de Vet HC.
Clinically important outcomes in low back pain.
Best Pract Res Clin Rheumatol 2005;19:593-607.Nyiendo J, Haas M, Goldberg B.
Pain, Disability, and Satisfaction Outcomes and Predictors of Outcomes:
A Practice-based Study hronic Low Back Pain Patients
Attending Primary Care and Chiropractic Physicians
J Manipulative Physiol Ther. 2001 (Sep); 24 (7): 433–439Hudak PL, Wright JG.
The characteristics of patient satisfaction measures.
Spine 2000;25:3167-77.Vickers A.
Parametric versus non-parametric statistics in the analysis of randomized trials
with non-normally distributed data.
BMC Med Res Methodol 2005;5:1-12.Barnett A, van der Pols J, Dobson A.
Regression to the mean: what it is and how to deal with it.
Int J Epidemiol 2005;34:215-20.McQuay H, Moore R.
Using numerical results from systematic reviews in clinical practice.
Ann Intern Med 1997;126:712-20.Altman D, Schultz K, Moher D.
The revised CONSORT statement for reporting randomised controlled trials:
explanation and elaboration.
Ann Intern Med 2001;134.Rubin DB.
Multiple imputation for nonresponse in surveys.
New York: Wiley; 1987
Return to LOW BACK PAIN
Return to WHAT IS USUAL CARE?
Since 9–15–2017


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |