

Biomechanics of Spinal Manipulative Therapy This section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Spine J. 2001 (Mar); 1 (2): 121–130 ~ FULL TEXT
John J. Triano, DC, PhD
Conservative Medicine,
Chiropractic Division,
Texas Back Institute,
6300 W. Parker Road,
Plano, TX 75093, USA.BACKGROUND CONTEXT: Modern scientific investigations into spinal manipulative therapy (SMT) began in 1975. Conditions often treated include acute and chronic low back pain, radicular pain, neck pain, and some forms of headache. The field of spinal manipulation has often been treated by the literature, incorrectly, as being homogeneous. Much of the confusion regarding this form of treatment can be traced to the ambiguity surrounding the procedures themselves. This report summarizes the clinical biomechanics of SMT and evidence for its associated manipulable lesion is reviewed. Finally, a classification system based on biomechanics is proposed that may facilitate more detailed research in the future.
PURPOSE: A categorization system for SMT was sought that would be more objective than is clinically available. Such a system may serve as a means to strengthen future studies, determine operating principles, applicability, treatment effectiveness, and nature of the manipulable lesion.
STUDY DESIGN: Literature synthesis.
METHODS: A search of the indexed biomechanical and medical literature as well as a hand search of published works was conducted. The criteria for article selection consisted of studies that included measurements of mechanical characteristics of treatment techniques used under the general headings of SMT or manual therapy. A second set of studies was identified that explored the biomechanics of buckling behavior of vertebral segments as a model of the manipulable lesion. Quantitative characteristics of SMT were extracted and grouped to form a basis for classification.
RESULTS: A total of 31 articles were identified that contained quantitative data on the biomechanical properties of SMT methods. An additional seven studies were found that quantified spinal buckling behavior. Common features of SMT procedures lead to a matrix that biomechanically characterizes the types of procedures in use. Buckling behavior was compared qualitatively with clinical observations to form a plausible and evidence-based hypothesis of the manipulable lesion.
CONCLUSIONS: There currently are a number of named systems of manual procedures. No current triage system is available that predicts which patient has the greater likelihood of benefiting from manual treatment or the procedure type. The biomechanical parameters of SMT form a systematic characterization of manual procedures. Such a system may be used in future studies to test hypotheses of treatment effect from quantitatively defined procedures.
Keywords: Spinal manipulation; Mobilization; Continuous passive motion; Manipulable lesion; Functional spinal lesion; Subluxation; Biomechanics
From the FULL TEXT Article:
Introduction
Manipulation of the spine has ancient roots and popularity that has varied over time. From the late nineteenth through the twentieth century, its use has been embroiled as a part of interprofessional medical controversies. [1] Modern scientific investigations in this area began with a federally supported conference in 1975. [2] For over two decades, the scientific debate over the value of manipulation in the treatment of spine-related disorders has been engaged. Anecdote and opinion are slowly yielding to a preponderance of evidence accumulated through basic science and clinical studies. At the moment (as of 2000), over 50 randomized controlled trials of spinal manipulative therapy (SMT) have been conducted. Modern, evidence-based guidelines and formal consensus documents on appropriate treatments list manipulation as a recommended or optional approach. Conditions where manipulation is offered include acute and chronic low back pain [3, 4], radicular pain [5], neck pain [6–8], and some forms of headache. [9] Manipulation is being offered to an increasingly broad case–mix, including the elderly and postsurgical patients. Debate continues on the relative effect strength and the appropriate indications for use of this form of care. Do we have sufficient information on SMT to move forward in understanding its operating principles, applicability, and possible risk?
Much of the current confusion regarding the use and effectiveness of SMT can be traced to the ambiguity surrounding the treatment procedures themselves. Few of the published reports provide a clear, objective description of the manipulation techniques used, the identity of those administering the procedures, or a means of assessing the skill of the manipulators. [4] The field of spinal manipulation has often been treated by the literature, incorrectly, as being homogeneous. A few authors have attempted to segregate the procedures selected for study by using such categorical names as Maitland, Gonstead, or diversified methods. Such classification, cumbersome in like manner to the naming of some new surgical techniques, fails to clarify the nature of the treatment intervention. It introduces confusion by preventing experimental methodology to be replicated easily for future study. A small number of publications (e.g., [10, 11]) have attempted to provide a more scientifically based description focusing on the perception of differences, velocity, and depth of local tissue displacement achieved by different methods.
Quantification of procedural differences in SMT, for the most frequently used forms, has begun to appear. Thus far, no systematic effort has been undertaken to organize this new information into clinically meaningful categories that may serve to clarify procedure descriptions in future manipulation research. Concise categories could improve quality of outcomes from care by empowering research into subpopulations that may receive more favorable benefit from specific types of procedures than others. This report summarizes the state of the art in clinical biomechanics of spinal manipulation. Based on clinical and laboratory evidence, the pathomechanics of the manipulable lesion are reviewed, a classification system based on biomechanical principles is proposed, and the substantive literature reviewed.
Methods
This work sought to consolidate more recent biomechanical information that might serve as a model of the manipulable lesion and to characterize quantitatively SMT. A search of the indexed biomechanical and medical literature as well as a hand search of published works was conducted. The criteria for article selection consisted of studies that included measurements of mechanical characteristics of treatment techniques used under the general headings of SMT or manual therapy. A second set of studies was identified that explored the biomechanics of buckling behavior of vertebral segments as a model of the manipulable lesion. Quantitative characteristics of SMT were extracted and grouped to form a basis for classification.
Results
A total of 31 articles were identified that contained quantitative data on the biomechanical properties of SMT methods. An additional seven works were found that quantified spinal buckling behavior. Common features of SMT procedures lead to a matrix that biomechanically characterizes the types of procedures in use. Buckling behavior was compared qualitatively with clinical observations to form a plausible and evidence-based hypothesis of the manipulable lesion.
Rationale of the treatment construct
Understanding the rationale behind any treatment approach requires knowledge of its underlying assumptions and supportive evidence. Spinal manipulation is thought to act on a manipulable lesion (often called a functional spinal lesion [FSL] or subluxation) that itself is conformable to specific forces and moments in such a way that the internal mechanical stresses that generate symptoms are reduced. [12] The ability to sustain these effects is supported by case management that promotes healing of nociceptive pain generators and promotes return to normal activity. Classic clinical management steps include a temporary reduction of spinal joint loads during daily activity, attention to comorbid conditions that may affect the body’s capacity to accommodate or to repair, and systematic use of exercise to restore the pre-injury distribution of loads through the tissues.

Table 1 Given the historical presumption of the FSL, it is somewhat surprising that there is not more information on its pathomechanical properties. The state of art with respect to the FSL is analogous to that of diabetes mellitus in the early twentieth century. General clinical characteristics were known, but the nature of the pathology remained a mystery, waiting further technological development. Over the past two decades, biomechanical and physiological studies have yielded supportive information. However, a pathognomonic diagnostic presentation remains elusive, requiring a successful trial of therapy to confirm its presence. Clinicians view the FSL as a set of individual disorders responsible for the patient’s symptoms [12, 13] or as a comorbid condition in common with classical pathoanatomical lesions (Table 1).
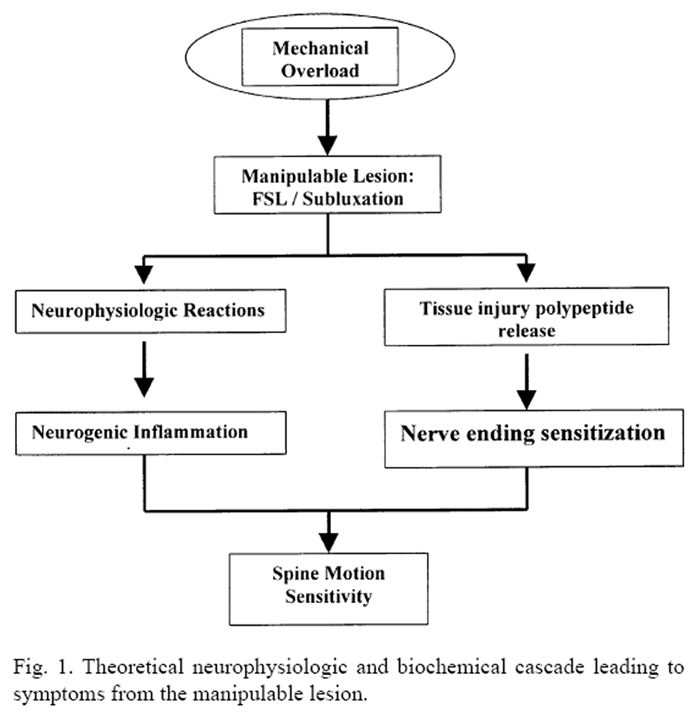
Figure 1 A brief review of modern theory provides the context in which spinal manipulation is currently used. The concepts of functional lesions of the spine are similar to that regarding the occurrence of compressive buckling injury to the wrist. [14–16] The functional spinal unit (FSU) fits the biomechanical criteria for a multimuscular, biarticular chain that may be subject to “zigzag” collapse [16, 17] or buckling. Localized joint buckling of the spine is opposed only by adequate timing in the recruitment of attached muscles appropriate to a biomechanical task at hand. The FSL begins with a mechanical overload (Figure 1) as either a single traumatic or cumulative event. Experiments in vitro have produced these effects under conditions where a single FSU is incrementally loaded at the balance point. [18–21] When a critical buckling load is reached, the linear force-displacement behavior is interrupted by a disproportionately large displacement. The total distance, however, remains within the normal intersegmental range. That is, when buckling occurs, the affected area of the spine reaches its maximum range under lower load conditions and is operating at its extreme, out of phase with the demands of the task. It is assumed that such a functional configuration may result in altered stress distribution within the FSU. [12]
Mechanical irritation to the tissues in and around the spinal joints results in neurogenic or nonneurogenic pain. [22, 23] Neuroactive chemicals (e.g., substance P, 11-amino neuropeptides) may generate inflammatory response. Vasoactive byproducts of tissue damage (bradykinin, serotonin, histamine, prostaglandins, and potassium ions) may trigger nerve-ending sensitivity and reduced response threshold. Mechanically, the motion segment behavior also is affected. Individual structural elements (disc, facet, ligament, nerve, muscle) may experience concentration of local stresses with reduced functional limits and symptom production specific to the tissue affected. The result is a state of dysfunction [24, 25] that leads to local inflammatory or biomechanical changes. If neural elements become inflamed or compromised, then remote symptoms may also appear. Consequently, the clinical presentation may be multifaceted and variable.
Spinal manipulation uses controlled forces and moments applied to the spine along with inertial forces generated by acceleration of relevant body segment mass. The algebraic sum of these loads are transmitted to the spine in a controlled manner and are designed to “unbuckle” motion segments and reduce local mechanical stresses within the functional spinal unit. [26–28]
BiomechanicsFunctional spinal lesions (FSL) Biomechanical studies from cadaver spines, computer simulations, and in vivo experiments give support to the concepts of the FSL. Wilder et al. [19–21] were among the first to demonstrate buckling behavior in isolated motion segments, depending on the load application point, load vector, load rate, and load magnitude. [29] Similar results have been shown to occur for the lumbar region as a whole with extreme displacements at all levels under the correct loading conditions. [30] In the case of regional spine function, Crisco et al. [30] also demonstrated buckling behavior. Moreover, damage to the L5–S1 disc resulted in the ability to create a buckled condition earlier, reaching maximum displacement of each segment at much lower total loads. Cholewicki and McGill [31] captured the occurrence of a painful buckling event while using videofluoroscopy to monitor lumbar spine kinematics during heavyweight lifting in a young volunteer.
The conditions that can accelerate spinal buckling resemble common circumstances reported by patients at the onset of their pain. It may arise from a single overload event or from prolonged static posture followed by a small increment in load. Overload events that result in buckling may be rate dependent, requiring about 500 pounds per second. Exposure to vibration, a known risk factor for back disorder, also enhances FSU buckling. [20] As mentioned earlier, previous disc injury may facilitate the buckling event.

Table 2 Types of spinal manipulative therapy The core concept of SMT is the application of controlled load vectors to the spine in effort to restore normal behavior and reduce [20] harmful mechanical stresses to the local tissues. The target lesion is identified through use of provocative maneuvers that reproduce the patient’s symptoms. The procedure selection is accomplished by matching the results of testing with consideration of comorbid factors, including patient age, stature, degenerative state, and pathology. Table 2 lists treatment options grouped by common biomechanical characteristics that lend themselves to quantification and scientific description. This new basis of classification involves quantifiable characteristics, including whether the procedure is applied manually, the type and the frequency of load application, and amplitude of displacement.
Treatment loads Quantifying manipulation forces and moments can be technically challenging. The total loads acting on the spine are the sum of applied treatment loads, inertial loads from accelerating the body segment mass, and the internal muscular tensions that may arise. Two experimental approaches have been taken. Direct load measurements use sensors imposed between the hand of the operator and the patient’s body. They have the advantage of providing the amplitude of force acting in a direction perpendicular to the surface. Their disadvantage is that they do not include estimates of the applied moments or knowledge with respect to the direction of the applied loads. The second method consists of an inverse dynamics approach that monitors body segment motions and the resulting forces and moments that pass through the body during the treatment procedure. Myoelectric activities, simultaneously recorded, permit an estimate of internally developed tensions from muscle activity. Loads acting on the spine are then calculated. The advantage of inverse dynamics is that it provides estimates of the moments and gives the directions of loads. The disadvantage is a sacrifice in accuracy of the estimated load amplitudes, particularly for moments.

Figure 2
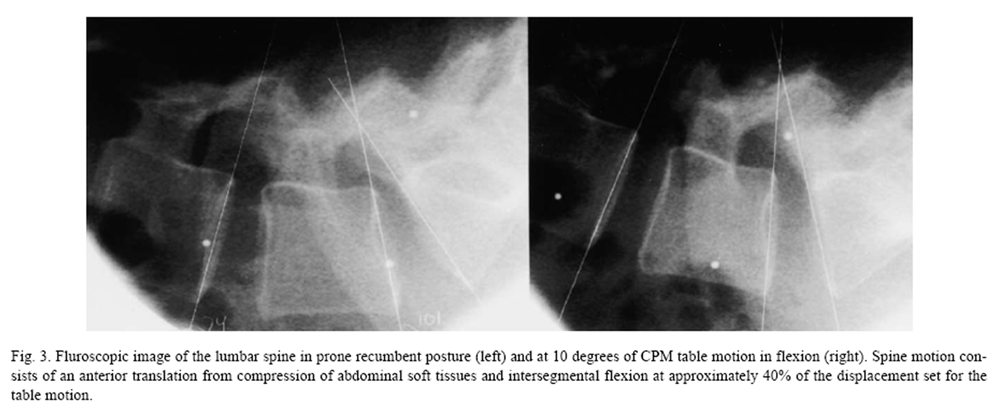
Figure 3 Figure 2 shows typical forces and moments passing through the lumbosacral region during continuous passive motion of the lumbar spine from a study of six volunteers (unpublished data). With the patient prone on a continuous passive motion (CPM) treatment table (Leader Health Technologies, Inc., Port Orchard, WA), the lower body was set in periodic motion at a rate of 0.224 Hz. An inverse dynamics model published previously [27, 28] was used to estimate the loads transmitted through the lumbar spine. The primary effect is an intermittent traction load ranging from a minor compressive force of 17.2 N to a maximum distraction force of 144.5 N. A small secondary flexion moment (10.1 Nm) was induced. Increasing the speed of CPM to 0.5 Hz increases the axial loads by 10% and the flexion moment by 30%. Separate fluoroscopic monitoring of spine motion demonstrates flexion of four degrees (Figure 3). Complex loads from altering the direction of motion, including extension and lateral bending, remain to be quantified.
Seated continuous passive motion (B>CPM also has been studied. [35] Small alternating rotational motions were imposed over a 0.6–degree range at a rate of 12.5 seconds per cycle by a motorized seat pan with a moment of 23.1 Nmm. Patients reported reduction of back pain. The authors concluded that minor stimulation is sufficient to mobilize the lumbar spine. In separate work the effects on sitting comfort were studied by Reinecke et al. [36] They evaluated the effects of cyclical posteroanterior pressure to the lumbar spine, alternately increasing and decreasing the lumbar lordosis.
Mobilization procedures differ from CPM by nature of the method by which movement is imposed. While CPM generally uses a treatment table powered mechanically or manually, mobilization manually applies loads to the local spinal tissues. The displacements resulting from forces of 150 N cycling between 0.5 and 2.0 Hz have been studied. Effects are discussed separately under the section on vertebral motions.
Most studies have been conducted with respect to high-velocity, low-amplitude (HVLA) methods. By direct measurement and inverse dynamics methods, the uniaxial forces applied to the patient from simple procedures have been determined. [33, 37–45 Peak amplitudes have ranged widely (41–889 N) depending on the spinal regions treated. Applied forces rise quickly with slopes ranging between 519 N/s and — 2907 N/s.

Table 3 Complex HVLA procedures have been studied by Triano [28] and Triano and Schultz [27] in both the cervical and lumbar regions. The assumption of symmetry in treatment delivery was studied by randomly applying 35 procedures to the C2 vertebral segment from the left and 31 from the right. Differences in mean forces and moments were insignificant, ranging from 1% to 16%. Their findings of mean peak forces at 111 N and 123 N reported for left- and right-sided maneuvers were similar to those of Kawchuk. [40] As a first effort to test ability to control amplitude, operators were randomly instructed to perform procedures with maximal permissible effort or with minimally effective effort, based on clinical judgment. Mean amplitudes stratified in this way were significantly different (.0000 < P < .0294). Table 3 gives the mean and standard deviation for transmitted neck loads.
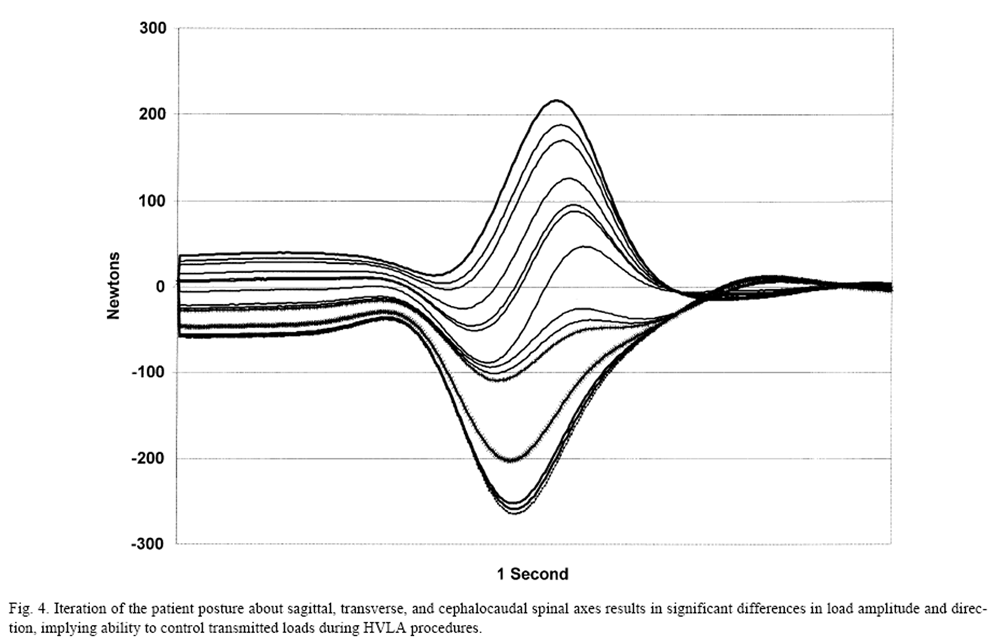
Figure 4 Manipulation control strategies were evaluated further in the lumbar spine by contrasting the differences in loads transmitted through the lumbosacral region by systematic changes in initial posture (axial twist) and in selection of the HVLA procedure. Results demonstrated differences between three commonly used methods and between patient positions. [27] Evaluation of combined postural variation in flexion, lateral bending, and axial twist were studied further [46] using computer models. Figure 4 shows the effects on transverse loads across the spine from systematic changes affecting multiple axes. A complete reversal in direction of the applied load can be achieved.
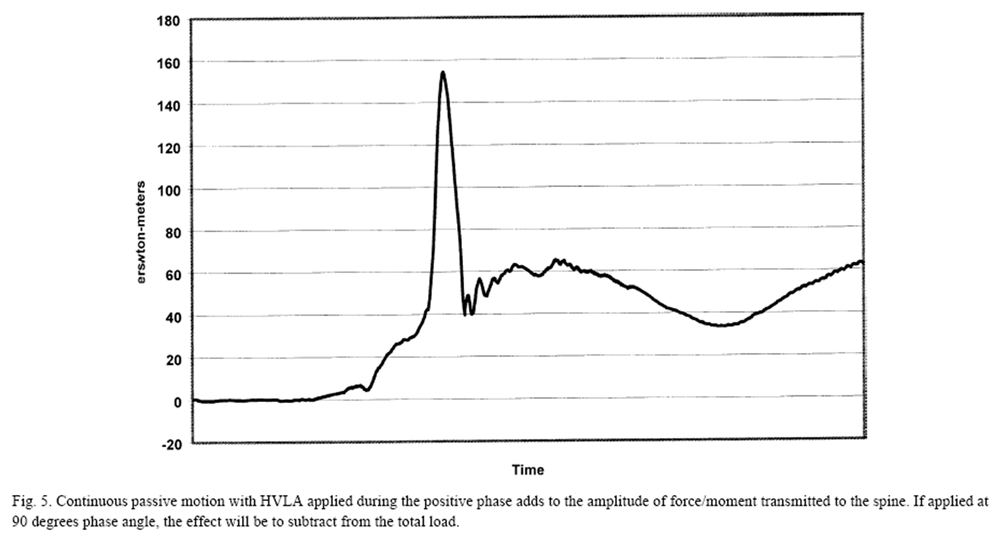
Figure 5 Mechanically assisted procedures include the use of impulse hammers, adapted from instruments designed to apply orthodontic appliances. These devices deliver a uniaxial load directed to a small area (1 cm square), effectively avoiding applied moments. In a study of 20 healthy subjects, Keller [47] demonstrated a range of loads to the lumbar spine from 83 N to 120 N with impulse duration of less than 20 ms. Kawchuk and Herzog [33] showed much lower amplitudes in the study of the cervical spine with mean amplitudes from 17 subjects of 41 N. Other forms of mechanical assistance are derived by combining the controlled motions from CPM with manually applied loads. Figure 5 shows the algebraic summation of loads that can be manipulated by varying the timing of the HVLA with respect to the phase of the passive motion.

Table 4 Muscle activity The behaviors of neck and torso muscles have the potential to alter the net loads acting on the spine during manual treatments. Clinical observations from patient care suggest that there are three intervals (Table 4) of muscle tension that, theoretically, may alter the action of a treatment procedure. Muscle activity may result from voluntary or reflex contraction. Increased spinal stiffness is likely to alter the ability of the doctor to obtain the desired initial joint positioning. Muscle action during the procedure would alter the amplitudes and direction of the loads transmitted through the spine and, if sufficiently strong, may stiffen the joint to prevent relative motions. Tension developed subsequent to the loading phase would be equivalent to applying a second or successor load of unknown amplitude and direction.
Unfortunately, little quantitative information is available with respect to the muscular behavior in patient populations. Studies, to date, have focused on healthy subjects and reveal myoelectric activity that is unpredictable both in terms of amplitude of response and muscular recruitment. No significant muscle activity has been observed in healthy subjects during the preload phase. Twitch responses have been reported for HVLA procedures during the loading and resolution phases [27, 28, 48–51] Onset of apparent reflex response occurs within 50 to 200 ms after impulse initiation and may last as long as 400 ms. Using a myoelectric and double-linear optimization model, Triano [28] found that muscle tension developed during twitch reaction in the neck was insufficient to significantly influence spinal loads.
Vertebral motions The spine is a viscoelastic system of linkages that are mechanically coupled. Forces and moments applied to a local site will result in different displacements along the length of the spine that are based both on the mechanical characteristics of the applied load and the properties of the tissues that concatenate the individual vertebral segments. [12] At higher rates of loading, the spine elastic stiffnesses increase and the time-dependent viscous behavior acts more as a nonviscous solid. Slower rates provide sufficient time for fluid compartments within the ligaments, muscles, and discs to respond. At high loading rates, the relative displacement will be dependent on the relative elastic tissue stiffnesses (bone versus disc versus ligament). Conversely, slower loads will allow loads to be transmitted between segments viscoelastically, effectively spreading the displacements across a broader spinal region.

Table 5 Some direct measurements of vertebral displacement, both translation and rotation, have been measured on cadavers and conscious volunteers. For low velocity forces, as seen with CPM and mobilization, displacement is distributed along a series of vertebra in a progressive manner. Maximum displacement occurs at the target segment and decreases with increasing distance from the application point (Table 5). In contrast, high velocity procedures resulted in statistically significant differences in the intersegmental motions indicating a target-specific, greater amplitude of motion. [21] Unfortunately, the vertebral movement distances observed in volunteers cannot be compared directly with those obtained from cadaver studies because of the changes in stiffness that occur after death. Cadaver measures may underestimate in vivo motions by as much as 30%. [54]
Biomechanics of safety Most evidence on serious safety concerns come from epidemiologic inference. [55–58] The primary complications consist of self-limiting symptomatic exacerbation or new local symptoms. Rarely, serious complications are attributed to spinal manipulation, including cauda equina syndrome and cerebrovascular accidents. Biomechanical evidence has been reported that benchmarks the loads transmitted through the spine during high-velocity, low-amplitude procedures with experimental data or activities of daily living.
Triano and Schultz [27] measured the loads transmitted through the torso at the lumbosacral spine for 66 HVLA procedures. Results were compared with spinal loads predicted by three-dimensional biomechanical models [59] for various activities of daily living. The asymmetrical task of a single-handed, 50–pound lift (20 degrees of flexion, 30 degrees axial twist, 12 degrees lateral bending) produced the same spinal loads as were observed for the entire trunk during manipulation.
The experimental neck loads from spinal manipulation are given in Table 3. Loads were modeled using an inverse dynamics approach [28] and compared with moment loads tolerated by human volunteers under test conditions. [60–62] Volunteers tolerated sudden neck moments as high as 94 Nm in the sagittal plane and 143 Nm in the coronal plane without incident. These moment levels exceed those observed during the spinal manipulation to the neck.
Spinal manipulation is a manual procedure that requires some level of skill to be performed expertly. Differences in novice versus expert performance have been recorded. [63, 64] Statistically significant differences occur in terms of amplitude, rate of force/moment development, and duration of loading. Moreover, like many psychomotor skills, manipulation performance is not transportable. That is, ability to skillfully perform one procedure does not imply ability to perform a new procedure equally well without substantial rehearsal. [44] Skillful operators are able to provide forces within 20% of desired levels over a range from 20% to 100% of maximum effort. [12]
Conclusions and future directions
Spinal tissues are consistently exposed to varying rates and amplitudes of loads. Manual treatment differs by the intent to apply loads locally, in an effort to alter mechanical stresses thought to contribute to symptoms. Studies over the past 10 to 15 years have provided some understanding of the different procedures used in treating spine-related disorders, including spinal manipulation/adjustment. Based on the little biomechanical evidence available and clinical observations, a biomechanical theory of the manipulable lesion has begun to emerge.
Most physicians are aware of the fact that some of their patients avail themselves of manipulative treatment. However, they may be uncertain of the mechanisms of action or the anticipated therapeutic effects. Spinal manipulation, by its very nature, is a physical process. In this article, the theoretical basis for using manipulation, the types of clinical procedures, and their biomechanical actions are described. Questions of loads that are applied and transmitted through the body during the procedures, motions that occur, skill of performance, and safety have been addressed. The size of loads that are observed range widely according to the region under treatment and are able to be controlled by the skillful operator. Control strategies include procedure type; selected load amplitudes, direction, and speed; patient posture; and mechanical assistance. The upper limits of loads have the potential to be significant but remain within the bounds of structural compatibility.
The clinical literature shows symptomatic relief with small spinal segment displacements and loads [35, 36], while others have been benefited with more intense procedures using higher velocity and loads (e.g., [Table 2 begins a systematic characterization of manual procedures that are detailed more completely in the review that follows. Such a system may be used in future studies to test hypotheses of treatment effect from quantitatively defined procedures. The operating principles of SMT are emerging. Many questions remain to be answered. What are the characteristics of patients that respond best to continuous passive motion, mobilization, or manipulation? Can knowledge of the mechanics of procedures that benefit subcategories of patients give insight as to the elusive nature of the pain generator that responds favorably to SMT? What are the dosage–duration characteristics of effective treatment? Can clinical effect sizes be optimized by systematic treatment modification or ancillary rehabilitative care? Refinement of our knowledge of patient care begins, on the one hand, with quantitative knowledge of the manipulable lesion and, on the other, with understanding of the methods that are used.
References:
Kaptchuk TJ, Eisenberg DM.
Chiropractic: Origins, Controversies, and Contributions
Arch Intern Med 1998 (Nov 9); 158 (20): 2215–2224Goldstein M.
The research status of spinal manipulative therapy.
Bethesda (MD): US Department of Health, Education, and Welfare,
DHEW Publication (NIH) 76-998, 1975.Stanley J. Bigos, MD, Rev. O. Richard Bowyer, G. Richard Braen, MD, et al.
Acute Lower Back Problems in Adults. Clinical Practice Guideline No. 14.
Rockville, MD: Agency for Health Care Policy and Research, [AHCPR Publication No. 95-0642].
Public Health Service, U.S. Department of Health and Human Services; 1994van Tulder MW, Koes BW, Bouter LM.
Conservative treatment of acute and chronic nonspecific low back pain:
a systematic review of randomized controlled trials of the most common interventions.
Spine 1997;22(18):2128–56.Poulsen PB.
Low-Back Pain Frequency, Management and Prevention from an HTA perspective
Copenhagen: Danish Institute for Health, 1999.Coulter ID, Shekelle PG, Mootz RD, Hansen DT.
Use of expert panel results: the RAND panel for appropriateness of manipulation
and mobilization of the cervical spine.
Top Clin Chiro 1995;2(3):54–62.Coulter, I, Hurwitz, E, Adams, A et al.
The Appropriateness of Manipulation and Mobilization
of the Cervical Spine
Santa Monica, CA: RAND Corporation; 1996 Document No. MR-781-CR.Shekelle PG, Coulter ID.
Cervical spine manipulation: summary report of a systematic review
of the literature and a multidisciplinary expert panel.
J Spinal Disord 1997;10:223–8.Nilsson N, Christensen HW, Hartvigsen J.
The Effect of Spinal Manipulation in the Treatment of Cervicogenic Headache
J Manipulative Physiol Ther 1997 (Jun); 20 (5): 326–330Hadler NM, Curtis P, Gillings.A.
Benefit of spinal manipulation as adjunctive therapy for acute low back pain:
a stratified controlled trial.
Spine 1987;12:703–6.Triano JJ, McGregor M, Hondras MA, Brennan PC.
Manipulative Therapy Versus Education Programs in Chronic Low Back Pain
Spine (Phila Pa 1976). 1995 (Apr 15); 20 (8): 948–955Triano J.
The mechanics of spinal manipulation.
In: Herzog W, editor.
Clinical biomechanics of spinal manipulation.
New York: Churchill Livingstone, 2000. p. 92–190.Bernard TN, Kirkaldy-Willis WH.
Recognizing specific characteristics of nonspecific low back pain.
Clin Orthop 1987;217:266–80.Kauer JMG.
Functional anatomy of the wrist.
Clin Orthop 1980;149:9.Kauer JMG.
The mechanisms of the carpal joint.
Clin Orthop 1986; 202:16.Landsmeer JMF.
Studies in the anatomy of articulation. I. The equilibrium of the “intercalated” bone.
Acta Morphol Neerl Scand 1961; 3:287–321.Kauer, TMJ, Landsmeer JMF.
Functional anatomy of the wrist.
In: Tubiana R, editor. The hand.
Philadelphia: WB Saunders, 1981.Ogon M, Bender BR, Hooper DM, et al.
A dynamic approach to spinal instability. Part II: hesitation and giving-way
during interspinal motion.
Spine 1997;22(24):2859–66.Wilder DG, Pope MH, Frymoyer JW.
Cyclic loading of the intervertebral motion segment.
Proceedings of the 10th Northeast Bioengineering Conference.
Hanover, (NY): Institute of Electrical and Electronic Engineers, 1982.Wilder DG, Pope MH, Frymoyer JW.
The biomechanics of lumbar disc herniation and the effect of overload and instability.
J Spinal Disord 1988;1:16–32.Wilder DG, Pope MH, Seroussi RE, Dimnet J, Krag MH.
The balance point of the intervertebral motion segment: an experimental study.
Bull Hosp Jt Dis Orthop Inst 1989;49(2):155–69.Saal JS.
The role of inflammation in lumbar pain.
Spine 1995;20(16): 1821–7.Siddall PJ, Cousins MJ.
Spine update spinal pain mechanisms.
Spine 1997;22(1):98–104.Kirkaldy-Willis WH, Hill RJ.
A more precise diagnosis for low-back pain.
Spine 1979;4(2):102–9.Yong-Hing K, Kirkaldy-Willis WH.
The pathophysiology of degenerative disease of the lumbar spine.
Orthop Clin North Am 1983; 14(3):491–504.Triano JJ, Schultz AB.
Motions of the head and thorax during neck manipulations.
J Manipulative Physiol Ther 1994;17(9):573–83.Triano J, Schultz AB.
Loads transmitted during lumbosacral spinal manipulative therapy.
Spine 1997;22(17):1955–64.Triano JJ.
Biomechanical analysis of motions and loads during spinal manipulation. 1998.
University of Michigan. Thesis/dissertation.Pope MH, Wilder DG, Krag MH,.
Biomechanics of the lumbar spine A: basic principles.
In: Frymoyer JW, editor.
The adult spine—principles and practice.
New York: Raven Press, 1991. p. 1487–501.Crisco JJ, Panjabi MM, Yamamoto I, Oxland TR.
Euler Stability of the human ligamentous lumbar spine. Part II experiment.
Clin Biomech 1992;7:27–32.Cholewicki J, Mcgill SM.
Lumbar posterior ligament involvement during extremely heavy lifts estimated
from fluoroscopic measurements.
J Biomech 1992;25(1):17–28.Lee M, Svensson NL.
Effect of loading frequency on response of the spine to lumbar posteroanterior forces.
J Manipulative Physiol Ther 1993;16(7):436–9.Kawchuk GN, Herzog W.
Biomechanical characterization (fingerprinting) of five novel methods of
cervical spinal manipulation.
J Manip Physiol Ther 1993;16:573–7.Fuhr AW, Colloca CJ, Green JR, Keller TS.
Activator methods chiropractic technique.
St. Louis: Mosby Books, 1997.van Deursen DL, Lengsfeld M, Snijders CJ, Evers JJM, Gordon MJ.
Mechanical effects of continuous passive motion on the lumbar spine in seating.
J Biomech 2000;33(6):695–700.Reinecke SM, Hazard RG, Coleman K.
Continuous passive motion in seating: a new strategy against low back pain.
J Spinal Disord 1994; 7(1):29–35.Wood J, Adams AA, Hansmeier D.
Force and time characterstics of pierce technique cervical adjustments.
J Chirop Res Clin Invest 1994;9:39–44.Hessel B, Herzog W, Conway PJW, Mcewan MC.
Experimental measurement of the force exerted during spinal manipulation using
the Thompson technique.
J Manipulative Physiol Ther 1990;8:448–53.Brennan PC, Kokjohn K, Kaltinger CJ, et al.
Enhanced Phagocytic Cell Respiratory Burst Induced by Spinal Manipulation:
Potential Role of Substance P
J Manipulative Physiol Ther 1991 (Sep); 14 (7): 399–408Kawchuk GN, Herzog W, Hasler EM.
Forces generated during spinal manipulative therapy of the cervical spine: a pilot study.
J Manip Physiol Ther 1992;1 5:275–8.Lee R, Evans J.
Load-displacement-time characteristics of the spine under posteroanterior mobilization.
Aust J Physio-ther 1992;38(2): 115–23.Conway PJW, Herzog W, Zhang Y.
Forces required to cause cavitation during spinal manipulation of the thoracic spine.
Clin Biomech 1993;8:210–4.Herzog W, Conway P, Kawchuk G, Zhang Y, Hasler E.
Forces exerted during spinal manipultive therapy.
Spine 1993;18:1206–12.Cohen E, Triano JJ, Mcgregor M, Papakyriakou M.
Biomechanical performance of spinal manipulation therapy by newly trained vs.
practicing providers: does experience transfer to unfamiliar procedures?
J Manipulative Physiol Ther 1995;18(6):347–52.Gal JM, Herzog W, Kawchuk GN, Conway PJ, Zhang Y.
Biomechanical performance of spinal manipulation therapy Forces and
relative vertebral movements during SMT to unembalmed post-rigor
human cadavers: peculiarities associated with joint cavitation.
J Manip Physiol Ther 1995;18(1):4–9.Triano JJ.
Effects of changing lumbar posture on spine loads during SMT.
Proceedings of the Consortium of Canadian Chiropractic Research Centres,
Toronto, Ontario, Canada,
Canadian Memorial Chiropractic College, 2000.Keller TS.
Engineering—In vivo transient vibration analysis of the normal human spine.
In: Fuhr A, Collaca CJ, Green JR, Keller TS, editors.
Activator methods chiropractic technique.
St. Louis: Mosby, 1997. p. 431–50.Triano JJ.
Studies on the biomechanical effect of a spinal adjustment.
J Manipulative Physiol Ther 1992;15(1):71–5.Suter E, Herzog W, Conway PJ, Zhang YT.
Reflex response associated with manipulative treatment of the thoracic spine.
J Neuromusculoskel Syst 1994;2:124–30.Herzog W, Scheele D, Conway PJ.
Electromyographic responses of back and limb muscles associated with spinal manipulative therapy.
Spine 1999;24:146–53.Herzog W.
The mechanical, neuromuscular and physiologic effects produced by the spinal manipulation.
In: Herzog W, editor. Clinical biomechanics of spinal maniuplation.
New York: Churchill Livingstone, 2000:191–207.Gal JM, Herzog W, Kawchuk GN, Conway PJ, Zhang Y.
Biomechanical studies of spinal manipulative therapy (SMT):
quantifying the movements of vertebral bodies during SMT.
J Can Chiro Assoc 1994;38:11–24.Gal J, Herzog W, Kawchuk G.
Movements of vertebrae during manipulative thrusts to unembalmed human cadavers.
J Manipulative Physiol Ther 1997;20:30–40.Keller TS, Holm SH, Hansson TJ, Senstad O.
The dependence of intervertebral disc mechanical properties on physiologic conditions.
Spine 1990;15(8):751–61.Haldeman S, Rubinstein SM.
Cauda equina syndrome in patients undergoing manipulation of the lumbar spine.
Spine 1992;17(12):1469–73.Haldeman S, Rubinstein SM.
The precipitation or aggravation of musculoskeletal pain in patints receiving
spinal manipulative therapy.
J Manip Physiol Ther 1993;16:47–50.Senstad O, Leboeuf-Yde C, Borchgrevink C.
Frequency and characteristics of side effects of spinal manipulative therapy.
Spine 1997; 22(4):435–41.Haldeman S, Kohlbeck FJ, McGregor M.
Risk Factors and Precipitating Neck Movements Causing Vertebrobasilar Artery Dissection
After Cervical Trauma and Spinal Manipulation
Spine (Phila Pa 1976) 1999 (Apr 15); 24 (8): 785–794Chaffin DB, Andersson G.
Occupational biomechanics.
New York: John Wiley & Sons, 1984. p. 182–7.Patrick LM, Chou CC.
Analytic: response of the human neck in flexion, extension and lateral flexion.
New York: SAE, Behild Research VRI 7.3, 1976.Sauces A., Weber RC, Larson SJ, Cusick JS, Myklebust JB, Walsh PR.
Bioengineering analysis of head and spine injuries.
CRC Crit Rev Bioeng 1981:5;79–122.Gadd CW, Culver CC, Nahum AM.
A study of responses and tolerances of the neck.
In: Backaitis SH, editor.
Biomechanics of impact injury and injury tolerances of the head-neck complex.
Warrendale (PA): SAE, 1993. 73–86Byfield D.
Cervical spine: manipulative skill and performance considerations.
Eur J Chiropr 1991;39:45–52.Triano J, Skogsbergh D, Mior S, Sportelli L.
Biomechanical parameters of skill in lumbar SMT.
Proceedings of the International Conference on Spinal Manipulation,
Palm Springs, CA. Arlington (VA): FCER Publishers. 1994
Return to ABOUT SPINAL ADJUSTING
Return to BIOMECHANICAL COMPONENT
Since 4-27-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |