

Articular Cartilage Surface Changes Following Immobilization
of the Rat Knee Joint. A Semiquantitative Scanning
Electron-microscopic StudyThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Acta Anat (Basel) 1996; 157 (1): 27–40 ~ FULL TEXT
Hong SP, Henderson CN.
Palmer Chiropractice University,
Davenport, IA 52803, USA.
Hong_S@Palmer.edu
Normal articular cartilage surfaces are not flat and smooth, but are contoured with various degrees of roughness. We applied the articular surface classification system developed by Jurvelin to evaluate contour and surface quality changes in rat patellae after varying periods of knee joint immobilization. In our study, one knee joint was immobilized in each of 20 rats, while the contralateral joint served as a free-moving control. Scanning electron-microscopic montages were constructed and the entire cartilage surface was subdivided by contour and surface quality areas. The total articular surface area and all contour and surface quality subclass areas were then calculated for both joints in each rat. Numerous studies have demonstrated that joint immobilization induces degenerative changes in articular cartilage. We found a correlation between the duration of immobilization and changes in the measured area of contour and surface quality subclasses. There was a statistically significant decrease of the even surface area in the weight-bearing, central region of the patellae. This corresponded to a statistically significant increase in the slightly uneven articular surface area usually found just peripheral to this weight-bearing region. There was also a less pronounced (but not statistically significant) decrease in the smooth surface area with a corresponding increase in the rough surface area of the patellae following immobilization. Closer examination of these surfaces revealed that the superficial cartilage layer in the central, weight-bearing areas had less ground substance than other areas. In addition, there appeared to be two different fiber diameters in the superficial cartilage layer of the rat patellae. These were predominantly collagen fibers (142.3 +/- 31.5 nm in diameter) generally running parallel to the long axis of the patella, occasionally random in orientation, and sometimes presenting on the cartilage surface as collagen tips and much more scarce fibrils (20.0 +/- 4.8 nm in diameter) randomly crossing the larger collagen fibers.
Key Words Knee joint, Articular cartilage, Patella, Immobilization, Scanning electron microscopy, Rat
From the FULL TEXT Article:
Introduction
The objective of this investigation was to identify and quantify changes in articular joint surfaces associated with reduced joint mobility. Basic investigations such as this are necessary to increase our understanding of normal joint structures and the pathology of joint disease. Ultimately, these basic investigations may allow us to evaluate the impact of daily working conditions, sports activities, and other life style characteristics on joint health. This information may also assist in the development of successful treatment strategies for degenerative joint disease. However, it must be noted that the findings of several studies suggest that articular changes associated with joint immobilization are different than those associated with degenerative joint disease [Müller et al.. 1994: Grumble et al., 1995].
There is some variation in the use of the term, ‘superficial layer of the articular surface". In rat joints, Gardner [1972] described the superficial articular cartilaginous surface as a thin layer of electron-dense material that was thought to be a synovial protein-hyaluronate complex, or a surface lamina, with a proteoglycan structure. Ghadially et al. [1982] described the superficial cartilage layer of the rabbit femoral condyles as a surface coat with a single layer of filamentous and particulate electron-dense materials. They noted that this unilaminated coat at limes penetrated a short distance into the superficially placed fibrils of the collagen substructure. In some instances, the superficial coat penetrated much deeper forming a multilaminated surface. Using TEM. the unilaminated coal measured between 0.03 and 0.1 um in thickness, while the total multilaminated coat extended to about 1 pm from the surface. Longmore and Gardner [ 1975] described the superficial layer of human femoral condyle articular cartilage as consisting of chondrocytes and collagen fibers with a complex ground substance. Coudane el al. [1987] described the surface layer of the human patella as corresponding to zone 1, which was poor in cells with a uniform structure showing primary and secondary undulations that gave it a regular wavy appearance. These descriptions essentially characterize the superficial layer as a thin amorphous matrix which overlies a much thicker, multilaminate collagenous substrata. However. Teshima et al. [1995] have described the superficial layer of human femoral heads as a 4- to X-pm-thick, acellular layer consisting of collagen fibrils (125-150 nm in diameter), without commenting on the amorphous matrix layer. In this article, the term ‘superficial cartilage layer’ refers to the 0.03-0.1 pm thick amorphous matrix overlying the collagenous substructure of the joint surface.
It is widely accepted that normal articular cartilage surfaces are irregular or rough when examined in vivo and in vitro with the light or scanning electron microscope. With respective increases in magnification, the surface irregularities of articular cartilage have been classified into primary through quaternary order |Gardner and McGillivray, 1971: Longmore and Gardner. 1975], However, these classifications did not readily permit quantification of joint surface changes. Jurvelin et al. [1983| introduced a semiquantitative method for evaluating articular cartilage surface changes. Their approach involved measuring the area of qualitatively determined articular surface features from large picture montages of rabbit patellae. Following a period of physical activity or immobilization, the articular surfaces of rabbit patellae were evaluated for evenness (flatness) of surface contours and smoothness (fine features) of the true articular surface [Jurvelin et al.. 1983].
These studies are particularly interesting because immobilization of injured and inflamed joints has been the predominant treatment of choice in medical history. Recently, this clinical approach has come under increasing criticism. In fact, researchers now use immobilization to produce arthritic changes in joints [Kiviranta et al.. 1987: van Lent et al.. 1991: Sakakibara et al.. 1994] or to induce weightbearing changes within the contralateral joint [Slowman and Brandt. 1986], It is also believed that surface morphology contributes to joint lubrication mechanisms and is. therefore, an important consideration in locomotion studies. It has been suggested that altered lubrication influences age-related cartilage changes and the onset of osteoarthrosis [Silberberg et al.. 1970: Fame et al.. 1977; Greisen et al., 1982], When an animal knee joint is immobilized for a period of time, degenerative changes appear in the cartilage [Evans et al.. 1960; Hall. 1964: Wigren and Wik. 1974: Candolin and Videman. 1980: Helminen et al.. 1983a. b: Jurvelin et al., 1983. 1985: Jozsa et al.. 1987], This is independent of whether or not the joint is physically stressed [Gardner and McGillivray, 1971; Broom and Myers. 1980; Candolin and Videman. 1980; Jurvelin et al.. 1983. 1985: Bouvier and Zimny. 1987: Coudane et al., 1987: Jozsa et al., 1987]. Destructive changes in synovial joints, resulting from prolonged immobilization, have been described in both humans and experimental animals [Finsterbush and Friedman. 1973: Wigren and Wik, 1974: Candolin and Videman. 1980: Palntoski and Brandt, 1982]. Therefore, immobilization studies, such as the one described here, provide essential information regarding the process of degenerative joint disease in humans.
Numerous methods have been used to immobilize extremities in experimental animals. The most common method employs a plaster cast. However, plaster casts do not work well with rats, because rats chew off the cast. Evans et al. [1960] were able to successfully immobilize the rat knee by placing an internal Plexiglas splint on the lateral aspect of the femur and tibia, secured to the bones with steel pins. This procedure is very time consuming and produces extensive local trauma. Hall [1964] fixed the knee in extension by tying fine stainless-steel wire around the rat's mid-tibia and mid-femur, and fastened the ends of the two wires together anterior to the knee joint. We adopted a modification of this procedure because it is simple and easily performed in the rat. and has the important feature of protecting the fixation device from chewing. We decided to study the patella because it readily permits examination of an entire articular surface and allows a comparison with similar studies by Jurvelin et al. [1983].
The specific aim of this investigation was to identify changes in the articular surfaces of rat patellae after one of the rat’s knee joints was immobilized by a surgical ligation of the tibia to the femur [after Hall. 1964], The articular surfaces of the immobilized and freely moving contralateral (control) knee joints were compared according to a modification of Jurvelin's articular surface classification [Jurvelin et al., 1983|.
Materials and Methods
Immobilization of the Knee
The right or left knee, chosen at random, was immobilized in 20 adult albino male rats. Under sodium pentobarbital anesthesia, the skin over the posterior aspect of the extended knee was opened (approximately 20 mm). The skin over the lateral aspect of the thigh was retracted and the quadriceps muscle mass was pierced to allow a suture (000 silk. Ethicon, Inc.) to be subcutaneously looped over the proximal femur. The knee was then maximally Hexed, without forceful compression of the soft tissues, and the suture was passed beneath the skin to the distal ankle, where it was subcutaneously looped around the ankle. The two ends of the suture were tied together in the posterior knee region (maintaining the knee in flexion) and the skin was closed over the suture fixation with Michel clips. Each rat was then returned to its cage (25x33 cm'). Postsurgical survival times were varied from 6 It to 6 months to produce a range of immobilization intervals. At the end of the immobilization interval, the effectiveness of the procedure was evaluated by measuring the residual angular motion of the fixated knee joint. This was accomplished by tracing the anterior outline of the lower limb, from the fixated knee to the distal tibia, after the knee was maximally flexed from its fixed position (with thigh stabilized). The residual motion angle was thus formed by the anterior, distal tip of the patella as the vertex, while the apices of the heel outlines formed the two limbs of the angle.
Scanning Electron Microscopy
Deep anesthesia was induced with sodium pentobarbital and the rats were perfused through the abdominal aorta with isotonic saline, followed by a 4'/ glutaraldehyde solution. Both patellae were excised and rinsed briefly in isotonic saline solution to remove blood and synovial fluid. The patellae were then immersed fora maximum of three days in 2c/< glutaraldehyde in Sorrenson's phosphate buffer (ph 7.4). Dehydration was carried out gradually in ascending concentrations of alcohol. Final drying, according to the critical point method, was accomplished using liquid carbon dioxide as a transition fluid (Tousimis AUTOSAMDRI-810). All patellae were then mounted on aluminum stubs and sputter coaled with gold, platinum, or palladium. The entire surface area of each patella was examined and photographed using a JEOL JSM-35 SEM or Uitach: HS500 SEM with a beam acceleration voltage of 15 kV. Nine to 12 SEM photographs (x60) were used to form a large picture montage of the patellar surface. Following photographic enlargement, the final montage magnification was x 120 (fig. la, b).
Surface Classification
The patellae articular surfaces were classified using a modification of the method described by Jurvelin et al. 1I983|. a descriptive label with an associated numerical tag. The subclasses we used were: Contour: even (01). slightly uneven (02). hillock) (03), folded (04): and Surface quality: smooth (II). rough (12). knobby (14). leafy (15). Examples of each subclass are provided in figures 2a-8a (x 200). Evaluation of a surface is highly dependent on the magnification factor. All of our evaluations were performed on montages with a x 120 final magnification. In this study, we used the first two classifications of Jurvelin (General Contours, and Quality of the True Articular Surface). Jurvelin's third classification. Splits in the Surface, was not used in our semiquantitative assessments, because we considered that these features were very likely to be procedural artifacts. Although we did not measure them as classifiable surface areas, splits in the articular surface were noted in the description of the patellar cartilages and have been addressed in the discussion.
Figure 1.
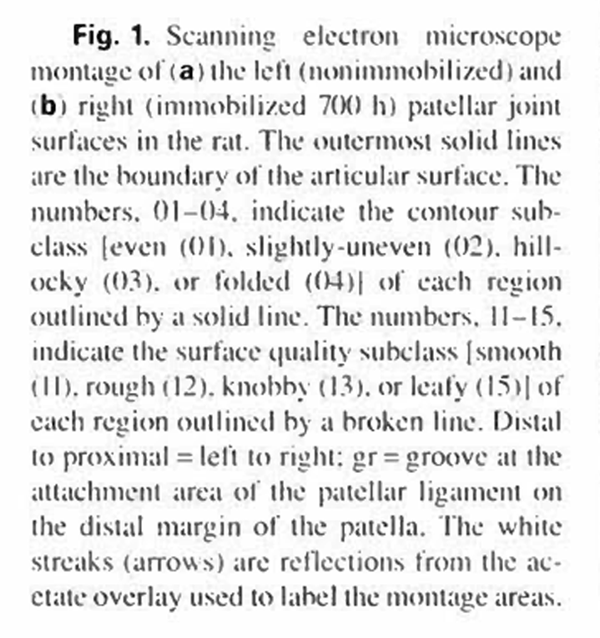
Figure 1.

Figure 2.
Mensuration and Statistical Analysis
The margins of the patellar cartilage, and the area of each subclass, were drawn onto a clear vinyl sheet placed over the montage. Solid lines were used to outline general contour areas and broken lines were used to outline surface quality areas (fig. 1 a. b). Adjacent areas of the same classification were measured collectively when they were separated by 8.3 urn (I cm on the montage) or less, and individually when the intervening area was greater than 8.3 pm. Area calculations were performed by digitizing the boundaries of the outlined areas with the cursor of a Digi-Pad 5 (GTCO Co.). These data were recorded on a Quattro Pro spread sheet (Borland International. Inc.). The spatial areas of each subclass were calculated relative to the area of the whole articular surface. These data were analyzed by paired t test, multivariate tests with Hotelling's confidence intervals. Pearson correlation, and linear regression analyses (SPSS Inc., 1993). We examined the association between the length of the immobilization interval and the surface area of general contour and surface qual ity subclasses. The resultant graphical plots (fig. 9, 10) were produced with SPSS Graphics (SPSS Inc.. 1993). Reproducibility of our methods w ere evaluated by examining the repeated digitizations of one observer (intraobserver reliability) and the equivalent measurements of five separate observers (interobserver reliability ). These data were analyzed using the paired Student t lest and Pearson correlation coefficient.
Results
Scanning Electron Microscopy
The mean articular cartilage surface areas (table I), derived from 20 pairs of patellae, were 11.58 ± 1.38 mnr for the immobilized joints and 11.65 ± 1.24 mnr’ for the contralateral (control) joints. These articular surfaces were essentially pear shaped (approximately 2.8x3.9 mm in diameter). with a longitudinal convexity. The proximal ends of the patellae were always wider than the distal ends. In addition. the distill ends of the patellae were continuous with the patellar ligament (fig. la. b). A small groove (gr) separated the articular surface of the bony patella and the ligament. The average percentage of the even and smooth surfaces were 43.1 ±8.1 and 54.1 ±7.7%, respectively, for the control knee joints, and 26.0± 16.8 and 46.6 ± 12.5%. respectively for the immobilized knee joints (table 1). Throughout this article, when both the contour and surface quality features are combined to describe an area, the contour term will be presented first followed by the surface quality term, separated by a slash (i.e. contour/surface quality).
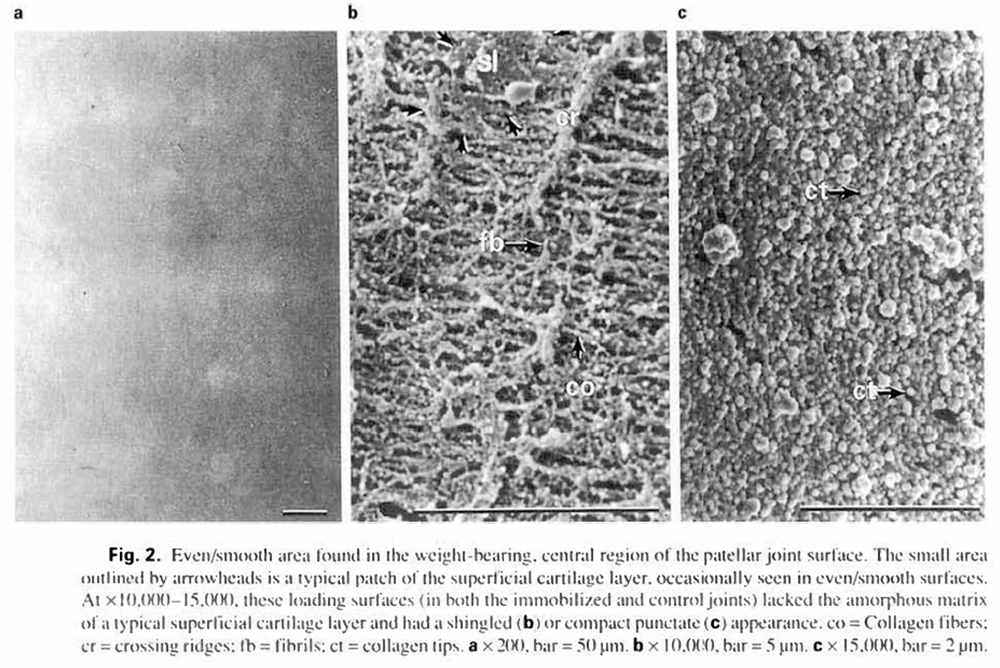
Even/smooth cartilage surfaces (01/11) could always be observed in the center portion (loading areas) of each patella. At x 10.000-15.000, these loading surfaces (in both the immobilized and control joints) lacked the amorphous matrix of a typical superficial cartilage layer and had a shingled (fig.2b) or compact punctate (fig. 2c) appearance. Even/smooth surfaces always contained some combination of the areas shown in figures 2b and c. with gradual transitions between them. Collagen fibers (co) were frequently discerned and appeared to be parallel to each other. These fibers were quite compact with occasional fibrils (fb) crossing them. The observed libers were typical of the superficial layer substructure observed in other areas of patellar cartilage: collagen fibers running parallel to the long axis of the patella (mean diameter, 142.3 ±21.5 nm), and fibrils randomly crossing the collagen fibers (mean diameter. 20.0 ±4.8 nm). In figure 2b. sparse patches of superficial layer (si) are observed, in which collagen fibers are not well resolved because they are embedded in amorphous ground substance. In some portions of the even/smooth area, the parallel network of collagen fibers were regularly interrupted by crossing ridges (cr) produced by the raised free ends of collagen sheets overlapping each other in shingled layers (fig. 2b). Other portions of this area appeared to be a dense blanket of collagen fiber tips (fig. 2c).
Slightly uneven/smooth surface (02/11) were generally peripheral to the even/smooth surfaces. These were transitional areas between the even/smooth and slightly un- even/rough (02/12) areas. At low magnification (fig. 3a). these areas had a slightly bumpy appearance. Only a few collagen fibers could be discerned at higher magnification (fig.3c). because the fibers were embedded in amorphous ground substance, although, numerous protruding collagen tips were usually visible (fig.3b). Occasional collagen fibers and fibrils were randomly scattered on the surface of this superficial cartilage layer.
The slightly uneven/rough surfaces (02/12) were located at the perimeter of the articular cartilage. These areas always contained a well developed superficial cartilage layer, which often appeared to be flaked away from the articular surface and/or appeared cracked (fig.4a. b). Collagen fibers were randomly arranged, less compact and more hidden by the superficial layer in these areas than the slightly uneven/smooth surfaces. Often, collagen tips could just be discerned beneath the well developed amorphous matrix (fig. 4c).
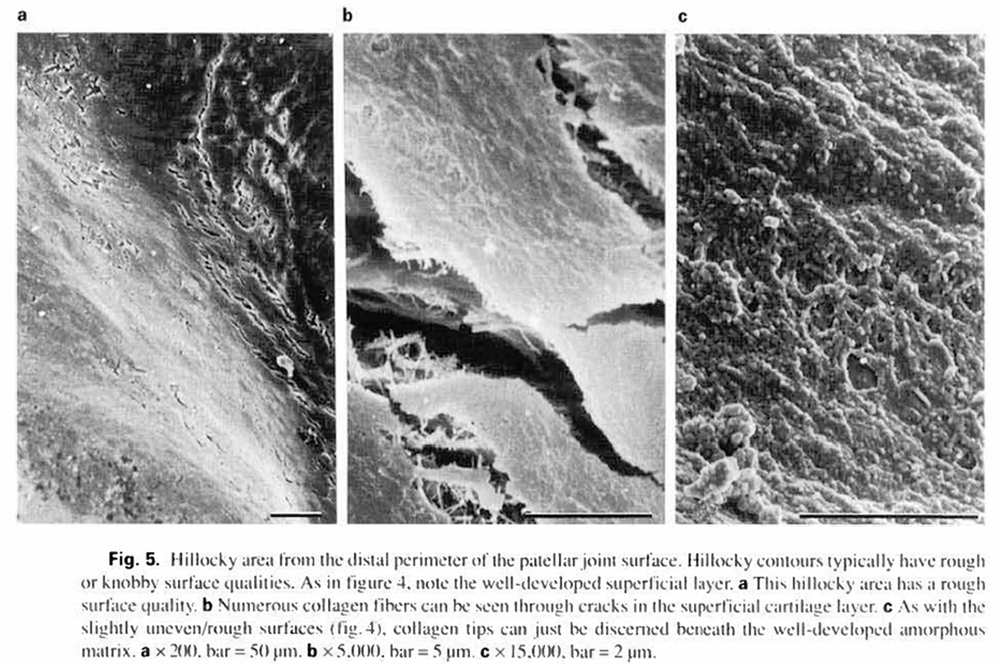
Hillocky surfaces (03) were generally located at the proximal and distal ends of the patellae (fig. la. b). These areas were usually rough (12) or knobby (14). but never smooth (11). They frequently had cracks within the superficial layer, through which collagen fibers were clearly seen (fig. 5a, b). In addition, numerous randomly oriented collagen fibers were noted on the surface of the superficial cartilage layer (fig.5b). As with the slightly uneven/rough surfaces (02/12). collagen tips could just be discerned beneath the well developed amorphous matrix (fig. 5c).
Figure 3.

Figure 4.
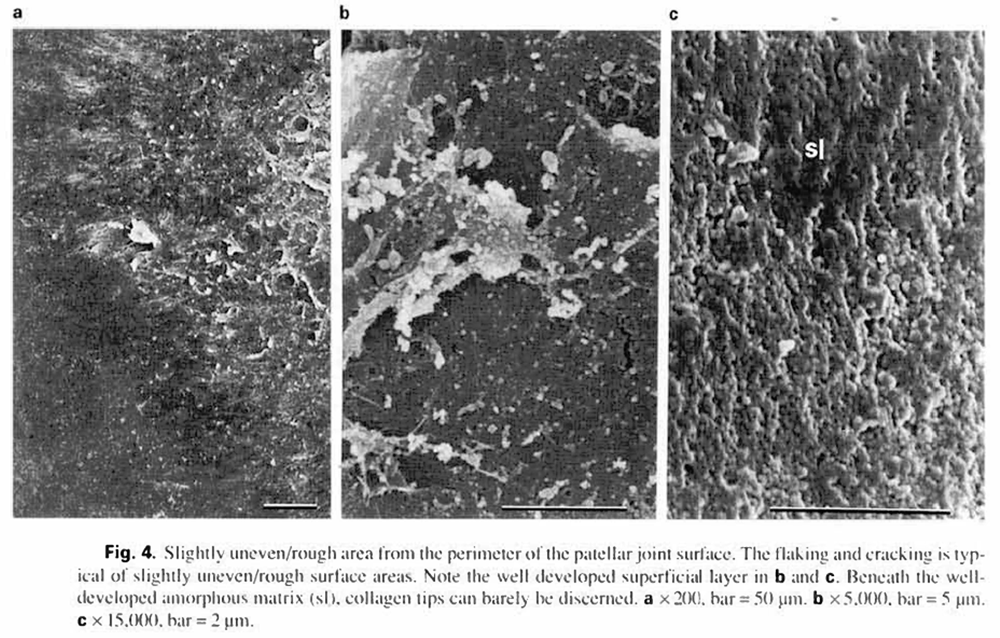
Figure 5.
Folded areas (04) generally had rough (12) surface features. These articular surfaces appeared ‘wrinkled’, and were located primarily at the medial distal and lateral distal corners of the patellae (fig. la. b, 6a). The superficial layer always retained its continuity in folded areas. However, this layer was thinner than that of hillocky surfaces. For this reason, the collagen substructure was more easily discerned in folded areas (fig.6b) than in hillocky areas (fig. 5b). Folded surfaces were found on both immobilized and control patellae in seven of the twenty rats tested. Among the remaining 13 rats, 2 animals had folded areas only on the immobilized side. 7 animals had folded areas only on the control side, and 4 animals did not have folded areas on either side.
The knobby cartilage areas (14) occupied only about 4% of the total articular surface. This surface quality was usually located in slightly uneven and/or hillocky cartilage areas, especially at the proximal and distal corners of the patellae (fig. la, b). At higher magnification, ‘knobs' were round or oval elevations (average diameter, I0.2x 14.0 pm; fig. 7a. b). Knobby surfaces showed numerous. parallel, densely packed, collagen fibers lacking crossing ridges.
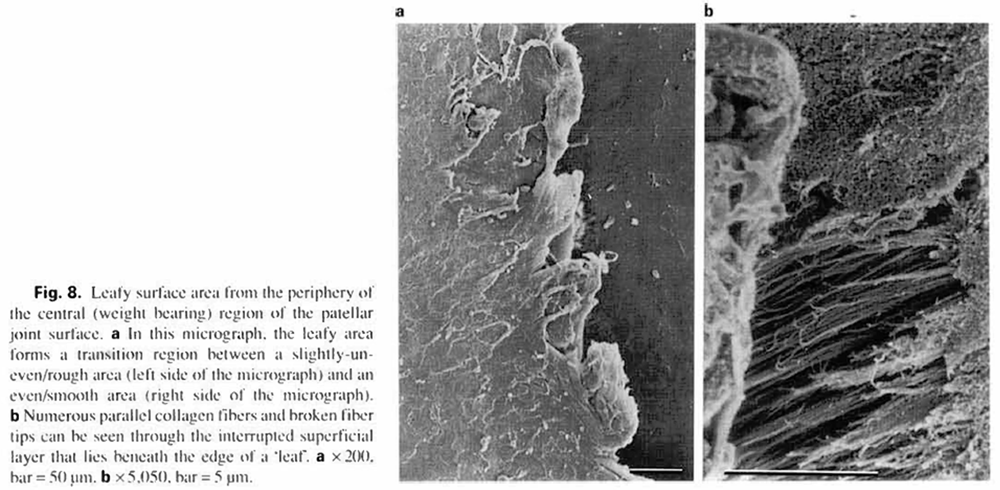
Leafy surfaces (15) were generally located on slightly uneven contours. The leafy surface areas were also very small (5%), residing in the proximal or distal perimeter of the central articular region (fig. la. b). The superficial layer of these surfaces was interrupted at the leafy free ends, and whole collagen fibers and broken collagen tips could be seen though the surface discontinuities (fig. 8a. b).
Figure 6.
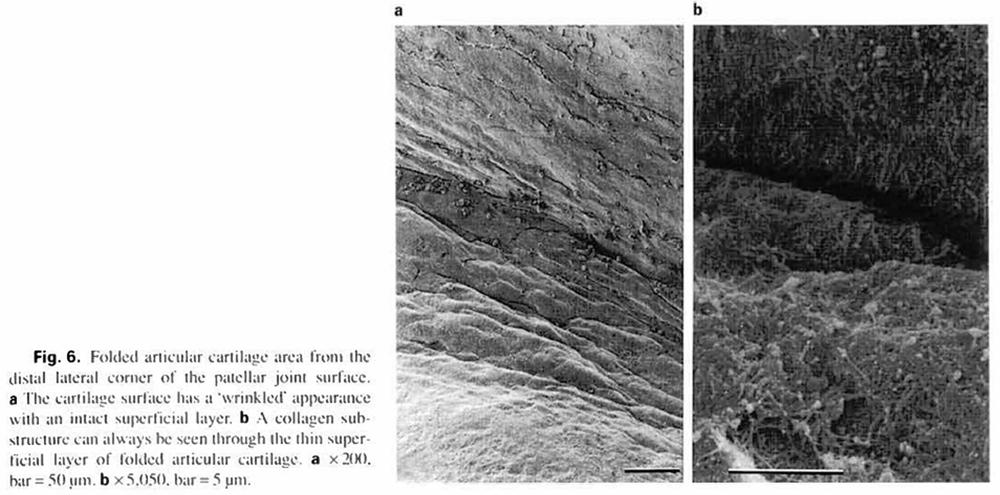
Figure 7.
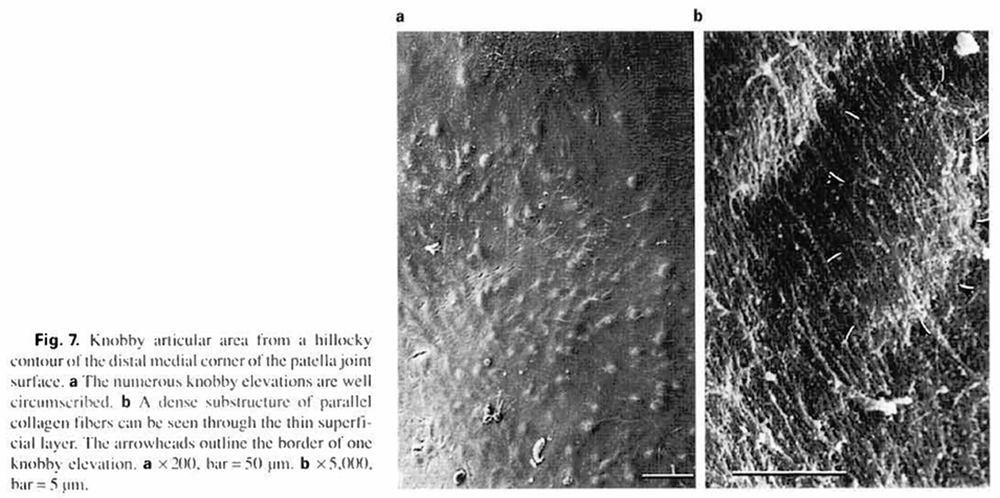
Figure 8.
Statistical Analysis
Analysis of the data indicated high intra- and interexam- incr reproducibility: intraexaminer, r = 0.80 (p = 0.00): interexaminer, r = 0.70 to 0.88 (p< 0.05).
Table I lists mean surface areas and associated standard deviations for patellar contour and surface quality subclasses in immobilized and control knee joints. Multivariate test results (Hotelling’s T:) with associated confidence intervals are also reported. There was no significant difference between the immobilized and control total articular surface areas. The multivariate confidence intervals revealed a significant change in the relative areas of two surface contours: even (95% Cl. -32.975 to -1.365) and slightly uneven (95% Cl. 3.301-31.919). By contrast, the surface qualities did not attain a statistically significant level. However, wide confidence intervals were obtained for these variables.
Linear regression analysis revealed correlations between survival time and changes in surface contour and quality, however, possibly due to small sample size, some of the sublevel correlations failed to achieve a statistically significant level. In figure 9 (surface contours), control regression lines demonstrate essentially no change in the even, slightly uneven, hillocky, and folded surface areas with increased survival time. The immobilization regression lines indicate that increased immobilization time produces a marked decrease in the evenness of the patellar surface (p = 0.000), with a commensurate marked increase in the slightly uneven surface area (p = O.(K)O) and a slight increase in the hillocky surface area (p = 0.03). There was no change in the folded surface area with increased immobilization time. Figure 10 presents regression plots for surface quality changes in control and immobilized joints with increasing survival time. Control regression lines failed to demonstrate statistically significant changes. Immobilization regression lines show that increased immobilization time was associated with a substantial decrease in patellar smoothness (p = 0.01). There was also a slight increase in the knobby, and leafy surface areas that almost attained a statistically significant level (p = 0.07 and p = 0.07. respectively).
Figure 9.
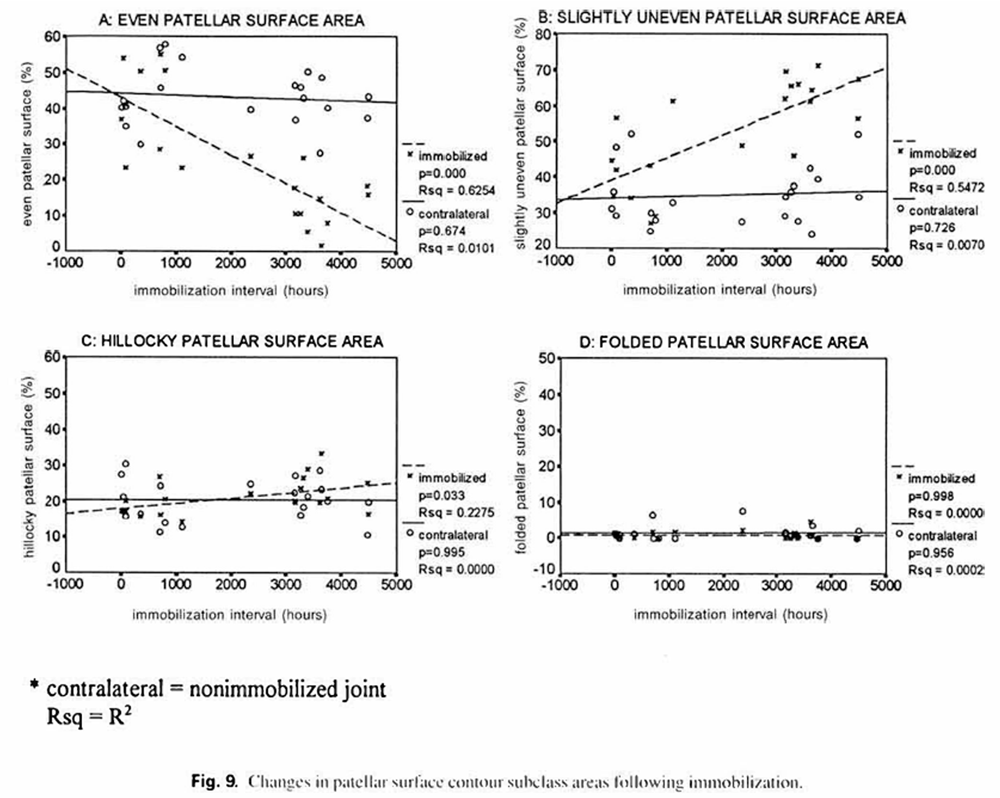
Figure 10.
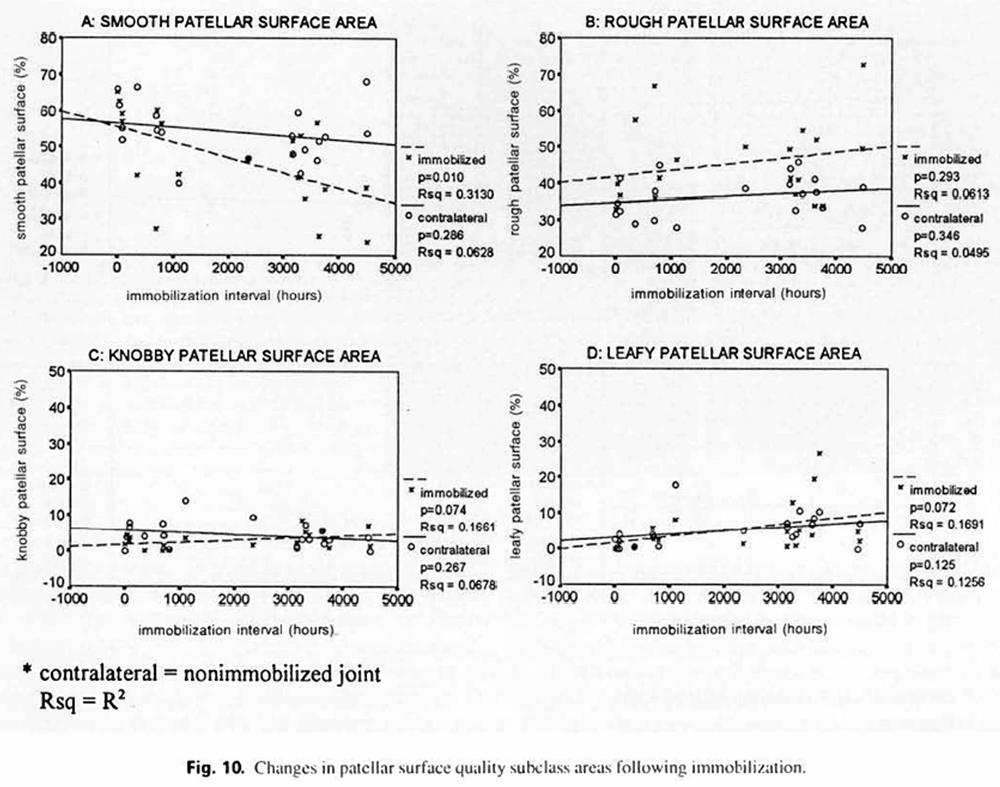
Table 1.

Discussion
The anatomical irregularity of articular cartilages has been demonstrated in vivo and in vitro. Longmore and Gardner [1975] organized these irregularities into four categories corresponding to increasing levels of magnification: primary contours, secondary irregularities, tertiary hollows, and quaternary ridges [Gardner and McGillivray. 1971; Gardner. 1972; Longmore and Gardner. 1975; Pidd and Gardner. 1987]. Later. Jurvelin et al. [1983. 1985] developed classifications of articular cartilages for contour (even, slightly uneven, hillocky, folded, fibrous, and unclassified), quality of surface (smooth, slightly rough, rough, knobby, slriated/leafy, and unclassified), and surface splits (intact, superficial, deep, and unclassified) to study the relative area changes following experimental treatment. Jurvelin's hillocky and folded surfaces are similar to Longmore's secondary irregularities. The knobby surfaces described by Jurvelin are thought to be produced by underlying chondrocyte lacunae. Chondrocyte lacunae are also thought to produce the tertiary hollows of Longmore’s classification system. The cause of these different presentations, elevations versus hollows, is controversial. Wigren and Wik [1974] reported oval depressions of 20-30 um diameter in rabbit articular cartilage, with the number of depressions decreasing after immobilization. By contrast. Jozsa et al. [1987] reported that immobilizing rat knee joints produced an increase in the number of these depressions. They attributed this finding to a necrotic effect on chondrocytes, with the collapsing superficial layer producing empty pits on the cartilage surface. Although we agree that the knobby surface is probably due to underlying chondrocytes, we observed elevations (knobby surfaces), but not hollows in our materials. Further, our regression analysis (fig. 10C) indicates a slight increase in knobby surface area in immobilized joints and a slight decrease in the control joints.
This investigation utilized the articular surface classification system introduced by Jurvelin et al. [1983, 1985]. However, we found it necessary to make some modifications in this system. We did not use Jurvelin's ‘fibrous surface’ label because we did not observe this feature in our material. Similarly, it was not necessary to label any surfaces ‘unclassified’. Finally, we did not use the label, ‘striated’. or Jurvelin’s third classification level. ‘Splits in the Surface', because we felt these features were likely to be histological artifacts. With these modifications, we observed excellent intra- and interexaminer reliability. As we noted previously, evaluation of a surface is highly dependent on the magnification factor. Jurvelin’s classification system does not address this issue. Longmore’s classification system reflects four levels of magnification: however, it lacks a sufficient number of descriptive subclasses for each magnification level. Therefore, we are developing a joint surface classification system that incorporates the benefits of both the Jurvelin and Longmore approaches. This system will be presented in a future article.
In this study, immobilization was achieved in a manner similar to the procedure described by Hall [1964]. However, the knee joints were maintained in marked flexion rather than extension. Full flexion was chosen because it places the patella firmly against the femoral condyle, increasing the stress on the articular surfaces. In our modification of Flail's procedure, it was readily apparent that when a suture was applied around the proximal end of the femur and the distal end of the tibia, rigid fixation could not be obtained, and in most cases there would be some residual motion (25° to 68°) after a few days. We recorded this residual motion for each experiment, but found no statistically significant correlation with the surface features reported here. This is consistent with a report by Evans el al. [1960] that, following knee joint fixation in 12 rats, residual motion did not effect the degenerative changes produced in the knee cartilage. However, in one preliminary experiment we compressed the intervening muscles much more than usual when we immobilized the limb. This produced a joint with very little residual motion (15°). When the knee joint was opened following an 8 weeks' survival time, we observed that the patella was totally adhered to the femoral condyle. We believe that this adhesion resulted from the knee being tightly fixed. Some investigators still consider that residual motion after a fixation procedure should influence the degree of articular degeneration associated with the joint fixation. For example. Wigren and Wik [1974] proposed that the variability they observed following immobilization studies of the rat knee might be explained by the fact that the Plaster of Paris fixations were sometimes insufficiently rigid. With these ambiguous results we conclude that, although it is reasonable to expect that residual motion will influence the amount of joint degeneration following a fixation procedure, it has not been determined how much residual motion is required to demonstrate this effect. Similarly. the position of the fixed joint (flexion versus extension) may produce substantially different results in studies of knee joint cartilage surfaces.
It is interesting that, in our study, contour surface features were more responsive to immobilization than were surface quality features. The data in table 1 demonstrate that area reductions associated with immobilization were greater for surface contour features than for surface quality features. We observed a significant decrease (40%) in the area of even surface contours with a commensurate increase (50%) in the slightly uneven surface area. By comparison. Jurvelin reported a 21% decrease in even surface area and a 36% increase in the slightly uneven surface area. We also observed a 14% decrease in the smooth surface area and a 25% increase in the rough surface area that did not attain a statistically significant level. However, the width of the 95% confidence intervals indicate a biologically significant change may be present (table 1). The relatively small sample size (n = 20) and relatively large number of covariables (4 subclasses each for contour and surface quality) make it very difficult to attain a statistically significant level in multivariate analysis. Jurvelin et al. [1985] reported a 31% decrease in the smooth surface area with a commensurate 586% (almost 6-fold!) increase in the area of the rough articular surface. They did not perform a multivariate analysis. The differences between our findings and those reported by Jurvelin and co-workers may reflect species differences (rat versus rabbit), the much longer immobilization time interval of our study (a maximum of 6 months versus 8 weeks), and differences in the position of the immobilized joint (flexed versus extended). We observed that changes in the relative surface area of contour features, as well as the relative area of smoothness, occurred in less than 1 week (168 h) of immobilization (fig. 9. 10). This finding was consistent with the results obtained by Jurvelin et al. [1985] - They reported that a 1-week immobilization period was sufficient to generate significant stereo microscopic and scanning electron microscopic alterations in the articular surface of the rabbit patella.
In our study, the central, weight-bearing area of both the immobilized and control patellae were typically even/ smooth surfaces with little or no superficial cartilage layer. In addition, control joints had a larger central area than immobilized joints (table 1). Our observation that the central weight-bearing area is even/smooth and decreases with immobilization is also in agreement with Jurvelin et al. [1985]. but these investigators did not comment on the condition of the superficial cartilage layer. Our observation on the absence of the superficial layer in the central joint area is, however, consistent with the findings of Longmore et al. [1975]. We also observed that, in normal (control) knee cartilage, approximately the central third of the joint surface was even (fig. la. b). while approximately another third of the joint surface was uneven and located at the periphery. Hillocky surfaces, which were generally located at the proximal and distal ends of the patellae, were usually rough with small knobby areas. In our study, the relative proportion of hillocky areas did not demonstrate a statistically significant change after immobilization. Jurvelin et al. 119851 reported an increase in the hillocky surface area following immobilization, but this did not reach a statistically significant level (univariate analysis). We also noted that folded articular surfaces were located primarily at the distal corners of control joint patellae. Immobilized joints frequently lacked folded areas. Moreover, immobilized joints that did have folded areas, had only about half the folded surface area demonstrated by the contralateral, control, joint. With the very small number of immobilized joints that had folded surfaces in our study (7 joints), this area reduction did not attain a statistically significant level. The appearance of leafy cartilage surfaces may lead an observer to conclude that these areas are degenerative changes. However. we observed very little change in the relative area of this surface feature after immobilization, and Jurvelin et al. [1983] actually reported a decrease (70%) in the relative area of leafy articular surface after immobilization.
In this study, increased load was a potential confounding variable for the control joints. However, changes suggested by the control regression lines with increasing survival times were very small and might simply reflect aging of the articular surfaces. Whatever the cause, these changes failed to achieved a statistically significant level. This is consistent with the findings of Slowman et al. 119X6]. He found no difference between the metabolite level of normal versus ‘increased load'joints, but there were substantial changes in the immobilized joints. Therefore. Slowman concluded that there was little ‘increased load' effect upon the joint contralateral to the immobilized knee.
Gardner and Woodward [1969] emphasized that the surface structure of articular cartilage was likely to vary from joint to joint and even within different areas of the same joint. They concluded that the articular regions selected for study must be well defined, and that reports by different workers must be compared on this basis. This study supports that view. We also noted a marked degree of variation. between and within animals, in both the proportionate area and location of articular surface features. For this reason. we discourage the common practice of obtaining a sample from one region of the joint surface and considering it to be representative of the entire articular surface.
We observed two different fiber types, independent of their location within the articular surface. These were: (1) collagen fibers running primarily parallel to the long axis of the patella (mean cross diameter = 142.3 ±21.5 nm), and (2) fibrils randomly crossing the collagen fibers (mean cross diameter 20.0 ±4.8 nm diameter). Meachim and Fer- gie 11974] noted that most collagen fibers showed either a predominantly unidirectional alignment or two major axes, one al a right angle to the other, which related to the direction of joint movement, and was indicative of ‘abrasive- adhesive’ wear. In the human joint. Redler |I974| reported that the most superficial portion of the cartilage surface was composed of sheets of fine collagen fibrils about 100 nm in thickness. These fibrils were oriented parallel to the joint surface and extended to depths varying between 50 and 200 pm from the surface, depending on the thickness of the cartilage. However. Teshima et al. (1995] reported that collagen fibers located in the superficial layer of the human femoral head were 125-150 nm in diameter, while deeper fibers were 75-100 nm in diameter. Note: These investigators include a portion of the fibrous substructure in their definition of the superficial layer. We noted that the collagen fibers in even/smooih cartilage were either regularly interrupted by crossing ridges (cr. fig. 2b) or appeared to be a dense blanket of collagen fiber tips (fig. 2c). These free ends were consistently greater in diameter (178.4 ±25.0 nm) than the diameter of the collagen fibers. Meachim and Fer- gic 11974] reported a similar observation, and proposed that it was due to modification of exposed collagen tips within the synovial cavity. They proposed that synovial or polymorphonuclear collagenases and the abrasiveness of joint movement altered the fiber tips. Jeffery et al. [1991] reported an overlapping, leaf-like orientation of the superficial cartilage layer in bovine metacarpal heads, in which the collagen fibers were linked by bridging fibrils similar to the crossing ridges we observed.
In rabbit joints, Finsterbush and Friedman [1973] and Wigren and Wik [I974] observed that degenerative change in the superficial cartilage layer is associated with loss of its amorphous hyaluronic acid coat and exposure of collagen fibers. We made a rough estimate of the superficial layer thickness from upturned fragments of the articular surface. These are rough estimates, since they are not derived from true cross-sectional views. The thickness of the superficial cartilage layer ranged from 67 nm in the peripheral (hillocky) region to 21 nm in patches of the superficial layer found in the central weight bearing region. Ghadially et al. [1982] reported a similar superficial layer thickness in the femoral condyles of rabbits. As noted above, we also observed differences in the superficial layer of the patellae that appear to reflect weight bearing and non-weight-bearing zonal differences. Within the weight-bearing area (the central articular area in contact with the femoral condyle) of both control and immobilized joints, we observed even/ smooth areas that lacked a superficial layer and slightly un- even/smooth areas with thin superficial layers and exposed collagen fibers. Previous investigators [Redler. 1974: Wigren and Wik. 1974; Longmore and Gardner, 1975: Finsterbush and Friedman. 1973: Jurvelin et al., 1985: Jozsa et al.. 1987; Pidd and Gardner. 1987] found that thinning, or partial loss, of the superficial layer was the principle degenerative change in the articular cartilage following immobilization. However, we feel that the absence of the superficial layer is more indicative of the functional nature of the area. The fact that the even/smooth areas (which lack a superficial layer) are found in the weight-bearing region of normal joints, and observation that the even/smooth areas of immobilized joints decrease, argues that the absence of a superficial layer is not necessarily indicative of degeneration. Rather, degeneration is signaled by replacement of even/smooth surfaces with rough surface areas.
We also observed that the slightly uneven/rough and hillocky areas had the thickest superficial layer (67 ±9 and 66 ±7 nm. respectively). We attribute this observation to the fact that these areas are on the peripheral, non-weight- bearing region of the patellar cartilage. It was interesting that these same areas were frequently cracked. We considered these cracks to be largely a result of the histological preparation process; however, it is possible that the surfaces that flaked or cracked may have done so because they had been weakened previously by immobilization. This possibility is supported by the observation that immobilized joints generally demonstrated greater areas of cracked surfaces. Finally, we noted that plica covered most of the peripheral joint surface (slightly uneven/rough, and hillocky areas). It is possible that growth of synovial plica and articular surface degeneration correlate. Moreover, several studies have evaluated histochemical changes in the matrix of immobilized and unstable joint models [Muller et al.. 1994; Grumble et al.. 1995]. These covariables will be evaluated in future studies.
References:
Bouvier. M.. iVl.L. Zimny (1987)
Effects of mechanical loads on surface morphology of the condylar cartilage of the mandible in rats.
Acta Anal 129:293—3(H).Broom. N.. D. Myers (1980)
Fibrous waveforms or crimp in surface and subsurface layers of hyaline cartilage maintained in its wet functional condition.
Connect Tissue Res 7: 165—175.Candolin. T.. T. Videman [1980] Surface changes in the articular cartilage of rabbit knee during immobilization: A scanning electron microscopic study of experimental osteoarthritis. Acta Pathol Microbiol Sound | A) SO: 291 297.
Çoudane. H.. D. Mole. J. Sommelet. D. Schmitt. G. Grignon. B. Foliguct (1987)
A study of the patellar articular cartilage by scanning electron-microscopy. Fr J Orthopaed Surg / 200-209. Evans. E..G. Eggers. J. Butler.J. Blumcl(I960) Experimental immobilization and remobilization of rat knee joints.
J Bone Joint Surg 42-A 737-758.Faure. G., P. Netter. B. Malaman, J. Steinmetz (1977)
Monocrystalline calcium hydrogen phosphate dihydrate in destructive arthropathies of chondrocalcinosis.
Lancet July 16: 142-143.Finsterhush. A.. B. Friedman [1973]
Early changes in immobilized rabbits knee joints: A light and electron microscopic study.
Clin Orthop Rel Res 92:305-319.Gardner. D. (1972)
The influence of microscopic technology on knowledge of cartilage surface structure.
Ann Rheum Dis 21: 235 -258.Gardner. D.. D. McGillivray (1971)
Living articular cartilage is not smooth.
Ann Rheum Dis 20: 3-9.Gardner. D.. D. Woodward (1969)
Scanning electron microscopy and replica studies of articular surfaces of guinea-pig synovial joints.
Ann Rheum Dis 28:379-391.Ghadially. F.. N. Yong. J. Lalonde (1982)
A transmission electron microscopic comparison of the articular surface of cartilage processed attached to bone and detached from bone.
J Anat 135:685-706.Greisen. II.A., B.A. Summers. G. Lust (1982)
Ultrastruclure of (lie articular cartilage and synovium in (he early stages of degenerative joint disease in canine hip joints.
Am J Vet Res 43: 1963-1971.Grumble. R.M., D.S. Howell, G.A. Howard. B.A. Roos. L.A. Setton. Van C. Mow, A. Ratcliffc. F.J. Muller. R.D. Altman (1995)
Cartilage métalloprotéases in disuse atrophy.
J Rheumatol 22(suppl43): 146-148.Hall M. (1964)
Articular changes in the knee of the adult rat after prolonged immobilization in extension.
Clin Orthop Rel Res 34: 184-195.Helminen. IL. J. Jurvelin. T. Kuusela. R. I leikkila. I. Kiviranta. M. Tammi (1983a)
Effects of immobilization for six weeks on rabbit knee articular surfaces as assessed by the semiquan- titativc stereomicroscopic method.
Acta Anat IIS: 327-335.Helminen. H.. J. Jurvelin. T. Kuusela. R. Ilcikkila. I. Kiviranta. M. Tammi (1983b)
Semiquantitative analysis of rabbit knee articular surfaces based on stereomicroscopic examination of the cartilage.
Acta Anat 115: 319-326.Jeffery. A.K.. G.W. Blunn. C.W. Archer. G. Bentely (1991)
Three-dimensional collagen architecture in bovine articular cartilage.
J Bone Joint Surg 73-B: 795-801.Jozsa. L.. M. Jarvinen. P. Kannus. A. Rcffy (1987)
Fine structural changes in the articular cartilage of the rat's knee following short-term immobilization in various positions: A scanning electron microscopical study.
Int Orthop II: 129-133.Jurvelin. J.. T. Kuusela. R. Heikkila. A. Pelttari. I. Kiviranta. M. Tammi. H. Helminen (1983)
Investigation of articular cartilage surface morphology with a semiquantitative scanning electron microscopic method.
Acta Anal 116 302-311.Jurvelin. J.. Fl. I lelminen, S. Laurisalo. I. Kiviranta. A. Saamanen. K. Paukkonen. M. Tammi ( 1985)
Influences of joint immobilization and running exercise on articular cartilage surfaces of young rabbits.
Acta Anal 122:62-68.Kiviranta. I.. J Jurvelin. M Tammi. Anna-M. Saamanen. H.J. Helminen (1987)
Weight bearing controls glycosaminoglvcan concentration and articular cartilage thickness in the knee joints of young beagle dogs.
Arthritis Rheum 30: 801-809.Longmorc. R.. D. Gardner (1975)
Development with age of human articular cartilage surface structure.
Ann Rheum Dis 34:26-37.Meachim. G.. I. Fcrgie (1974)
Scanning light microscopy of fibrillated articular cartilage.
J Bone Joint Surg 56:582.Muller. F.J.. L.A. Setton. D.ll. Manicourt. Van C. Mow. D.S. Ilowell. J.C. Pita (1994)
Centrifugal and biochemical comparison of proteoglycan aggregates from articular cartilage in experimental joint disuse and joint instability.
J Orthop Res 12:498-508.Palmoski. M.. D. Brandt (1982)
Aspirin aggravates the degeneration of canine joint cartilage caused by immobilization.
Arthritis Rheum 25: 1222-1342.Pidd. J.. D. Gardner (1987)
Surface structure of baboon (PAPIO ANUBIS) hydrated articular cartilage: Study of low temperature replicas by transmission electron microscopy.
J Med Pri- matol 16:301-309.Redler. I. (1974)
A scanning electron microscopic study of human normal and osteoarthritic articular cartilage.
Clin Orthop Rel Res 103: 262-268.Sakakibara. Y.. T. Miura. H. Iwata. T. Kikuchi. T. Yamaguchi. T. Yoshimi. H. Itoh (1994)
Effect of high-molecular-weight sodium hyaluronate on immobilized rabbit knee.
Clin Orthop Rel Res 299:282-292.Silbcrbcrg. R.. W. Stamp. P. Lesker. M. Hasler (1970)
Aging changes in ultrastructure and enzymatic activity of articular cartilage of guinea pigs.
J Gerontol 25: 184-198.Slow man. S.. K. Brandt (1986)
Composition and glyeosaminoglycan metabolism of articular cartilage from habitually loaded and habitually unloaded sites.
Arthritis Rheum 29:88-94.Teshima. R.. T. Olsuka. N. Takasti. N. Yamagata. K. Yamamoto (1995)
Structure of the most superficial layer of articular cartilage.
J Bone and Joint Surgery 77-11 460-464.van Lent. P.L.E.M.. F.A.J. van de Loo. L. van den Bcrsselaar. W. B. van der Berg (1991)
Chondrocyte nonresponsiveness of arthritic articular cartilage caused by short term immobilization.
Rheumatology I ft: 709-715.Wigren. A., O. Wik (1974)
The influence of hyaluronic acid on immobilized knees: An experimental study on adult rabbits.
UPPSALA: 1-27. 4 0 Acta Anal 19%: 157:27-40
Return to SUBLUXATION DEGENERATION
Since 6-17-2004


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |