

Multimodal Chiropractic Care of Pain and Disability
for a Patient Diagnosed With Benign Joint
Hypermobility Syndrome: A Case ReportThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Chiropractic Medicine 2014 (Mar); 13 (1): 35–42 ~ FULL TEXT
OPEN ACCESS Richard G. Strunk, DC, MS, Mark T. Pfefer, RN, DC, MS, Derrick Dube
Research Clinician Cleveland Chiropractic College,
Overland Park, KS.OBJECTIVE: The purpose of this case report is to describe multimodal chiropractic care of a female patient diagnosed with benign joint hypermobility syndrome (BJHS) and a history of chronic spine pain.
CLINICAL FEATURES: A 23-year-old white female presented for chiropractic care with chronic low back pain, neck pain, and headaches. The patient was diagnosed with BJHS, including joint hypermobility of her thumbs, elbows, right knee, and lumbopelvic region. A 6-year history of low back pain and varicose veins in her posterior thighs and knees were additional significant diagnostic findings of BJHS.
INTERVENTIONS AND OUTCOMES: The treatment consisted of spinal and extremity manipulation, Graston technique, and postisometric relaxation combined with sensory motor stimulation and scapular stabilization exercises. The patient was seen 15 times over an 18-week period. After 18 weeks of care, the Revised Oswestry Low Back Questionnaire and Headache Disability Index demonstrated clinically important improvements with her low back pain and headache; but little change was noted in her neck pain as measured by the Neck Disability Index.
CONCLUSION: This patient with BJHS who had decreased disability and spine pain improved after a course of multimodal chiropractic care.
KEYWORDS: Chiropractic; Joint hypermobility; Low back pain; Manipulation; Neck pain
From the FULL TEXT Article:
Introduction
Benign joint hypermobility syndrome (BJHS) is a hereditary connective tissue disorder defined by pain and hypermobility in multiple joints. [1–4] Benign joint hypermobility syndrome is also known as joint hypermobility syndrome and hypermobility syndrome.
Furthermore, it is important to be aware that BJHS shares similar clinical features to Ehlers-Danlos syndrome–hypermobility type(EDS-HT). Both BJHS and EDS-HT can present with generalized joint hypermobility, abnormal skin features, recurring joint dislocations, chronic joint/extremity pain, and a positive family history. The close association between BJHS and EDS-HT causes many to view them as the same condition. [5, 6] However, with the lack of readily available clinical testing, the association between BJHS and EDS-HT is not definitive.

Figure 1 Benign joint hypermobility syndrome is considered to be more common in African, Asian and Middle Eastern populations and is also more common in females and younger individuals. [4, 7–11] The true prevalence of BJHS is unknown, but estimates range from 5% in the United States to 43% in African populations. [7, 12] It is diagnosed by patient history and/or physical examination findings such as generalized joint hypermobility, chronic limb and or back pain, and connective tissue lesions. These history and examination findings are grouped into “major” and “minor” criteria called the Brighton criteria (Figure 1). [13]

Figure 2 Within the Brighton criteria, the amount of joint hypermobility is calculated through the Beighton score (Figure 2). The Beighton score is used to quantify the extensiveness of joint hypermobility on a “0 to 9” scale where 0 indicates no hypermobility and 9 indicates joint hypermobility in multiple body sites such as the low back, elbows, hands, and knees. Studies have shown adults and children with BJHS frequently experience arthralgia, with adults also being prone to generalized back pain. [14–21] There are also some studies that suggest BJHS is associated with fibromyalgia, [22, 23] and a prospective cohort study concluded that joint hypermobility in adolescence was a risk factor for musculoskeletal pain at the shoulder, knee, and ankle/foot joints. [24]
Research on the conservative treatment of BJHS is quite limited, with most high-level studies (randomized controlled trials) focused on knee pain and low-level studies (single-group prospective cohort, case series, case reports) focused on the spine or full body pain. [25–33] Proprioceptive or balance exercises have been the most widely studied treatment approach, showing some promise for knee pain. [25–27] As a result of the limited amount of high-level studies, standardized treatment guidelines are not available, with treatment recommendations based on low levels of evidence. [34] It is generally recommended that, for patients with BJHS and chronic pain, a multidisciplinary approach consisting of physiotherapy (strengthening and proprioceptive exercises), cognitive behavioral therapy, and pharmacology is best.
Regarding research on chiropractic spinal manipulative therapy and patients with spinal pain and BJHS, only 3 case reports have been published. [28, 32, 33] Despite the limited amount of research, the results from these case reports showed a pain reduction from multimodal chiropractic care. The small quantity of clinical research on the conservative management of BJHS and pain calls for more research on this topic. The purpose of this case report is to describe multimodal chiropractic treatment of a patient diagnosed with BJHS and spinal pain.
Case Report
Patient History
A 23–year-old white female presented with persistent headaches and right neck and low back pain. All areas were rated a 4/10 for average pain, 5/10 for worst pain, 1/10 for best pain, and 2/10 for pain at time of initial visit using the numeric pain rating scale. The numeric pain rating scale is commonly used and has shown to be reliable and valid. [35, 36] Her neck pain was achy and sometimes sharp, localized to the right upper trapezius, with referral into the right upper neck and shoulder region. The neck pain began insidiously 9 months ago while working on patients at a dental hygienist school. Resting helps decrease the pain, whereas seated postures at the dental school increase the pain. The Neck Disability Index (NDI) was scored at 10% at the initial visit. The NDI is a 10–item questionnaire with good reliability, as well as good construct and concurrent validity in an ambulatory clinic population. [37] It has also been shown to possess stable psychometric properties and is thought to provide an objective means of assessing the disability of patients with neck pain. [38]
Her dull achy headaches were localized to the suboccipital and temporal area and occurred with the neck pain at least 3 times per week. No photophobia/phonophobia or other symptoms were reported with the headaches. The 25–item Headache Disability Index (HDI) score was 12%. The HDI questionnaire is designed to measure the disability levels among patients with headache. It has been shown to have strong internal reliability and construct validity as well as acceptable levels of test-retest reliability. [39] The deep and dull low back pain was localized to the right lower lumbar spine and sacroiliac joint. The pain that began 6 years ago from childbirth is made worse by prolonged standing and sitting and temporarily decreased by hot showers and previous chiropractic care. The patient’s initial Revised Oswestry Low Back Questionnaire (OLBQ), which measures low back pain disability, was 16%. This 10–item questionnaire has demonstrated good reliability [40] and has shown to be responsive to clinically meaningful change. [41–45] Except for a horse-back riding fall as a child, a low weekly exercise level, and a low daily fruit and vegetable intake, the patient's past medical history and health habits were unremarkable.
Initial Examination
The orthopedic examination of the cervical spine and lumbopelvic regions indicated mechanically induced pain in both regions. In the cervical spine, pain was reproduced with stretching tests (shoulder depression/costoclavicular test) in the right upper trapezius muscle. Lumbopelvic pain was reproduced at the lumbosacral junction and right sacroiliac joint with lumbosacral extension tests. Cervical and lumbar active and passive range of motion demonstrated no limitations in motion or pain except for sharp and deep pain at the right sacroiliac joint and lumbosacral junction with active lumbar extension. Postural examination revealed a forward flexion bias as observed by anterior head carriage, internally rolled shoulders, anteriorly translated torso and anteriorly rotated pelvis in the sagittal plane. The Janda muscle movement pattern testing [46] demonstrated bilateral hyperactivity of the upper trapezius muscles, whereas the motion palpation end-play examination [47] revealed joint hypomobilities in the cervical, thoracic, and lower lumbar spinal joints as well as the right sacroiliac joint. The soft tissue palpation examination indicated multiple areas of hypertonicity and tenderness in the thoracic and lumbar paraspinals, as well as the right suboccipital, bilateral levator scapula, bilateral sternocleidomastoid, and right upper trapezius muscles. The patient was initially diagnosed with cervical/lumbar myalgia; cervicogenic headaches; sacroiliac arthralgia; and joint dysfunction of the cervical, thoracic, and lumbopelvic regions.
Examination for BJHS
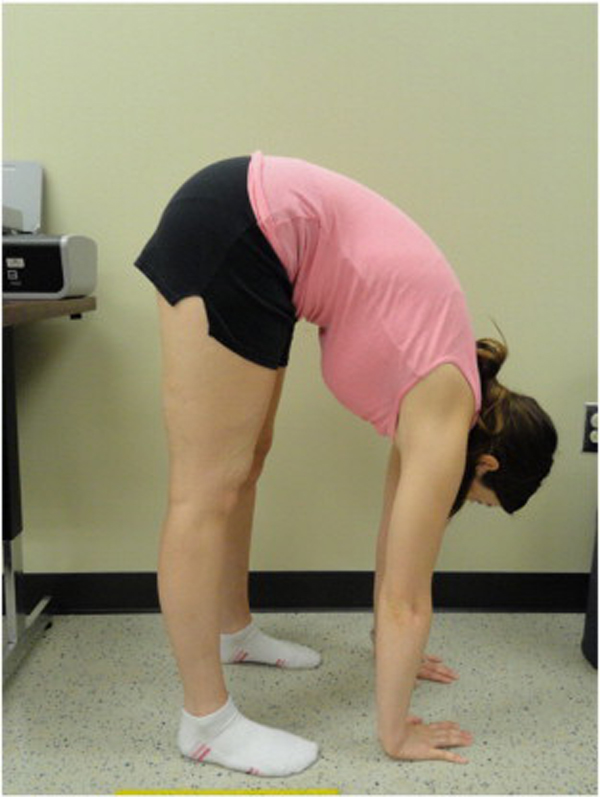
Because BJHS examination procedures were not part of the standard examination performed on the initial visit by interns, a screening examination (Beighton score and Brighton criteria) for BJHS was performed on the patient’s third visit because of the patient’s age, sex, and history of spinal pain. The screening examination was later confirmed by a focused BJHS examination with goniometry on the patient’s seventh visit. The patient’s Beighton score was a “6” out of “9.” Her elbows, knees, and fifth metacarpophalangeal (MCP) joint hypermobility was measured with a 2–arm goniometer, whereas thumb opposition and lumbopelvic flexion were visually observed. Left and right elbow (humeroulnar joint) hyperextension was 20° (Figures 3 and 4), right knee hyperextension was 10° (Figure 5), left knee hyperextension was 9°, left fifth MCP dorsiflexion was 85°, and right fifth MCP dorsiflexion was 75°. Visually, both the left and right thumbs displayed full passive opposition to each respective forearm (Figures 6 and 7), and lumbopelvic flexion was full with palms flat on the floor and knees fully extended (Figure 8). The physical examination of the patient’s legs revealed varicose veins bilaterally in the posterior knee region and distal quarter of the posterior thigh.
Figure 3. Left elbow extension.
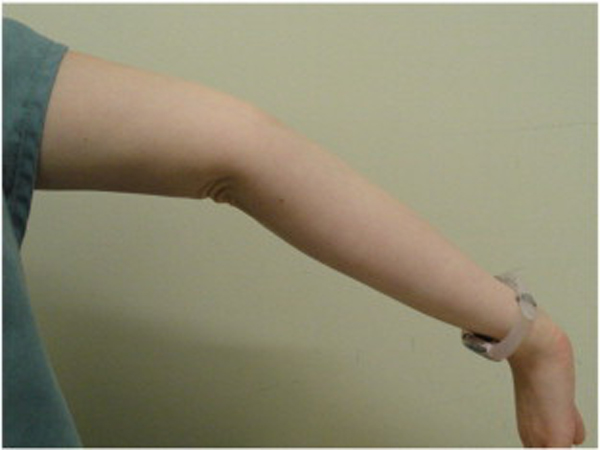
Figure 4. Right elbow extension.
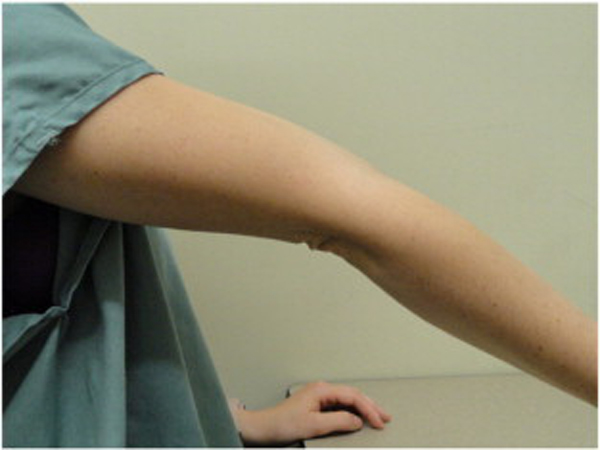
Figure 5. Right knee extension.
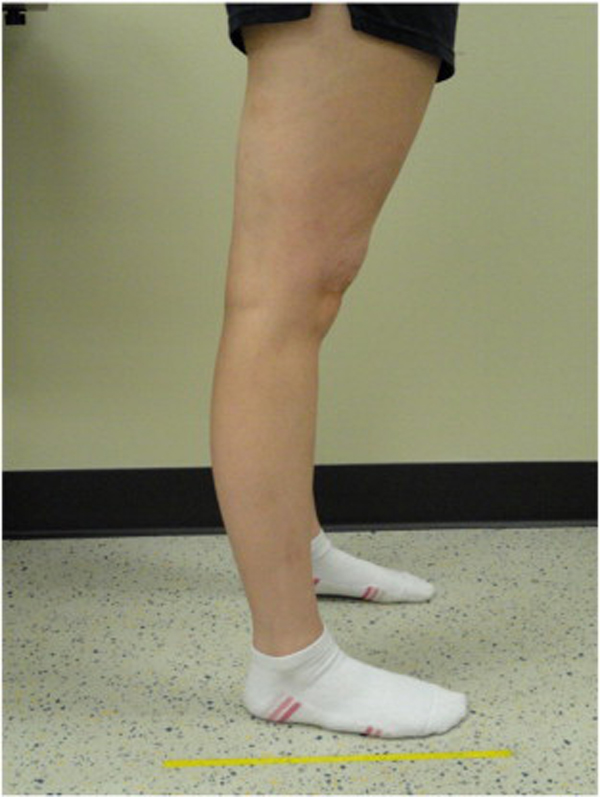
Figure 6. Right thumb hypermobility.
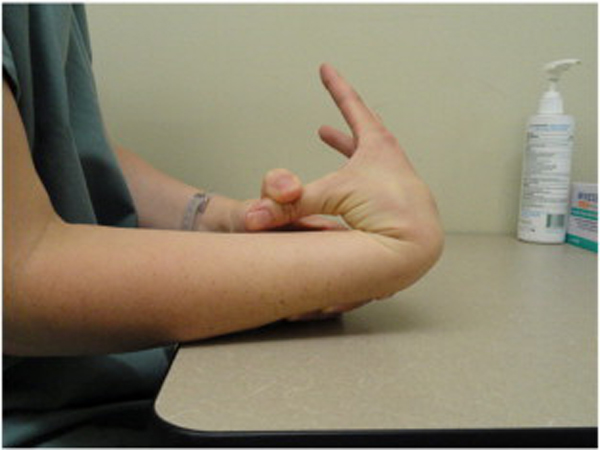
Figure 7. Left thumb hypermobility.
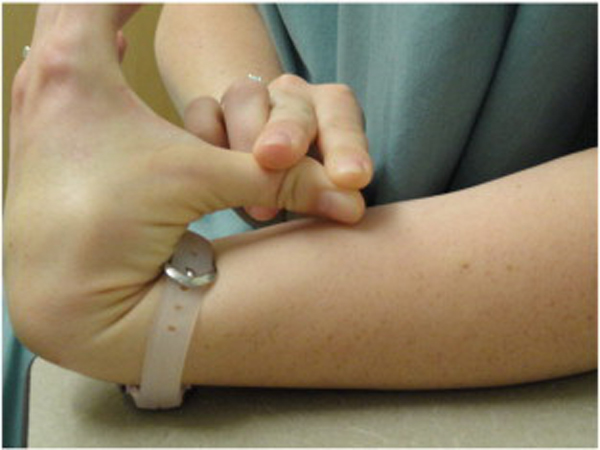
Figure 8. Trunk flexion hypermobility with palms flat on the floor and knees fully extended.
The diagnosis of BJHS was made by 1 major Brighton criterion (Beighton score = 6) and 2 minor Brighton criteria (varicose veins and a 6–year history of low back pain).
Treatment
Multimodal treatment consisted of joint manipulation and soft tissue/muscle energy treatment combined with scapular and sensory motor stimulation exercises. The most common type of manipulation (visits 4 through 15) administered was a mechanical-force, manually assisted technique applied by the Activator IV adjusting instrument to the spine, pelvis, and extremities. [48] This instrument allows for a specific, low-force impulse to the vertebral joint and may be preferred in patients with connective tissue fragility and systemic joint hypermobility. [28, 49] High-velocity, low-amplitude spinal manipulation to the cervical, thoracic, and lumbar spine (HVLA SM) and drop technique to the pelvis [47] were performed on the second and third visits before the patient was diagnosed with BJHS. Because of the patient’s new diagnosis of BJHS on the third visit and minor symptomatic change from treatment, the Activator method was used subsequently. In conjunction with the spinal manipulative techniques (Activator method and HVLA SM), a soft tissue technique was used each visit to the upper trapezius and upper thoracic paraspinal muscles. Graston technique was used for the first 8 visits, and then postisometric relaxation technique was applied at the remaining visits to help reduce hypertonicity. [50] Reevaluation of patient progress was performed 3 times throughout care; and because of the results of functional tests at these reevaluations, active care exercises were administered. Because of a failed “Vele’s Reflex Stability Test of the Transverse Arch” and the inherent hypermobility/laxity of the knees and low back, approximately 8 minutes of sensory motor training using a wobble board was used each visit starting on the ninth visit. The sensory motor training was initially performed with the goal of maintaining a neutral posture without any external stimulation. Once the 2–legged neutral posture was maintained without much difficulty, the patient was progressed to playing catch using medicine balls while maintaining an upright posture on the wobble board. The degree of difficulty with the medicine ball catch game was increased over the last 6 to 7 weeks by using a graded approach starting with 2–lb balls and concluding with 6.6–lb balls.
At about the same time as the sensory motor training was introduced, the patient was given home balance exercises and scapular stability exercises. The home balance exercises were to be done using a beginner-level foam stability pad. The patient was instructed to hold a neutral posture while performing a single-leg stance on the stability pad for 5 to 10 min/d. Once the single-leg stance exercise became easy, the patient was instructed to progress to performing hip extension, flexion, and abduction exercises while maintaining a neutral upright posture for 5 to 10 min/d on the same stability pad. To facilitate stability of the scapula, seated scapula depression/retraction exercises and serratus anterior wall push-up plus facilitation exercises were given to the patient to perform on a daily basis. For both scapular exercises, the patient was instructed to perform 1 set of 15 to 20 repetitions and progress to more and in the prone position (for retraction/depression) as the difficulty decreased. Other recommendations made to the patient were to increase weekly aerobic exercise levels to 4 times a week and increase daily fruit and vegetable serving intake to 3 times a day. All examinations (except for the BJHS examinations) and treatment were performed at a chiropractic college outpatient clinic by qualified chiropractic interns under the direction of a licensed doctor of chiropractic. The BJHS examinations were performed by the lead author.
Outcomes

Table 1 The HDI, NDI, and the Revised OLBQ were administered at follow-up intervals of 8, 15 and 18 weeks. The patient received treatment approximately over an 18–week period where she averaged 1 visit per week for a total of 15 visits despite missing multiple visits during the first couple of months of care including a 3½–week period of no care during the second month. The HDI and Revised OLBQ scores improved the most from the start of care to week 18 (Table 1). Whereas the Revised OLBQ demonstrated a clinically meaningful improvement (a decreased of 12 points), [41, 45] the NDI increased at week 8 and demonstrated little change at weeks 15 and 18. The Patient Global Impression of Change scale (PGIC) was administered at weeks 15 and 18. At week 15, the scale indicated that the patient was “much better”; and at week 18, it indicated that the patient was “a little better.” The PGIC is a self-reported 7–point Likert scale where a patient assesses his or her degree of change since starting treatment, ranging from very much better to very much worse. The PGIC has been well validated and has been commonly used by pain researchers as a standard outcome instrument. [51–54] Although the patient reported exercising 4 times a week and lessened her daily “carbohydrate/sugar” levels, it is not known how compliant the patient was with the home balance and scapular stability exercise program. The patient gave consent for this study to be published.
Discussion
This is one of the first reported cases of the chiropractic management of a patient with BJHS. The results of the outcome indices showed that the patient had a clinically important improvement in her headache and low back disability levels over an 18–week course of multimodal chiropractic care. Although the patient’s headache and low back pain improved, the patient’s neck disability levels varied since the start of care. The varied outcome seen with this patient has also been observed in other patients who have this condition as documented in a retrospective single-group cohort study [31] and in other case reports. [28, 29, 32, 33]
Patients with BJHS typically display a fluctuating pain pattern characterized by exacerbations and remissions and many times do not make a full recovery from treatment. Even with the varied outcome, this case report is important to health care providers especially doctors of chiropractic because of the low number of case reports and nonexistence of experimental research on effective conservative management of this condition (related to the spine) or similar conditions. [28, 29, 32, 33]
Diagnosis of BJHS
Because of the reported high prevalence rates of BJHS in certain populations and the underdiagnosis of BJHS, [61] screening for BJHS would likely add value in determining the best course of treatment for patients with chronic pain and this entity. Hakim and Grahame [62] developed a simple 5–item questionnaire to screen patients for joint hypermobility. The questionnaire has been shown to have good sensitivity and specificity for hypermobility in patients when 2 or more of the responses to the questions are affirmative. When screening with this questionnaire, a health care provider would not have to use the Brighton criteria on every patient with pain, just on patients with 2 or more affirmative responses on the questionnaire. Screening for BJHS might be helpful for patients with chronic pain who do not respond to an initial module of care. Practitioners who practice spinal manipulative procedures might apply specific techniques to this population of patients with better results if they know their patient has BJHS. Because of the nature of BJHS where articular hypermobility is present and joint stability is uncertain, HVLA SM represents a relative contraindication to the involved area of pathology. [49] More research is needed to determine the best course of treatment for patients with BJHS.
Limitations
Without intervention, headaches, neck pain, and low back pain tend to follow a recurrent and persistent natural course. [55–60] It is possible that the patient may have improved because of the natural course of the disorder or other factors that were not measured or that we are unaware of. Because this was a single case report, it is not appropriate to generalize the effects from this patient to other patients with BJHS and spinal pain. Further observation and experimental research with larger sample sizes are needed to determine what the effects multimodal chiropractic care has on patients with BJHS and chronic spinal pain.
Conclusion
This case report describes the response to multimodal chiropractic treatment of a patient diagnosed with BJHS and low back pain, neck pain, and headaches. The patient experienced clinically important improvements in her low back and headache disability levels posttreatment.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
References
Child A.H.
Joint hypermobility syndrome: inherited disorder of collagen synthesis.
J Rheumatol. 1986;13:239–243Hakim A.J., Cherkas L.F., Grahame R., Spector T.D., MacGregor A.J.
The genetic epidemiology of joint hypermobility: a population study of female twins.
Arthritis Rheum. 2004;50:2640–2644Prockop D.J., Kivirikko K.I.
Collagens: molecular biology, diseases, and potentials for therapy.
Annu Rev Biochem. 1995;64:403–434Beighton P., Solomon L., Soskolne C.L.
Articular mobility in an African population.
Ann Rheum Dis. 1973;32:413–418Castori M.
Ehlers-Danlos syndrome, hypermobility type: an underdiagnosed hereditary
connective tissue disorder with mucocutaneous, articular, and systemic manifestations.
ISRN Dermatol. 2012:1–22Tinkle B.T., Bird H.A., Grahame R.,
Lavallee M., Levy H.P., Sillence D.
The lack of clinical distinction between the hypermobility type of
Ehlers-Danlos syndrome and the joint hypermobility syndrome
(a.k.a. hypermobility syndrome)
Am J Med Genet A. 2009;149A:2368–2370Birrell F.N., Adebajo A.O., Hazleman B.L., Silman A.J.
High prevalence of joint laxity in West Africans.
Br J Rheumatol. 1994;33:56–59Jansson A., Saartok T., Werner S., Renstrom P.
General joint laxity in 1845 Swedish school children of different ages:
age and gender specific distributions.
Acta Paediatr. 2004;93:1202–1206Seow C., Chow P., Khong K.
A study of joint mobility in a normal population.
Ann Acad Med Singapore. 1999;28:231–236Wordsworth P., Ogilvie D., Smith R., Sykes B.
Joint mobility with particular reference to racial variation and inherited
connective tissue disorders.
Br J Rheumatol. 1987;26:9–12Grahame R., Hakim A.
Joint hypermobility syndrome is highly prevalent in general rheumatology clinics,
its occurrence and clinical presentation being gender, age and race related.
Ann Rheum. 2006;65:263.Jessee E.F., Owen D.S., Sagar K.B.
The benign hypermobile joint syndrome.
Arthritis Rheum. 1980;23:1053–1056Grahame R., Bird H.A., Child A.
The revised (Brighton 1998) criteria for the diagnosis of benign joint
hypermobility syndrome (BJHS)
J Rheumatol. 2000;27:1777–1779El-Garf A.K., Mahmoud G.A., Mahgoub E.H.
Hypermobility among Egyptian children: prevalence and features.
J Rheumatol. 1998;25:1003–1005Al-Rawi Z.S., Al-Aszawi A.J., Al-Chalabi T.
Joint mobility among university students in Iraq.
Br J Rheumatol. 1985;24:326–331Larsson L.G., Baum J., Mudholkar G.S., Kollia G.D.
Benefits and disadvantages of joint hypermobility among musicians.
N Engl J Med. 1993;329:1079–1082Gedalia A., Press J.
Articular symptoms in hypermobile schoolchildren: a prospective study.
J Pediatr. 1991;119:944–946Seckin U., Tur B.S., Yilmaz O., Yagci I., Bodur H., Arasil T.
The prevalence of joint hypermobility among high school students.
Rheumatol Int. 2005;25:260–263Acasuso Diaz M., Collantes Estevez E., Sanchez Guijo P.
Joint hyperlaxity and musculoligamentous lesions: study of a population of
homogeneous age, sex and physical exertion.
Br J Rheumatol. 1993;32:120–122Bravo J.F., Wolff C.
Clinical study of hereditary disorders of connective tissues in a Chilean population:
joint hypermobility syndrome and vascular Ehlers-Danlos syndrome.
Arthritis Rheum. 2006;54:515–523El-Metwally A., Salminen J.J., Auvinen A.,
Kautiainen H., Mikkelsson M.
Prognosis of non-specific musculoskeletal pain in preadolescents:
a prospective 4-year follow-up study till adolescence.
Pain. 2004;110:550–559Gedalia A., Press J., Klein M., Buskila D.
Joint hypermobility and fibromyalgia in schoolchildren.
Ann Rheum Dis. 1993;52:494–496Sendur O.F., Gurer G., Bozbas G.T.
The frequency of hypermobility and its relationship with clinical
findings of fibromyalgia patients.
Clin Rheumatol. 2007;26:485–487Tobias J.H., Deere K., Palmer S., Clark E.M., Clinch J.
Joint hypermobility is a risk factor for musculoskeletal pain during adolescence:
findings of a prospective cohort study.
Arthritis Rheum. 2013;65:1107–1115Sahin N., Baskent A., Cakmak A., Salli A., Ugurlu H., Berker E.
Evaluation of knee proprioception and effects of proprioception exercise in patients
with benign joint hypermobility syndrome.
Rheumatol Int. 2008;28:995–1000Ferrell W.R., Tennant N., Sturrock R.D.
Amelioration of symptoms by enhancement of proprioception in patients with
joint hypermobility syndrome.
Arthritis Rheum. 2004;50:3323–3328Barton L., Bird H.
Improving pain by the stabilization of hyperlax joints.
J Orthop Rheumatol. 1996;9:46–51.Colloca C.J., Polkinghorn B.S.
Chiropractic Management of Ehlers-Danlos Syndrome:
A Report of Two Cases
J Manipulative Physiol Ther 2003 (Sep); 26 (7): 448–459Simmonds J.V., Keer R.J.
Hypermobility and the hypermobility syndrome, part 2: assessment and management
of hypermobility syndrome: illustrated via case studies.
Man Ther. 2008;13:e1–e11Celletti C, Castori M, Galli M, et al.
Evaluation of balance and improvement of proprioception by repetitive muscle
vibration in a 15-year-old girl with joint hypermobility syndrome.
Arthritis Care Res (Hoboken);63:775–9Ashton S, Hakim AJ.
Undiagnosed, joint hypermobility syndrome patients have poorer outcome than
peers following chronic back pain rehabilitation.
Proceedings of the Soft tissue and regional musculoskeletal disease and
fibromyalgia Conference; 2005 April 21 p.109.Miller J.E., Mathews S.L.
Joint hypermobility syndrome: which intervention?
J Clin Chiropr Pediatr. 2011;12:915–918.Morley J., Perrault T.
Chiropractic management of Ehlers-Danlos Syndrome: a case report.
J Am Chiropr Assoc. 2010;47:6–15.Castori M., Morlino S., Celletti C.
Management of pain and fatigue in the joint hypermobility syndrome
(a.k.a. Ehlers-Danlos syndrome, hypermobility type): principles and proposal
for a multidisciplinary approach.
Am J Med Genet A. 2012;158A:2055–2070Jensen M.P., Karoly P., Braver S.
The measurement of clinical pain intensity: a comparison of six methods.
Pain. 1986;27:117–126Bijur P.E., Latimer C.T., Gallagher E.J.
Validation of a verbally administered numerical rating scale of acute pain
for use in the emergency department.
Acad Emerg Med. 2003;10:390–392Vernon H., Mior S.
The Neck Disability Index: A Study of Reliability and Validity
J Manipulative Physiol Ther 1991 (Sep); 14 (7): 409–415Hains F., Waalen J., Mior S.
Psychometric properties of the neck disability index.
J Manipulative Physiol Ther. 1998;21:75–80Jacobson G.P., Ramadan N.M., Aggarwal S.K., Newman C.W.
Headache Disability Inventory (HDI):
The Henry FordHospital Headache Disability Inventory (HDI)
Neurology. 1994 (May); 44 (5): 837-842Hsieh C.Y., Phillips R.B., Adams A.H., Pope M.H.
Functional outcomes of low back pain: comparison of four treatment groups
in a randomized controlled trial.
J Manipulative Physiol Ther. 1992;15:4–9Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole MR.
Clinical Importance of Changes in Chronic Pain Intensity
Measured on an 11-point Numerical Pain Rating Scale
Pain 2001 (Nov); 94 (2): 149-158Meade T., Browne W., Mellows S.
Comparison of chiropractic and outpatient management of low back pain:
a feasibility study.
J Epidemiol Commun Health. 1986;40:12–17Manniche C., Asmussen K., Lauritsen B.
Low back pain rating scale: validation of a tool for assessment of low back pain.
Pain. 1994;57:317–326Roland M., Fairbank J.
The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire.
Spine. 2000;25:3115–3124Fritz J.M., Irrgang J.J.
A comparison of a modified Oswestry Low Back Pain Disability Questionnaire
and the Quebec Back Pain Disability Scale.
Phys Ther. 2001;81:776–788Page P., Frank C.C., Lardner R.
Champaign; Human Kinetics: 2010.
Assessment and treatment of muscle imbalance: the Janda approach.Peterson D., Bergman T.
Mosby; St. Louis: 2002.
Chiropractic technique: principles and procedures. 2nd edition.Fuhr A.
Mosby Elsevier; St. Louis: 2009.
Activator method. 2nd ed.Haldeman S., Chapman-Smith D., Petersen D.J., editors.
Guidelines for Chiropractic Quality Assurance and Practice Parameters
Aspen Publishers; Gaithersburg, MD: 1993.Hammer W. 3rd ed.
Sudbury; Jones and Bartlett: 2007.
Functional soft-tissue examination and treatment by manual methods.Farrar J.T., Young J.P., Jr., LaMoreaux L.,
Werth J.L., Poole R.M.
Clinical importance of changes in chronic pain intensity measured on an 11-point
numerical pain rating scale.
Pain. 2001;94:149–158Goldsmith C.H., Boers M., Bombardier C., Tugwell P.
Criteria for clinically important changes in outcomes: development, scoring
and evaluation of rheumatoid arthritis patient and trial profiles.
OMERACT Committee. J Rheumatol. 1993;20:561–565Jenkinson C., Peto V., Coulter A.
Measuring change over time: a comparison of results from a global single item
of health status and the multi-dimensional SF-36 health status survey questionnaire
in patients presenting with menorrhagia.
Qual Life Res. 1994;3:317–321Juniper E.F., Guyatt G.H., Willan A., Griffith L.E.
Determining a minimal important change in a disease-specific
Quality of Life Questionnaire.
J Clin Epidemiol. 1994;47:81–87Kienbacher C., Wober C., Zesch H.E.
Clinical features, classification and prognosis of migraine and tension-type
headache in children and adolescents: a long-term follow-up study.
Cephalalgia. 2006;26:820–830Lyngberg A.C., Rasmussen B.K., Jorgensen T., Jensen R.
Prognosis of migraine and tension-type headache: a population-based follow-up study.
Neurology. 2005;65:580–585Cote P., Cassidy J.D., Carroll L.J., Kristman V.
The annual incidence and course of neck pain in the general population:
a population-based cohort study.
Pain. 2004;112:267–273Von Korff M., Saunders K.
The course of back pain in primary care.
Spine. 1996;21:2833–2837. discussion 8–9Philips H.C., Grant L.
The evolution of chronic back pain problems: a longitudinal study.
Behav Res Ther. 1991;29:435–441Bot S.D., van der Waal J.M., Terwee C.B.
Predictors of outcome in neck and shoulder symptoms: a cohort study in general practice.
Spine. 2005;30:E459–E470Grahame R., Bird H.
British consultant rheumatologists' perceptions about the hypermobility syndrome:
a national survey.
Rheumatology (Oxford) 2001;40:559–562Hakim A.J., Grahame R.
A simple questionnaire to detect hypermobility: an adjunct to the assessment
of patients with diffuse musculoskeletal pain.
Int J Clin Pract. 2003;57:163–166
Return to CASE STUDIES
Since 3-06-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |