Real-Time Visualization of Joint Cavitation This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: PLoS One. 2015 (Apr 15); 10 (4): e0119470 ~ FULL TEXT
OPEN ACCESS Gregory N. Kawchuk, Jerome Fryer, Jacob L. Jaremko,
Hongbo Zeng, Lindsay Rowe, Richard Thompson
Department of Physical Therapy,
Faculty of Rehabilitation Medicine,
University of Alberta,
Edmonton, Alberta, Canada
Cracking sounds emitted from human synovial joints have been attributed historically to the sudden collapse of a cavitation bubble formed as articular surfaces are separated. Unfortunately, bubble collapse as the source of joint cracking is inconsistent with many physical phenomena that define the joint cracking phenomenon. Here we present direct evidence from real-time magnetic resonance imaging that the mechanism of joint cracking is related to cavity formation rather than bubble collapse. In this study, ten metacarpophalangeal joints were studied by inserting the finger of interest into a flexible tube tightened around a length of cable used to provide long-axis traction. Before and after traction, static 3D T1-weighted magnetic resonance images were acquired. During traction, rapid cine magnetic resonance images were obtained from the joint midline at a rate of 3.2 frames per second until the cracking event occurred. As traction forces increased, real-time cine magnetic resonance imaging demonstrated rapid cavity inception at the time of joint separation and sound production after which the resulting cavity remained visible. Our results offer direct experimental evidence that joint cracking is associated with cavity inception rather than collapse of a pre-existing bubble. These observations are consistent with tribonucleation, a known process where opposing surfaces resist separation until a critical point where they then separate rapidly creating sustained gas cavities. Observed previously in vitro, this is the first in-vivo macroscopic demonstration of tribonucleation and as such, provides a new theoretical framework to investigate health outcomes associated with joint cracking.
Enjoy this live video demonstration
From the FULL TEXT Article
Introduction
Background
Sounds emitted from human synovial joints vary in their origin. Joint sounds that occur repeatedly with ongoing joint motion arise typically when anatomic structures rub past one another. In contrast, cracking sounds require time to pass before they can be repeated despite ongoing joint motion. Although various hypotheses have been proposed over many decades regarding the origin of cracking sounds, none have been validated; the underlying mechanism of cracking sounds remains unknown.
History
In 1947, Roston and Wheeler Haines [1] published the first scientific study toward describing the origins of joint cracking. Their experiment used serial radiography to visualize joint cracking when distraction forces were applied to metacarpophalangeal (MCP) joints. Their results characterized the sequence of gross articular events that define joint cracking. The process begins with the resting phase where joint surfaces are in close contact. In this stage, a light distraction force will barely separate the joint surfaces. With a greater distraction force, the surfaces resist separation until a critical point after which they separate rapidly. It is during this rapid separation phase that the characteristic cracking sound is produced. Following cracking, the joint is in a refractory phase where no further cracking can occur until time has passed (approximately 20 minutes). Importantly, post-cracking distraction also reveals the presence of a clear space assumed by Roston and Wheeler Haines to be a vapour cavity. This cavity, described by some as a bubble, has been thought to form as distraction forces decrease pressure within the synovial fluid to the point were dissolved gas comes out of solution. Importantly, Roston and Wheeler Haines linked the production of the cracking sound to the formation of this clear space, a phenomenon first described in 1911 [2] but thought by some to occur only in unhealthy joints [3] until demonstrated to also occur in normal joints [4].
This interpretation of joint cracking stood as the standard for 24 years until 1971 when Unsworth, Dowson and Wright [5] refuted this view by stating that the exact mechanism of joint cracking was in doubt. Although Unsworth et al. used a similar radiographic procedure to confirm the same sequence of events described by Roston and Wheeler Haines, they arrived at a different conclusion. Specifically, Unsworth et al. speculated that the formation of a clear space, or bubble, was not the source of joint cracking, but rather cracking was caused by the subsequent collapse of the bubble. This idea was likely influenced by the realization that bubble collapse could cause damage in surfaces adjacent to the bubble itself [6]. First described by Rayleigh in 1917 [7], cavitation collapse came into the fore in the late 1960s as a source of significant damage in marine equipment [6] such as propellers, hydrofoils [8].
As a result, publications since 1971 have referenced Roston [911] or Unsworth [1224] or both [5, 11, 2539] when describing joint cracking. Adding to the confusion, others [25] have suggested that sound produced during joint cracking occurs through ligamentous recoil. Still others [18, 19, 25, 26] advocate for an additional mechanism known as viscous adhesion or tribonucleation [40, 41], a process that occurs when two closely opposed surfaces are separated by a thin film of viscous liquid. When these surfaces are distracted, viscous adhesion or tension between the surfaces resist their separation. Then, as distraction forces overcome the adhesive forces, the surfaces separate rapidly creating a negative pressure. This negative pressure, combined with the speed with which the surfaces separate, can create a vapour cavity within fluid much like a solid that has been fractured [4244
]. Unfortunately, no direct evidence exists to resolve these differing perspectives regarding the mechanism of joint cracking. While many have used various radiographic means to record events associated with joint cracking [1, 5, 10, 45], these techniques have a number of limitations which conspire to obscure intra-articular events due to low space-time resolution, insufficient contrast and superimposition of structures.
Given the above, the objective of this study was to characterize the events associated with joint cracking within the joint itself using real-time cine magnetic resonance imaging (cine MRI). Here we present direct evidence from cine MRI that the mechanism of joint cracking is related to cavity formation rather than bubble collapse.
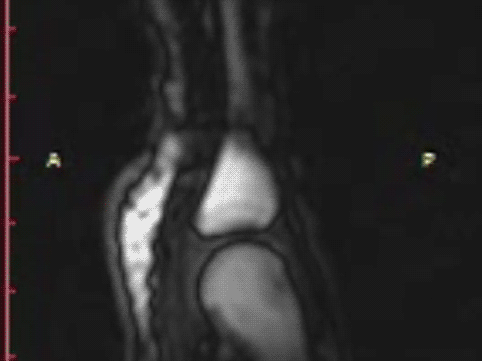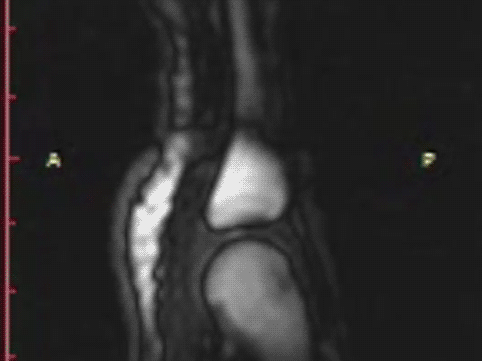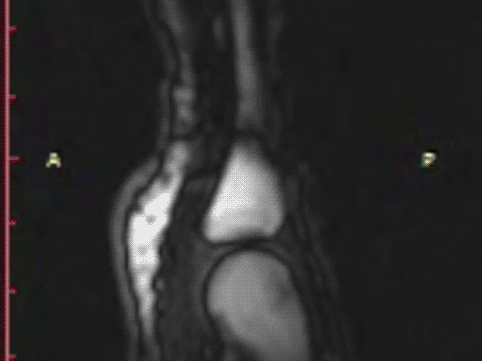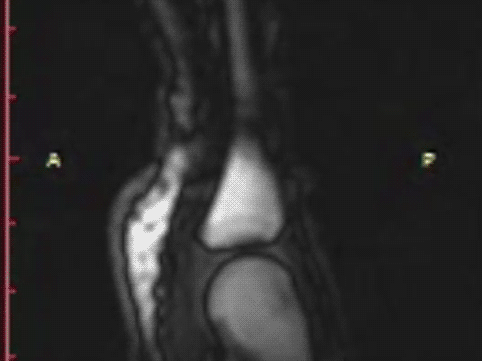
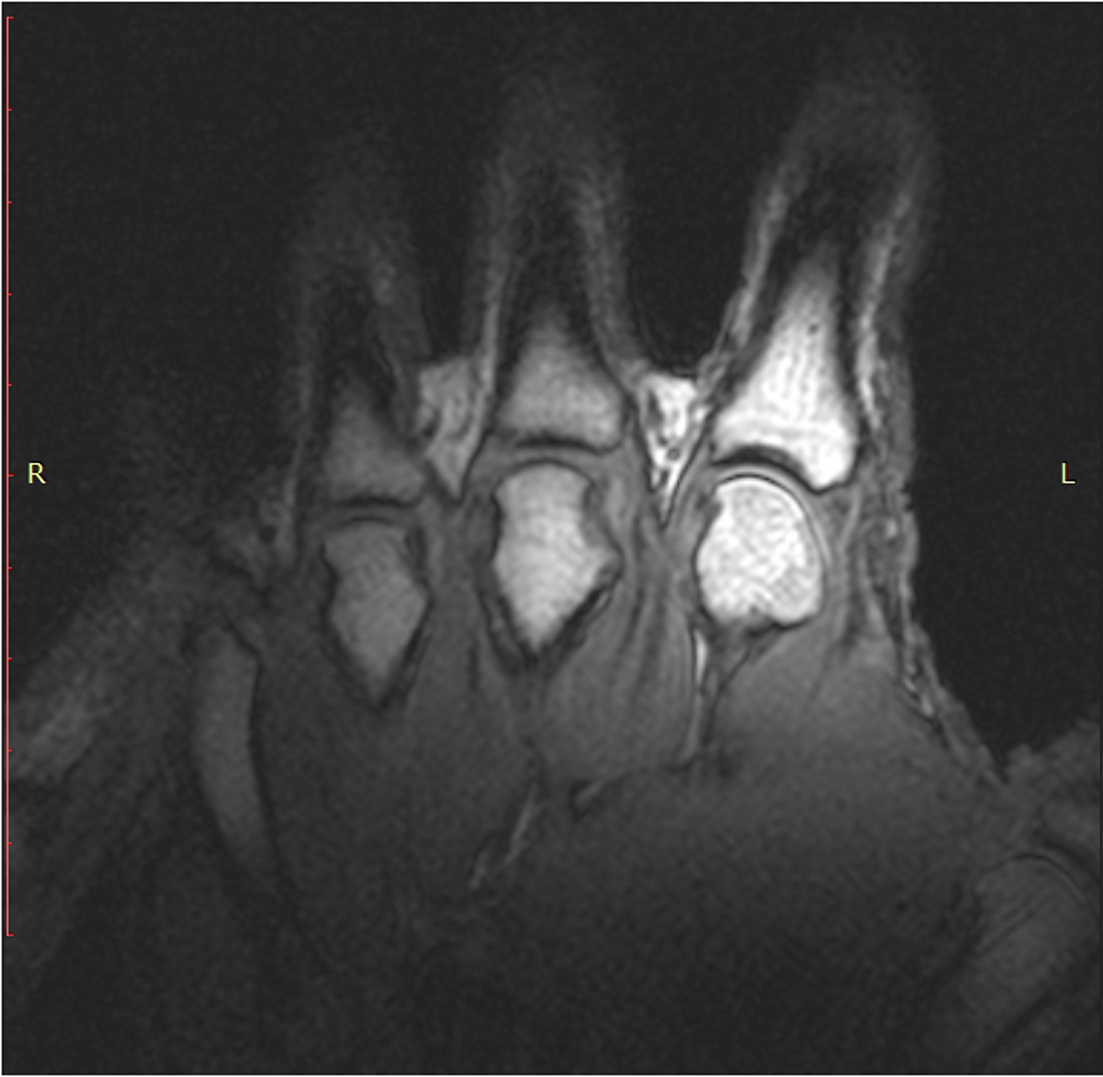
T1 static images of the hand in the resting phase before cracking
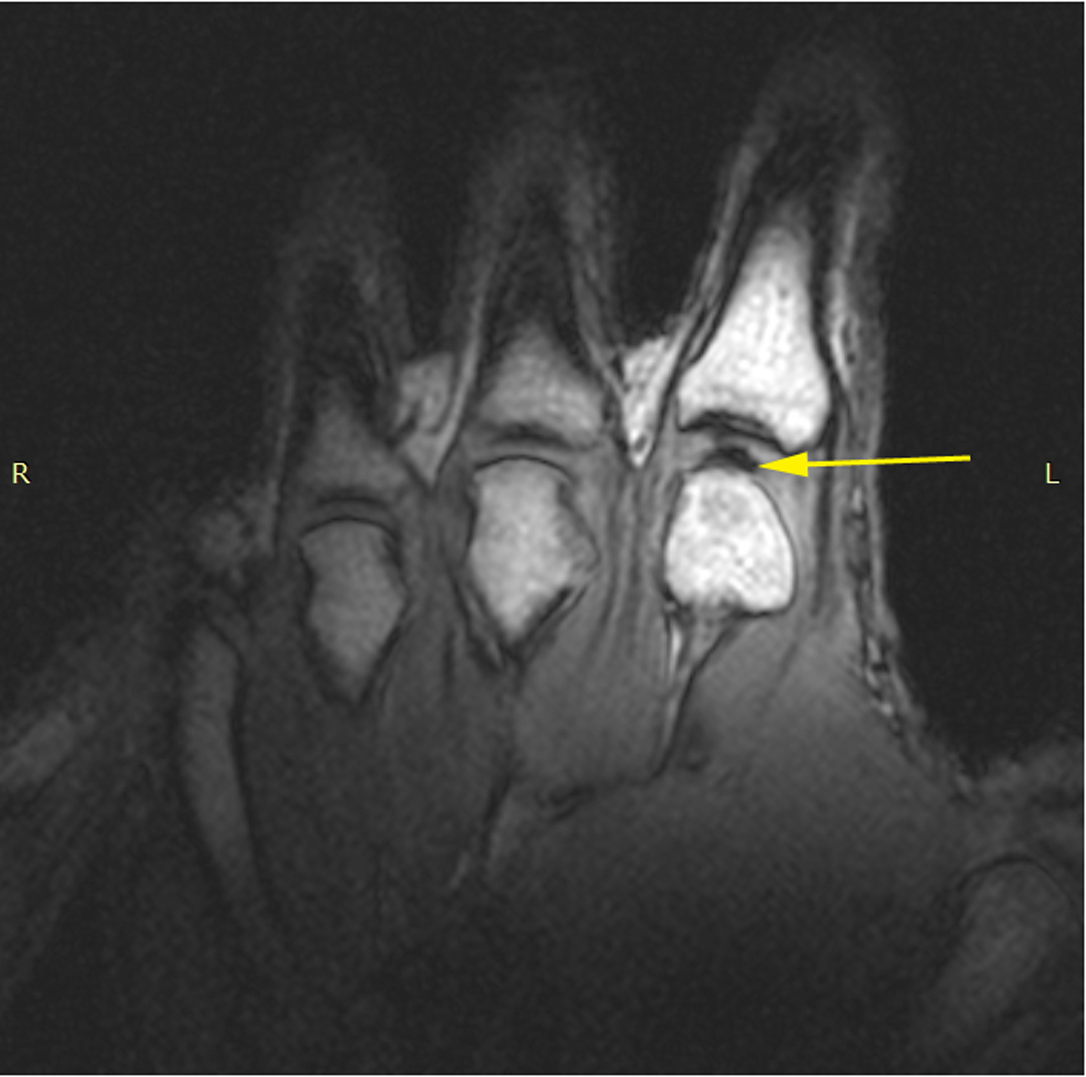
The same hand following cracking with the addition of a post-cracking distraction force.
Note the dark, interarticular void (yellow arrow).
Discussion
This study employed cine MRI to visualize joint cracking in real time. To our knowledge, cine MRI has not been used previously to characterize this phenomenon. Congruent with historic results, cine MRI demonstrated minimal joint surface separation in the resting phase prior to joint cracking followed by rapid joint separation during the crack itself. Incongruent with the prevailing perspective from the last half century, cine MRI revealed rapid cavity inception associated with concurrent sound production and joint separation. Following these events, the resulting cavity was never seen to collapse; the cavity formed at the time of rapid joint separation then persisted past the point of sound production.
Dark signal intensities in the joint immediately following cracking on both cine MRI imaging (a balanced SSFP pulse sequence with a characteristic mixed (T2/T1) weighting) as well as in the higher resolution T1 weighted static images, supports the presence of an air region of interest. Specifically, a significant and rapid increase in the fluid T1 values, which could reduce the signal intensity in both of these acquisitions, is implausible, and thus the reduction in signal is most likely due to a reduction in spin density associated with the formation of an air space. The gradual increase in signal intensity in the same region just prior to the cracking is suggestive of fluid accumulation during this phase of the finger cracking.
Events consistent with tribonucleation
Our results offer direct experimental evidence that joint cracking is the result of cavity inception within synovial fluid rather than collapse of a pre-existing bubble. These observations are consistent with tribonucleation, a known process where opposing surfaces resist separation until a critical point where they separate rapidly resulting in vapor cavities that do not collapse instantaneously.
Specifically, tribonucleation explains each phase of the joint cracking sequence described originally by Roston and Wheeler Haines [1]. The resting phase where distraction forces result in minimal joint separation is explained by viscous attraction between joint surfaces. With sufficient distraction force, those adhesive forces are overcome which explains the rapid separation of joint surfaces. The resulting drop in synovial pressure allows dissolved gas to come out of solution which explains the clear space (a.k.a. bubble, cavity, void, fluid fracture) created within the joint. This cavity persists past the point of sound production; a subsequent collapse is never visualized. Importantly, the cavity does disappear from the region of interest with subsequent cessation of distraction forces, but well after joint cracking has occurred.
Interpretation of prior studies
Our results are consistent with those of Roston and Wheeler Haines [1]. Their classic study using serial radiographs correctly identified the sequence of events that characterizes joint cracking. Although technical limitations did not allow them to see formation of the cavity during sound production, but only its presence after its formation, they correctly identified creation of the clear space as the defining event of joint cracking. Furthermore, many of their speculations were consistent with tribonucleation. These included prophetic comments that the 1) distraction force must be applied to overcome tension within the synovial fluid (not within the soft tissues) before cracking can occur and that 2) the inherent tension forces that kept the joint surfaces together add stability to the joint itself.
Alternatively, the suggestion by Unsworth et al. [5] that joint cracking was the result of cavity collapse, is a sensible one given the tremendous amount of work at the same time that defined bubble collapse to be a source of damage in marine equipment. While the 1971 paper from Unsworth et al. [5] made significant contributions in terms of the role of joint symmetry in joint cracking, composition of synovial gases and providing an explanation for the refractory period, they did not provide any direct evidence of a cavity collapse despite their conclusion. Given that the cavity which forms after joint cracking disappears from view when distraction forces are removed, but then appears again with additional distraction, Unsworth et al. [5] may have mistaken this disappearance for bubble collapse. Even if the bubble is reabsorbed after joint cracking to then be reformed in some fashion with subsequent distraction, the appearance and persistence of a cavity following rapid joint separation does not support bubble collapse as a mechanism of joint cracking. We also observed that the joint space before and after testing did not change significantly. This finding suggests that the resting joint orientation is not changed by the cracking event in the MCP. This is in disagreement with Unsworth et al. [5] who suggested that resting MCP joint space increases following cracking.
While our work provides new insights into defining the mechanism underlying joint cracking, this new visualization technique opens novel avenues for investigation. Specifically, cine MRI revealed a new phenomenon preceding joint cracking; a transient bright signal in the intra-articular space. While not likely visualized gas given the imaging parameters employed, we do not have direct evidence to explain this observation. We speculate this phenomenon may be related to changes in fluid organization between cartilaginous joint surfaces and specifically may result from evacuation of fluid out of the joint cartilage with increasing tension. If so, this sign may be indicative of cartilage health and therefore provide a non-invasive means of characterizing joint status.
Limitations
The slice thickness used for cine MRI prevented us from visualizing the joint in its entirety. As such, it was not possible to see what happened within all regions of the joint during cracking. Future studies that image peripheral areas of the MCP may reveal the fate of the cavity formed after rapid joint separation which does not collapse at the time of joint cracking, but disappears from the region of interest when distraction forces on the joint are removed. The current slice thickness in cine MRI cannot establish if the cavity formed after joint cracking migrates to the peripheral region of the joint or is resorbed when distraction forces cease. Similarly, when distraction forces are provided in the refractory phase, our data does not assist us in determining if the observed cavity reforms from gas nuclei migrating together from the periphery of the joint or if a new cavity is formed de novo from solution.
In addition, we presume that rapid joint separation with cavity formation does not occur at the same traction force in each finger. Unfortunately, traction forces were not measured in this experiment due to incompatibility of available force measuring equipment with MRI.
Last, this work does not explain the magnitude of the sound caused by cavity formation. Although some have noted the production of sound during cavity formation through tribonucleation [4850], the amplitude of the generated sound from these experiments would appear to be small whereas joint cracking can easily be heard across a room. Given the above, our in vivo results may be the largest example of tribonucleation and subsequent sound production observed to date.
Conclusions
Our data support the view that tribonucleation is the process which governs joint cracking. This process is characterized by rapid separation of surfaces with subsequent cavity formation, not bubble collapse as has been the prevailing viewpoint for more than a half century. Observed previously in vitro, this work provides the first in-vivo demonstration of tribonucleation on a macroscopic scale and as such, provides a new theoretical framework to investigate health outcomes associated with joint cracking. This framework will allow scientists to compare and contrast this process against tribonucleation observed between inanimate surfaces, an approach that may reveal how joint cracking affects cartilaginous joint surfaces. Presently, the literature in this area is confusing in that the energy produced during joint cracking is though to exceed the threshold for damage [51], but habitual knuckle cracking has not been shown to increase joint degeneration [52]. Ultimately, by defining the process underlying joint cracking, its therapeutic benefits, or possible harms, may be better understood.
References:
Roston JB, Haines RW.
Cracking in the metacarpo-phalangeal joint.
J Anat. 1947;81: 16573Fick R.
Zum Streit um den Gelenkdruck. Anat Hefte.
Springer-Verlag; 1911;43: 397414Christen T.
Richtigstellung zum Streit um den Gelenkdruck.
Anat Hefte. 1911;43: 379396Nordheim Y.
Eine neue Methode den Gelenkknorpel besonders die Kniegelenkmenisken rontgenologisch darzustellen.
Fortschr Rontgenstr. 1938;57.Unsworth A, Dowson D, Wright V.
Cracking joints A bioengineering study of cavitation in the metacarpophalangeal joint.
Ann Rheum Dis. 1971;30: 348358Benjamin TB, Ellis AT.
The Collapse of Cavitation Bubbles and the Pressures thereby Produced against Solid Boundaries.
Philos Trans R Soc London Ser A, Math Phys Sci. 1966;260: 221240Lord Rayleigh VIII.
On the pressure developed in a liquid during the collapse of a spherical cavity.
Philos Mag Ser 6. 1917;34: 9498Knapp R, Daily J, Hammitt F.
Cavitation.
New York: McGraw-Hill; 1970. p. 728LaFond E, Smith GK, Gregor TP, McKelvie PJ, Shofer FS.
Synovial fluid cavitation during distraction radiography of the coxofemoral joint in dogs.
J Am Vet Med Assoc. 1997;210: 12941297Mierau D, Cassidy J, Bowen V, Dupuis B, Noftall F.
Manipulation and mobilization of the third metacarpophalangeal joint: a quantitative radiographic and range of motion study.
Man Med. 1988;3: 135140Evans DW, Lucas N.
What is manipulation? A reappraisal.
Man Ther. Elsevier Ltd; 2010;15: 28691Barrow MS, Bowen WR, Hilal N, Al-Hussany A, Williams PR, Williams RL, et al.
A study of the tensile properties of liquids in confined spaces using an atomic force microscope.
Proc R Soc A Math Phys Eng Sci. 2003;459: 28852908Bronfort, G, Haas, M, Evans, R, Kawchuk, G, and Dagenais, S.
Evidence-informed Management of Chronic Low Back Pain with Spinal Manipulation and Mobilization
Spine J. 2008 (Jan); 8 (1): 213225Castellanos J, Axelrod D.
Effect of habitual knuckle cracking on hand function.
Ann Rheum Dis. 1990;49: 3089Crisco JJ, Fujita L, Spenciner DB.
The dynamic flexion/extension properties of the lumbar spine in vitro using a novel pendulum system.
J Biomech. 2007;40: 276773Cramer GD, Kim R, Raju PK, Cambron J, Cantu JA, Bora P, et al.
Quantification of cavitation and gapping of lumbar zygapophyseal joints during spinal manipulative therapy.
J Manipulative Physiol Ther. 2012;35: 614621DiGiorgi D.
Spinal manipulation under anesthesia: a narrative review of the literature and commentary.
Chiropr Man Therap. 2013;21: 14Evans DW, Breen AC.
A biomechanical model for mechanically efficient cavitation production during spinal manipulation: prethrust position and the neutral zone.
J Manipulative Physiol Ther. 2006;29: 7282Evans DW.
Mechanisms and Effects of Spinal High-velocity, Low-amplitude Thrust Manipulation:
Previous Theories
J Manipulative Physiol Ther 2002 (May); 25 (4): 251262Swezey R, Swezey S.
The Consequences of Habitual Knuckle Cracking.
West J Med. 1975;122: 377379Levick J.
Microvascular architecture and exchange in synovial joints.
Microcirculation. 1995;2: 217233Rubinstein SM, Terwee CB, Assendelft WJJ, de Boer MR, van Tulder MW.
Spinal manipulative therapy for acute low back pain: an update of the cochrane review.
Spine (Phila Pa 1976). 2013;38: E15877Trevena D.
Cavitation and the generation of tension in liquids.
J Phys D Appl Phys. 1984;17: 21392164Werner D, Kozin SH, Brozovich M, Porter ST, Junkin D, Seigler S.
The Biomechanical Properties of the Finger Metacarpophalangeal Joints to Varus and Valgus Stress.
J Hand Surg Am. 2003;28: 10441051Brodeur R.
The audible release associated with joint manipulation.
JMPT. 1995;18: 155164Reggars JW.
The manipulative crack. Frequency analysis.
Australas Chiropr Osteopathy. 1996;5: 3944Watson P, Kernohan WG, Mollan R a.
The effect of ultrasonically induced cavitation on articular cartilage.
Clin Orthop Relat Res. 1989; 28896Cascioli V, Corr P, Till A.
An investigation into the production of intra-articular gas bubbles and increase in joint space in the zygapophyseal joints of the cervical spine in asymptomatic subjects after spinal manipulation.
J Manipulative Physiol Ther. 2003;26: 35664Bereznick DE, Pecora CG, Ross JK, McGill SM.
The refractory period of the audible crack after lumbar manipulation: a preliminary study.
J Manipulative Physiol Ther. 2008;31: 199203Meal GM, Scott RA.
Analysis of the joint crack by simultaneous recording of sound and tension.
JMPT. 1986;9: 189195Sandoz R.
Some physical mechanisms and effects of spinal adjustment.
Ann Swiss Chiropr Assoc. 1976;6: 91141Flynn TW, Fritz JM, Wainner RS, Whitman JM.
The audible pop is not necessary for successful spinal high-velocity thrust manipulation in individuals with low back pain.
Arch Phys Med Rehabil. 2003;84: 10571060Hung W-C, Chang C-H, Hsu A-T, Lin H-T.
The Role of Negative Intra-Articular Pressure in Stabilizing the Metacarpophalangeal Joint.
J Mech Med Biol. 2013;13: 1350049Flynn TW, Childs JD, Fritz JM.
The audible pop from high-velocity thrust manipulation and outcome in individuals with low back pain.
J Manipulative Physiol Ther. 2006;29: 405Gibbons P, Tehan P.
Spinal manipulation?: indications, risks and benefits.
J Bodyw Mov Ther. 2001;5: 110119Beffa R, Mathews R.
Does the adjustment cavitate the targeted joint? An investigation into the location of cavitation sounds.
J Manipulative Physiol Ther. 2004;27: e2Reggars J.
Multiple channel recording of the articular crack associated with manipulation of the metacarpophalangeal joint. An observational study.
Australas Chiropr Osteopat. 1999;8: 1620Protapapas MG, Cymet TC.
Joint cracking and popping: Understanding noises that accompany articular release.
J Am Osteopath Assoc. 2002;102: 283287Watson P, Kernohan WG.
A study of the cracking sounds from the metacarpophalangeal joint.
Proc Inst Mech Eng, Part H J Eng Med. 1989;203: 109118Ikels K.
Production of gas bubbles in fluids by tribonucleation.
J Appl Physiol. 1970;28: 524527Campbell J.
The tribonucleation of bubbles.
J Phys D Appl Phys. 1968;1: 1085Chen YL, Kuhi T, Israelachvili J.
Mechanism of cavitation damage in thin liquid films:
collapse damage VS. inception damage. 1992;153: 3151Lung Y, Israelachvili J.
New Mechanism of Cavitation Damage.
Science. 1991;252: 11571160Zeng H, Zhao B, Israelachvili JN, Tirrell M.
Liquid- to Solid-Like Failure Mechanism of Thin Polymer Films at Micro- and Nanoscales. Macromolecules.
American Chemical Society; 2009;43: 538542Watson P, Mollan RAB.
Cineradiography of a cracking joint.
Br J Radiol. The British Institute of Radiology; 1990;63: 145147Davies MS, Saxby TS.
Arthroscopy of the first metatarsophalangeal joint.
J Bone Joint Surg Br. 1999;81: 2036Schneider C, Rasband W, Eliceiri K.
NIH Image to ImageJ: 25 years of image analysis.
Nat Methods. 9: 671675Aljishia S, Tatarkiewiczb J.
Why does heating water in a kettle produce sound? 1991; 628632Ramamurthy AS, Balachandar R, Ram HSG.
Some Characteristics of Flow Past Backward Facing Steps Including Cavitation Effects.
J Fluids Eng. 1991;113: 278284Ramamurthy AS, Bhaskaran P.
Velocity Exponent for Erosion and Noise Due to Cavitation.
J Fluids Eng. 1979;101: 6975Watson P, Kernohan WG, Mollan RA.
A study of the cracking sounds from the metacarpophalangeal joint.
Proc Inst Mech Eng H. 1989;203: 10918Deweber K, Olszewski M, Ortolano R.
Knuckle cracking and hand osteoarthritis.
J Am Board Fam Med. 2011;24: 16974
Return to SUBLUXATION ANATOMY
Since 4-16-2015


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |