

Spine Adjusting Instrument (Impulse®) Attenuates
Nociception and Modulates Oxidative Stress
Markers in the Spinal Cord and Sciatic
Nerve of a Rat Model of Neuropathic PainThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Pain Medicine 2021 (May 16); pnab167
Francielle B O da Silva, BS, Maria do Carmo Q Santos, MS, Thaisla Cristiane Borella da Silva et. al.
Laboratório de Neurobiologia Comparada,
Departamento de Fisiologia,
Instituto de Ciências Básicas da Saúde,
Universidade Federal do Rio Grande do Sul,
Porto Alegre, Rio Grande, do Sul.Objective: Oxidative stress plays an important role in neuropathic pain. Spinal manipulative therapy (SMT) can exert beneficial effects in pain outcomes in humans and animal models. SMT can also modulate oxidative stress markers in both humans and animals. We aimed to determine the effect of Impulse®-assisted SMT (ISMT) on nociception and oxidative stress biomarkers in the spinal cord and sciatic nerve of rats with neuropathic pain (NP).
Methods: NP was induced by chronic constriction injury (CCI) of the sciatic nerve. Animals were randomly assigned to naive, sham (rats with sciatic nerve exposure but without ligatures) and CCI, with and without ISMT. ISMT was applied onto the skin area corresponding to the spinous process of L4-L5, 3 times/week, for 2 weeks. Mechanical threshold, latency to paw withdrawal to thermal stimulus and oxidative stress biomarkers in spinal cord and sciatic nerve were the main outcomes evaluated.
Results: ISMT significantly increased mechanical threshold and withdrawal latency after CCI. In the spinal cord, ISMT prevented the increase of pro-oxidative superoxide anion generation and hydrogen peroxide levels. Lipid hydroperoxide levels both in the spinal cord and in the sciatic nerve were attenuated by ISMT. Total antioxidant capacity increased in the spinal cord and sciatic nerve of CCI rats with and without ISMT. CCI and ISMT did not significantly change the total thiol content of the spinal cord.
Conclusions: Our findings suggest reduced oxidative stress in the spinal cord and/or nerve may be an important mechanism underlying a therapeutic effect of SMT to manage NP non-pharmacologically.
Keywords: Antinociception; Antioxidant; Pain; Pro-oxidant; spinal manipulation.
From the FULL TEXT Article:
Introduction
Neuropathic pain (NP) is defined as pain caused by a lesion or disease in the peripheral or central somatosensory nervous system. [1] NP affects about 7 to 10% of the population, and it is clinically characterized by abnormal pain sensation, such as spontaneous ongoing pain or shooting pain, and by abnormal evoked pain responses, such as hyperalgesia and allodynia, within the somatotopic representation of the injured nervous system. [2] NP is associated with several pain conditions, such as postherpetic neuralgia, trigeminal neuralgia, painful radiculopathy, chronic low back pain subpopulations, diabetic neuropathy, HIV infection, leprosy, amputation, peripheral nerve injury pain, and stroke. [1, 2] The underlying mechanisms taking place in NP are complex. [3] The clinical management of NP is interdisciplinary and encompasses pharmacological and nonpharmacological approaches. [4] Pharmacological options show limited efficacy, and many patients may endure long-term side effects. [1] Therefore, it is important to determine new strategies to help manage NP.
Among nonpharmacological treatments, spinal manipulative therapy (SMT) is largely sought by patients with NP symptoms related to lumbar spine pain. [5, 6] SMT is embedded in international clinical guidelines as part of the nonpharmacological care package for the management of adults with low back pain with NP symptoms. [7, 8] SMT is often used in chiropractic care. [9] As a core therapeutic approach, chiropractic care embraces spinal manipulation, either manually or with the use of an instrument (e.g., Impulse® Adjusting Instrument, Neuromechanical Innovations, Phoenix, AZ, USA). [10] This device is an electromechanical instrument that produces shockwave pulses when triggered and is used for the management of musculoskeletal disorders. [10–13] Impulse® produces a controlled average peak force of 200 N, depending on its force setting (low, medium, or high), delivered in less than 5 ms with a driving frequency of 126 Hz. [10, 12, 13]
The mechanism of action of spinal mechanical shockwave pulse therapy on pain modulation has been suggested to involve stimulation of mechanoreceptors located at the spine and surrounding tissues, triggering a gate-control response at the segmental level in the spinal cord and dorsal root ganglia (DRG). [10, 14] Indeed, clinical and preclinical studies using instrument-assisted SMT observed improvement of pain and function outcomes in populations with spine-related pain [15, 16], improvement of secondary mechanical hyperalgesia and modulation of oxidative stress markers in the blood of rats after hind limb immobilization [17], and inhibition of interleukin-1β and tumor necrosis factor-α levels in the DRG and the spinal cord in an animal model of NP. [14] Despite the benefits of the instrument-assisted SMT in pain management, its underlying mechanisms remain to be further elucidated.
Oxidative stress may be defined as an excessive amount of reactive oxygen species (ROS), which is the net result of an imbalance between the production and destruction of these species. [18] ROS are generated as part of normal cell metabolism and serve both normal physiological and pathophysiological functions. Preclinical studies of NP observed that superoxide anion (one of the ROS) plays a role in mediating long-term potentiation in excitatory neurons and long-term depression in inhibitory GABAergic interneurons of the spinal cord. [19] Hydrogen peroxide (another ROS) appears to enhance the frequency of action potentials in primary neurons involved in NP. [20] Thiols, which are organic compounds that contain a sulfhydryl group and are important in the cell’s protection against oxidative stress [21], are reduced in the sciatic nerve and spinal cord in sciatic nerve–induced NP. [22, 23] Interestingly, attenuation of oxidative stress in the spinal cord and sciatic nerve contributed to ameliorating NP symptoms in rats. [24, 25]
Despite the alteration of oxidative stress in the spinal cord and sciatic nerve in NP, and the capability of SMT to modulate oxidative stress, no study to date has explored the direct relationship between SMT and oxidative stress biomarkers in the spinal cord and sciatic nerve in NP. Thus, the present study aimed to determine whether Impulse®-assisted SMT (ISMT) reduces NP symptoms and modulates oxidative stress biomarkers in the spinal cords and sciatic nerves of rats with chronic constriction injury (CCI) of sciatic nerve–induced NP, a commonly used animal model of NP. [26] We hypothesized that ISMT would modulate oxidative stress markers in the spinal cord and sciatic nerve in parallel with an antinociceptive effect. Understanding the relationship between ISMT and biomarkers of oxidative stress may add important insight into the underlying mechanisms contributing to the benefits of ISMT management of NP.
Methods
Animals
Experiments were performed on a total of 36 three-month-old male Wistar rats, with body weights between 200 and 250 g. All experiments were approved by the Ethics Committee for Animal Experimentation of the Universidade Federal do Rio Grande do Sul (CEUA-UFRGS #32773). Rats were divided into three groups: naive (rats that did not undergo surgical manipulation), sham (rats that underwent all surgical procedures involved in CCI except the ligature), and CCI (rats in which four ligatures were tied loosely around the right common sciatic nerve). Each group was further divided into two subgroups (n = 6/subgroup), one that received ISMT and one that did not. Then, the groups were as follows: naive, naive+ISMT, sham, sham+ISMT, CCI, and CCI+ISMT. All rats were housed in a room with a stable temperature of 23 ± 1°C and fed ad libitum with a regular pellet diet. Rats were acclimated for at least 1 week before starting the study.
ISMT
The treatment was carried out with the Impulse® Adjusting Instrument (U.S. Food and Drug Administration approval #K023462, Neuromechanical Innovations, Phoenix, AZ, USA). This is a handheld electrically assisted mechanical chiropractic instrument that has been used clinically as an alternative to manually maneuvering a person’s spine. Impulse® is a microprocessor-controlled electromechanical adjusting instrument that delivers a shockwave impulse with a faster force rate, lower amplitude, and shorter duration than those of manually applied SMT. [10, 13] It is equipped with a force adjustment switch that allows for the selection of three force settings (I, low; II, medium; III, high), thereby controlling the peak force given (ranging from 132.5 to 380.2 N). [13] A recent report studying the biomechanical characteristics of the Impulse® on analogous/model spine tissue observed that ISMT delivers a shockwave pulse width of 4.08 ms, a velocity of 1.22 m/s, a plunger travel of 1.24 mm, and a peak force of 129 N with a driving frequency of 126 Hz at its maximum force setting. [10] For the present study, Impulse® was adjusted by the manufacturer to develop a minor peak force suitable to rodent studies. No additional changes were made to the Impulse® instrument, and external and internal features similar to the device used in the clinic and approved by the U.S. Food and Drug Administration were maintained. The ISMT intervention was conducted according to previous preclinical studies using other types of instrument-assisted SMT. [17, 27]
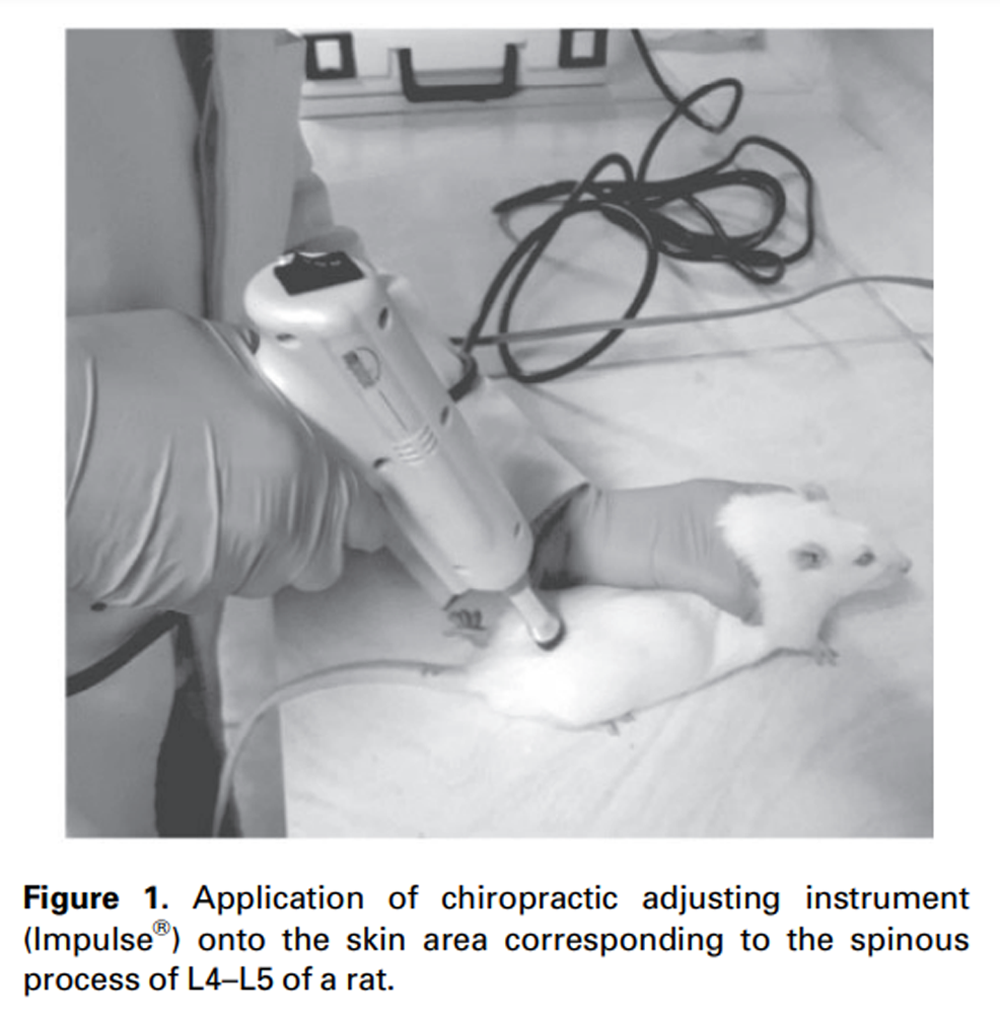
Figure 1 Impulse® was applied onto the skin area overlaying the spinous process of L4–L5 at a rostral direction at an angle of approximately 40° to 50° to the vertebral horizontal line. [17, 27] The ISMT started 72 hours after surgery to avoid manipulation immediately after surgery. The ISMT was consistently applied to the same region for 2 weeks, three times per week, at force setting III (Figure 1). The treatment was consistently applied at the same time (5:00 PM) by the same researcher. Each treatment session consisted of one single application of the ISMT per animal. The entire procedure lasted 1–2 minutes, including 1) animal handling, 2) localization of the target location (skin area corresponding to the L4–L5 spinous process), 3) instrument placement and angulation, 4) ISMT application, and 5) returning the animal to its cage. Rats were not anesthetized for the intervention [17], and no signs of discomfort in response to treatment were observed. The treatment protocol was based on a clinical study showing beneficial effects of manual SMT on pain and blood inflammatory biomarkers in patients with chronic low back pain. [28]
Determination of the Impulse® Peak Force and Pressure
Given that a rat has a mass approximately 350× less than that of a human weighing 70 kg [29] and that the Impulse® is a device for clinical use, the chiropractic device was adjusted by the manufacturer (Neuromechanical Innovations, Phoenix, AZ, USA) to deliver a body size–scaled force that would be more suitable for the rat spine. To estimate the pressure being delivered to the rat spine, we measured the peak force value of the device when operated by an investigator using an S-type load cell with 30-kgf capacity, as described by Duarte et al. [29] Similar to Duarte et al. [29], we determined peak force with the Impulse® in each of its force settings (I, low; II, medium; III, high). The test was repeated five times. Data were collected by a 16-bit analog converter (USB1608HS2AO, Measurement Computing, Norton, MA, USA) connected to a signal conditioner with five inputs (manufactured by GMAP, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil), linked to a current amplifier (Model TL074, Texas Instruments Inc., Dallas, TX, USA) and a computer. Data were analyzed with Agilent software (UEE Pro 7.5, Agilent Technology, Santa Clara, CA, USA), and 50,000 points per second were collected in each test. [29] From peak force data, the pressure values were calculated as pressure=peak force value / area of Impulse® tip.
Induction of CCI of the Sciatic Nerve
CCI of the sciatic nerve was induced as described by Bennett and Xie [30], with some modifications. [22] Briefly, a surgical procedure was performed under anesthesia (90 mg/kg ketamine and 10 mg/kg xylazine, intraperitoneally). For CCI, the right sciatic nerve was exposed at mid-thigh level, and four ligatures (4.0 chromic catgut, Shalon Fios Cirúrgicos Ltda., São Luis de Montes Belos, GO, Brazil) were placed around it with a 1.0- to 1.5-mm interval between each ligature. After the muscle and skin layer was sutured, a topical antibiotic (Ryfamicin SV 10 mg/mL, Sanofi-Aventis Farmacêutica Ltda, Brazil) was applied to the wound, and each rat received oral aspirin (acetylsalicylic acid, 100 mg/kg). Sham rats had the sciatic nerve exposed but did not receive ligatures. All rats made an uneventful recovery after surgery. After regaining consciousness, rats returned to their cages and were maintained in the same conditions previously described. These animals were checked daily by the same researcher.
Nociceptive Behavioral Assessments
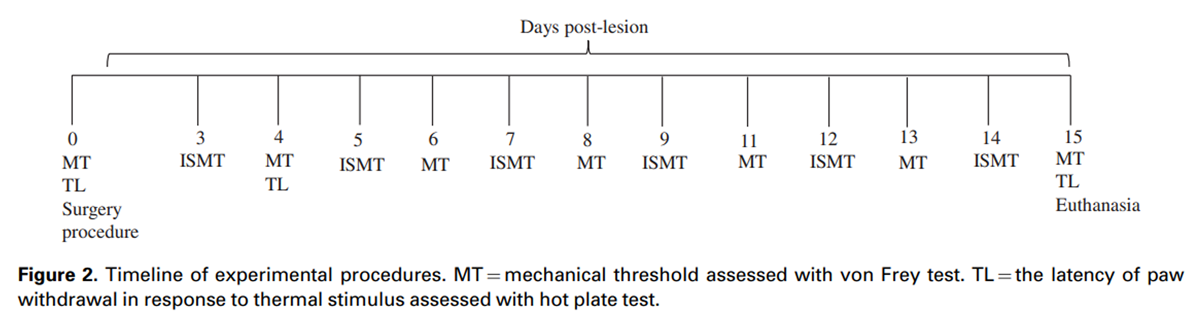
Figure 2 NP nociceptive changes were assessed with electronic von Frey and hot plate tests, which assessed mechanical threshold and latency to paw withdrawal in response to thermal stimulation, respectively. [24] The mechanical threshold was assessed in all groups with an electronic von Frey apparatus (Insight, Brazil) before surgery (day 0) and at days 4, 6, 8, 11, 13, and 15 after surgery, always at 7:00 AM. A single trial consisted of five applications of the plastic tip, once every 30 s, under the hind paw of the ipsilateral side of the CCI. A positive response was defined as the reflex withdrawal of the paw. The mean of five readings was taken as the threshold for a particular trial in each rat. Data were log-transformed for analyses. [31] The latency to paw withdrawal in response to the thermal stimulus was assessed in all groups before the surgery (day 0) and at days 4 and 15 after surgery, always at 8:00 AM. The withdrawal response to a heat stimulus was measured by placing the rats on the hot plate maintained at 50°C, and withdrawal latency (in seconds) was recorded. A positive withdrawal response was determined by observation of the rat’s behavior of jumping or licking the hind paw, regardless of the side. A cutoff time of 30 s was used to prevent tissue injury. All animals were tested by the same researcher in a room with a stable temperature of 23 ± 1°C. A timeline of the experimental procedures is provided in Figure 2.
Sample Preparation
Approximately 18 hours after the end of the last intervention, animals were euthanized by decapitation. The whole lumbosacral spinal cord and a segment (~7 mm) of the proximal portion of the sciatic nerve (superior to the ligatures) ipsilateral to the surgery were collected. This was done to offer insights into the general changes of the spinal cord and ipsilateral nerve. The spinal cord was divided transversely into three parts. The same portion always received the same preparation. Two parts of the spinal cord were immediately cooled in liquid nitrogen and processed to determine superoxide anion generation (SAG) and H2O2 levels. The third part was homogenized in 1.15% KCl diluted 1:5 (w/v) containing 1 mmol/L phenylmethylsulfonyl fluoride and was centrifuged at 1,000 g for 20 min at 4°C, and the supernatant was used for assays of lipid hydroperoxide levels, total antioxidant capacity (TAC), and total thiol content, as described previously. [22] The sciatic nerve received similar homogenization and preparation. The resulting supernatant was used for assays of lipid hydroperoxide levels and TAC, as described previously. [32]
Estimation of SAG
The lumbosacral spinal cord SAG was estimated with nitroblue tetrazolium to form formazan as an index of SAG, as described by Wang et al. [33] with some modification. [22] Absorbance was read at 540 nm. The SAG was determined as follows: SAG = A × V/(T×Wt × ε× l), where A is the absorbance of blue formazan at 540 nm, V is the volume of the solution, T is the period (90 min) during which the sections were incubated with nitroblue tetrazolium, Wt is the blotted wet weight of the spinal cord portion, ε is the extinction coefficient of blue formazan (i.e., 0.72 L/mmol/mm), and l is the length of the light path. Results are reported as reduced nitroblue tetrazolium mM/min/mg tissue.
Determination of H2O2
The lumbosacral spinal cord H2O2 levels were estimated with horseradish peroxidase, as described by Pick and Keisari [34] with some modification. [35] Absorbance was read at 610 nm. The results are reported as µM H2O2/g tissue.
Determination of Lipid Hydroperoxide Levels
Lipid hydroperoxides were measured in the sciatic nerve and lumbosacral spinal cord with xylenol orange dye, as described by Jiang et al. [36] and used in our previous studies. [22, 32] Absorbance was read at 560 nm. The assay was replicated three times. Results are reported as nM/g tissue.
Determination of TAC
TAC was determined in the sciatic nerve and lumbosacral spinal cord with 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical cation, as described by Erel [37] and used in our previous studies. [22, 32] Absorbance was read at 660 nm. The assay was replicated three times. Results are reported in µM eq trolox/g tissue.
Determination of Total Thiol Levels
Total thiol content was determined in the lumbosacral spinal cord with 5,5'-ditiobis (2-nitrobenzoic) acid, as described by Aksenov and Markesbery [38] and used in our previous studies. [22, 35] Absorbance was read at 412 nm. The assay was replicated three times. Results are reported as mM/mg tissue.
Statistical Analysis
Data are presented as mean ± standard error mean (SEM), except for peak force and pressure. ISMT peak force and pressure between force settings (I, II, and III) were analyzed by one-way analysis of variance (ANOVA) followed by Dunnet’s multiple variation test. Results of behavioral tests and oxidative stress biomarkers were analyzed with two-way ANOVA (factors: surgery and treatment) followed by the Tukey test. Differences were considered statistically significant when P was <0.05. Statistical analyses were carried out with SigmaPlot version 11.0 software (Systat Software Inc., Chicago, IL, USA).
Results
All animals maintained their body mass at every time point after sham or CCI surgery interventions. Autotomy was not present in the CCI groups.
Peak Force and Pressure Values

Table 1

Table 2
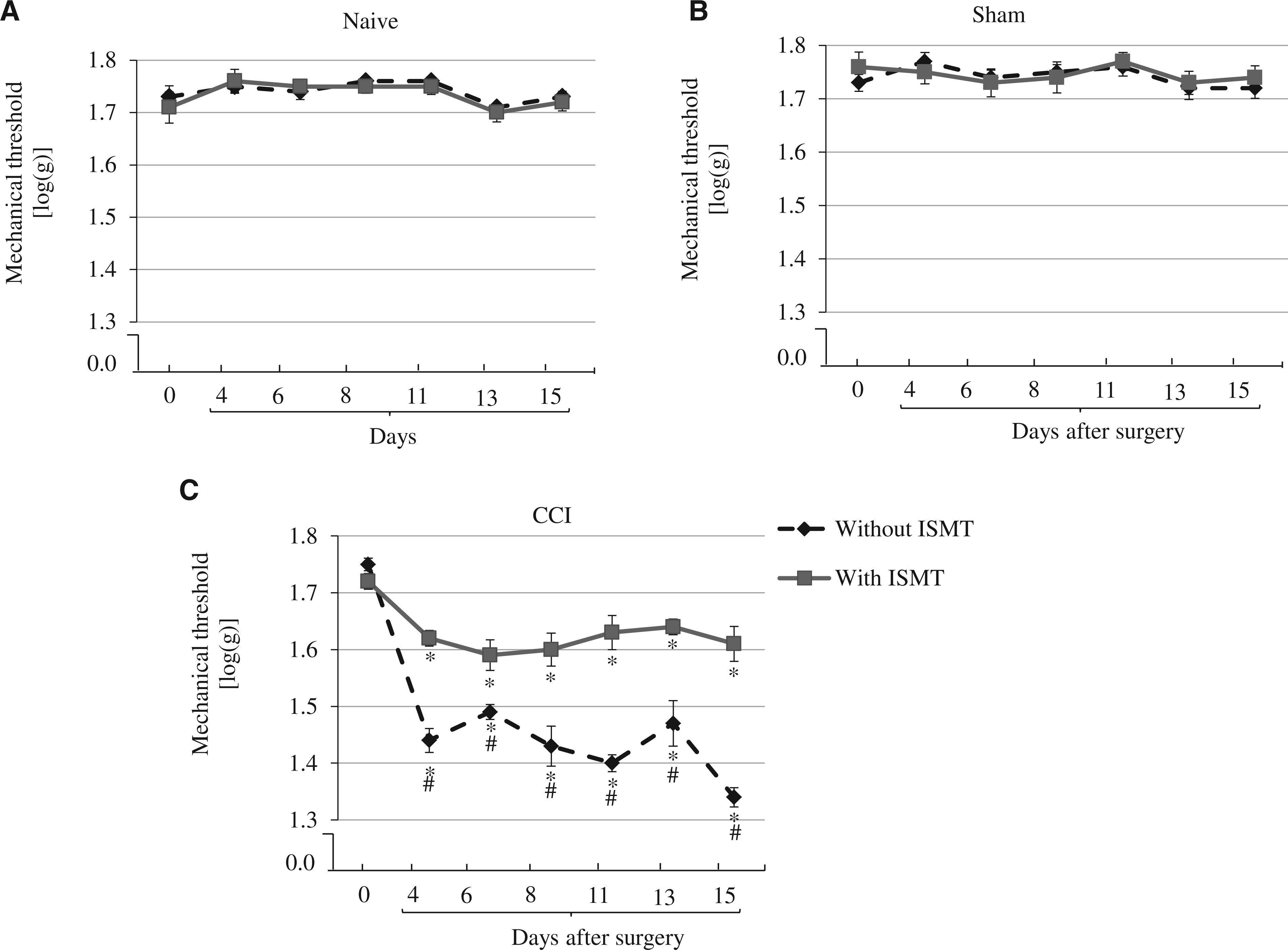
Figure 3
page 6
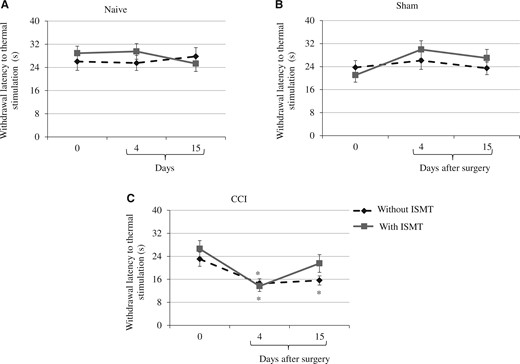
Figure 4
page 7The mean and standard deviation of the mean of the peak force and pressure of the ISMT at each force setting are listed in Table 1. There was a significant increase of peak force values when the force setting was set up to medium and high (settings II and III) compared with setting I (P = 0.010 and 0.001, respectively). There was also a difference between settings II and III, (P = 0.049). With regard to the pressure applied by the ISMT, there was a significant increase of pressure values when the force setting was set up to medium and high (settings II and III) compared with setting I (P < 0.001 and 0.001, respectively). No difference was seen between settings II and III (P = 0.051). Mean differences and 95% confidence intervals (CIs) are presented for all possible comparisons between force settings in Table 2.
Nociceptive Behavioral Assessments
There was a main effect of CCI on mechanical threshold between groups (F2,53 = 210.567, P < 0.001), a main effect of treatment (F2,53 = 10.588, P = 0.001), and an interaction between lesion and treatment on mechanical threshold (F2,53 = 6.826, P < 0.001). The post hoc test revealed that although the mechanical threshold did not exhibit any significant change in naive (1.7 ± 0.02) and sham (1.7 ± 0.02) rats over time (Figure 3A, 3B), this threshold significantly (P < 0.001) decreased in CCI rats (Figure 3C). The mechanical threshold was decreased at all time points: 4 (1.4 ± 0.01), 6 (1.5 ± 0.02), 8 (1.4 ± 0.03), 11 (1.4 ± 0.01), 13 (1.5 ± 0.04), and 15 (1.3 ± 0.02). In contrast, ISMT attenuated the decrease of mechanical threshold after CCI. Relative to CCI, CCI+ISMT rats had an increased mechanical threshold at all assessed times points: 4 days (1.6 ± 0.01), 6 days (1.6 ± 0.03), 8 days (1.6 ± 0.03), 11 days (1.6 ± 0.03), 13 days (1.6 ± 0.01), and 15 days (1.6 ± 0.03). Therefore, the ISMT attenuated the decrease in mechanical threshold by approximately 53% compared with CCI rats that did not receive the therapy.
There was a main effect of lesion on thermal withdrawal latency between groups (F2,53 = 7.224, P < 0.001), but no treatment effect (F2,53 = 0.739, P = 0.482) was observed. However, a significant interaction between lesion and treatment (F2,53 = 2.925, P = 0.005) was observed. The post hoc test revealed that the thermal withdrawal latency did not change in naive (27.2 ± 2.7) and sham (25.2 ± 2.7) rats (Figure 4A, 4B). However, the latency decreased in CCI rats (Figure 4C). On day 4, the thermal withdrawal latency was 13.6 ± 2.2 and 14.5 ± 2.4 in CCI+ISMT and CCI, respectively. On day 15, although no recovery was found in the withdrawal responses of CCI (15.6 ± 2.4), an increase was observed in CCI+ISMT (21.5 ± 2.4) rats compared with the pre-nerve lesion. Therefore, the ISMT attenuated the decrease in thermal withdrawal latency by approximately 38% (day 15) compared with CCI rats that did not receive the therapy.
Oxidative Stress Markers in the Sciatic Nerve
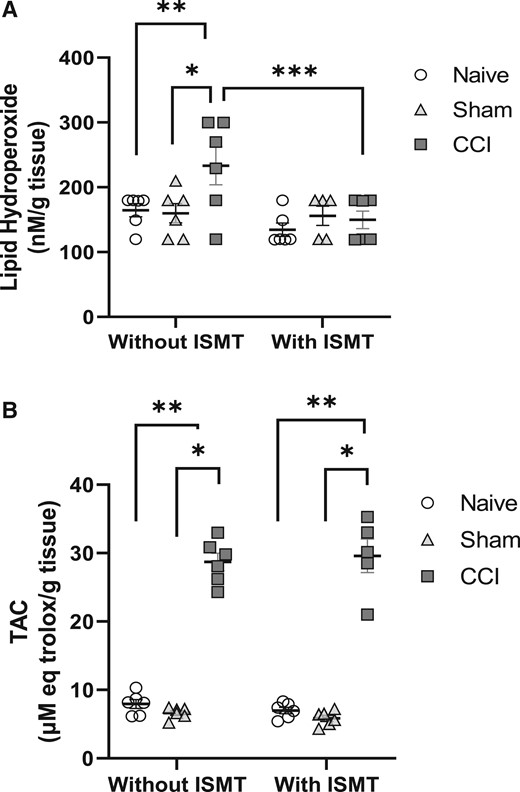
Figure 5
page 8
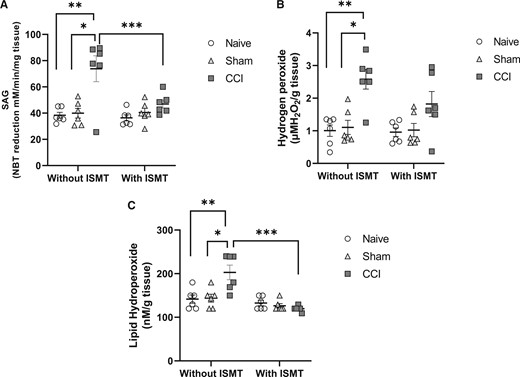
Figure 6
page 9A main effect of surgery (F2,53 = 3.484, P = 0.044) and treatment (F2,53 = 7.985, P = 0.008) in lipid hydroperoxide levels in the sciatic nerve was observed, with no interaction effect between factors (F2,53 = 2.836, P = 0.075) (Figure 5A). Post hoc testing revealed that the lipid hydroperoxide levels increased in the sciatic nerves of the CCI rats (233.9 ± 20.4) compared with the naive (149.9 ± 22.8, P = 0.012) and sham (157.9 ± 19.5, P = 0.035) groups. In contrast, no increase of lipid hydroperoxides was observed in the CCI+ISMT group (149.9 ± 22.8), whereas the lipid hydroperoxide values were comparable to those in the sham (P = 0.979) and naive (P = 0.888) groups. Statistical difference between the CCI and CCI+ISMT groups was observed (P = 0.015).
In addition, there was a main effect of surgery on TAC levels in the sciatic nerve (F2,53 = 807.677, P < 0.001), but no treatment effect was observed (F2,53 = 0.483, P = 0.497), and there was no interaction effect between lesion and treatment (F2,53 = 1.317, P = 0.296) (Figure 5B). Post hoc testing revealed that TAC increased in the sciatic nerves of the CCI group (28.7 ± 0.6) compared with the naive (7.5 ± 0.6, P < 0.001) and sham (6.3 ± 0.3, P < 0.001) groups. TAC also increased in the sciatic nerves of the CCI+ISMT group (29.5 ± 0.7, P < 0.001) compared with the naive and sham groups.
Pro-Oxidative Markers in the Spinal Cord
There was a main effect of surgery on SAG levels in the spinal cord between groups (F2,53 = 30.667, P < 0.001), a main effect of treatment (F2,53 = 13.147, P = 0.002), and an interaction effect between lesion and treatment on SAG levels (F2,53 = 13.807, P < 0.001) (Figure 6A). Post hoc testing showed that the levels of SAG significantly increased in the spinal cords of CCI rats (73.9 ± 3.2, P < 0.001) compared with naive (37.9 ± 2.9) and sham rats (38.4 ± 3.2), contrasting with CCI+ISMT (45.8 ± 3.2), which prevented the increase of SAG in the spinal cord, bringing SAG to values comparable to naive (P = 0.155) and sham (P = 0.484) rats. Also, there was a significant difference between CCI and CCI+ISMT (P = 0.006).
We also observed a main effect of surgery (F2,53 = 10.511, P < 0.001) and treatment (F2,53 = 4.809, P = 0.043), with a significant interaction effect between them (F2,53 = 7.489, P = 0.005) on the spinal cord H2O2 levels (Figure 6B). Post hoc testing revealed that H2O2 levels significantly increased in the spinal cords of CCI rats (2.5 ± 0.3, P < 0.001) compared with the naive (1.0 ± 0.3) and sham (1.1 ± 0.2) groups. In contrast, no significant increase of H2O2 levels was observed in the CCI+ISMT group (1.8 ± 0.3) compared with the naive (P = 0.855) and sham (P = 0451) groups. Also, there was no significant difference between CCI and CCI+ISMT (P = 0.292).
When lipid hydroperoxide levels in the spinal cord were assessed, no main effect of the surgery was found between groups (F2,53 = 3.299, P = 0.056) (Figure 6C). In contrast, a significant difference was found for treatment (F2,53 = 16.201, P < 0.001), with a significant interaction effect between surgery and treatment (F2,53 = 6.034, P = 0.008). The post hoc testing revealed a significant increase in the lipid hydroperoxide levels in the spinal cord of CCI rats (202.5 ± 12.2) compared with the naive (137.2 ± 11.4, P = 0.06) and sham (134.9 ± 10.9, P = 0.006) groups. No statistical difference was observed between the CCI+ISMT (119.9 ± 10.8) and naive and sham groups. Also, there was a significant difference between CCI and CCI+ISMT (P < 0.001).
Antioxidant Markers in the Spinal Cord
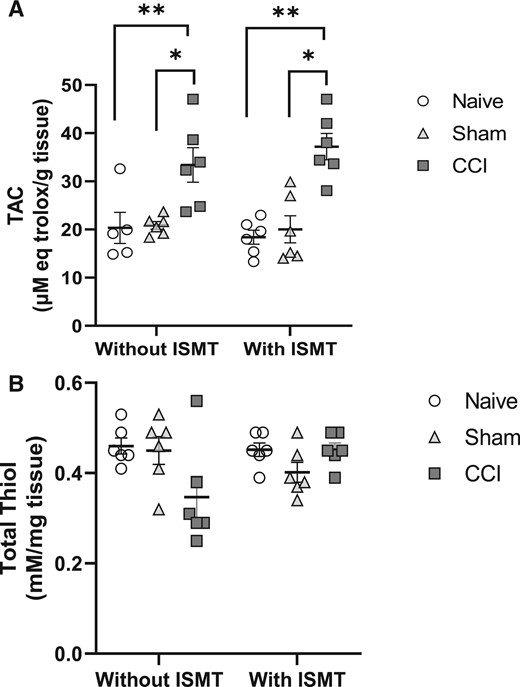
Figure 7
page 10In the spinal cord, a significant main effect of surgery between groups was observed for TAC level (F2,53 = 20.092, P < 0.001), but neither a treatment effect (F2,53 = 0.0586, P = 0.810) nor an interaction effect between lesion and treatment (F2,53 = 1.049, P = 0.364) was observed (Figure 7A). Despite the increase of TAC levels in both the CCI+ISMT (37.1 ± 2.7) and CCI (33.4 ± 2.7) groups compared with the sham (19.6 ± 3.3, P = 0.001 and P = 0.008, respectively) and naive (19.7 ± 2.8, P < 0.001 and P = 0.015, respectively) groups, no statistical difference between the CCI and CCI+ISMT groups was observed (P = 0.343).
There was no effect of surgery between groups (F2,53 = 1.442, P = 0.262) or for treatment (F2,53 = 0.184, P = 0.673); no interaction between lesion and treatment (F2,53 = 3.258, P = 0.062) was observed on total thiol levels in the spinal cord (Figure 7B). Total thiol content of the spinal cord was as follows: naive (0.5 ± 0.03), naive+ISMT (0.4 ± 0.03), sham (0.4 ± 0.03), sham+ISMT (0.4 ± 0.02), CCI (0.3 ± 0.02), and CCI+ISMT (0.4 ± 0.03).
Discussion
The present study demonstrated for the first time that Impulse®-assisted SMT (ISMT) increases the mechanical threshold of rats exposed to NP. ISMT also attenuated the increase of lipid hydroperoxides in the sciatic nerve and spinal cord in addition to diminishing the increase of SAG and H2O2 in the spinal cord after CCI-induced NP. These findings support our study hypothesis that ISMT would provide an antinociceptive effect and modulate oxidative stress markers in the peripheral and central nervous system in a rat model of NP.
A growing number of preclinical SMT studies are improving our understanding of the functional effects and molecular mechanisms involved in SMT. The decrease of the mechanical threshold of the plantar aspect of the hind paw and the diminished paw withdrawal latency in response to thermal stimulation that we observed after CCI is in line with previous studies. [22, 32, 35] We observed that lumbar ISMT—performed three times per week for 2 weeks—promoted a protective effect in rats undergoing CCI-induced NP, increasing the hind paw mechanical threshold. Previous studies using other types of instrument-assisted SMT observed a similar protective effect on secondary mechanical hyperalgesia after hind limb immobilization–induced pain hypersensitivity and intervertebral foramen inflammation and chronic compression of the DRG-induced NP. [14, 17, 29] Von Frey testing is widely used as a way to measure the mechanical threshold in clinical and preclinical studies of NP and is useful in determining the presence of cutaneous hyperalgesia (an increase in the pain elicited by a noxious stimulus) or allodynia (pain evoked by a normally innocuous stimulus). [39] CCI leads to the manifestation of secondary hyperalgesia, a hallmark of central sensitization. [40] This phenomenon in the CCI-induced NP model is thought to be caused by an abnormal barrage of neuron discharge, either in the peripheral nerve or at the DRG level, leading to sensitization of central neurons in the spinal cord. [40] Our findings of an increased mechanical threshold in the CCI+ISMT group relative to the CCI group suggests attenuation in nociceptive response and consequently modulation of the function of the somatosensorial system. Furthermore, the act of choosing which paw to lift to avoid the thermal stimulus in hot plate testing is believed to require supraspinal structures. [39] This suggests that the hot plate test goes beyond a spinal cord–mediated reflex, requiring integration between supraspinal and spinal cord structures. [39]Thus, ISMT may exert a beneficial effect at both the spinal and supraspinal levels. Further studies on the possible role of the ISMT on spinal circuitry and supraspinal areas are recommended.
ROS are important in the development of NP symptoms. [41] Deficiency in the ROS-producing enzyme NOX4 led to an attenuation of mechanical hypersensitivity induced by CCI. [42] The development of mechanical hyperalgesia appears to be related to the levels of superoxide anion and hydroxyl radicals in the spinal cords of mice with NP. [19] In agreement with previous studies using a similar animal model of NP [22, 32, 35], we observed elevated levels of SAG and H2O2 in the spinal cords of the CCI group, while ISMT prevented their increase. A previous study showed attenuation in the mechanical threshold parallel to a decrease in SAG in the spinal cord of CCI rats. [22] Data from the present study showing attenuated SAG and H2O2 concomitant with attenuated hypersensitivity to mechanical and thermal nociception suggest that the decrease in SAG and H2O2 levels may be contributing to the antinociceptive effect of ISMT.
Increased pro-oxidant markers, such as superoxide anions and H2O2, may be related to the increased lipid hydroperoxide levels that we observed in the injured sciatic nerve and the spinal cord. Lipid hydroperoxides are an early intermediate in the lipid peroxidation process. [43] As ROS concentration is lowered by antioxidants [18], increased pro-oxidant markers may be related to an increase in TAC in the sciatic nerves and the spinal cords of CCI rats. TAC reflects total antioxidant system function. [44] However, TAC increased similarly in the nerves and spinal cords of CCI and CCI+ISMT rats, despite ROS levels in CCI+ISMT being similar to those in naive rats. There are some spectrophotometric methods to evaluate the TAC of a sample. [45] The Trolox equivalent antioxidant capacity assay measures mainly non-enzymatic components of the antioxidant system, such as albumin, uric acid, ascorbic acid, α-tocopherol, and bilirubin. [45] A series of clinical and preclinical studies with SMT observed that a course of treatment with manual SMT increased enzymatic antioxidant levels, such as superoxide dismutase and glutathione peroxidase activities, in the red blood cells of patients with chronic back or neck pain. [46] In addition, instrument-assisted SMT (Activator 4) increased glutathione peroxidase activity in the red blood cells of rats that underwent hind limb immobilization–induced pain hypersensitivity. [17] Taken together, these results suggest that manual SMT and instrument-assisted SMT increase the activity of different antioxidant systems in the systemic circulation. Here, CCI elevated antioxidant capacity in nervous tissue (and in a NP model), but there was no effect of SMT; thus, the different results may be related to the examined tissue or the presence of an NP condition. Nevertheless, we analyzed TAC using only one assay. It is recommended that various assays should be used for TAC evaluation. [45] Thus, further research is needed to better understand the relationship between the antioxidants and SMT on the backdrop of different pain conditions.
Thiols are important in the cell’s protection against oxidative stress. [21] However, no significant change occurred in total thiol content in the spinal cord of CCI+ISMT rats compared with CCI rats. This result suggests that ISMT did not alter total thiol content in the spinal cord of CCI rats. However, total thiol content was lower in the spinal cords of CCI (0.3 ± 0.02) rats than in those of CCI+ISMT (0.4 ± 0.03) rats, and this last value was similar to that found in naive (0.45 ± 0.03) rats. Thus, we cannot exclude the hypothesis that ISMT plays a beneficial effect on thiol groups in the spinal cord after CCI-induced NP.
Although our findings point to the modulation of pro-oxidant and antioxidant biomarkers in the spinal cords and sciatic nerves of CCI+ISMT rats as an underlying factor in the amelioration of nociception, we cannot exclude the contribution of other factors. A recent study using a spinal nerve ligation mouse model suggested that mechanical hyperalgesia is the result of the combination of long-term potentiation in excitatory spinothalamic neurons and long-term depression in inhibitory GABAergic interneurons of the spinal cord. [19] Thus, the increase in mechanical threshold may be related to the effect of ISMT on GABAergic neurons of the spinal cord. Skyba et al. [47] showed that joint mobilization produced its antinociceptive effects through a non-opioid system in a model of capsaicin-induced central sensitization. Joint mobilization and SMT have distinct mechanical characteristics (e.g., peak force, pre-load, and thrust) and therefore likely work throughout different systems, with joint mobilization likely affecting more superficial axial muscles and SMT stimulating receptors within deep intervertebral muscles. [48] Nevertheless, joint mobilization and SMT may also share common antinociceptive modulatory pathways. Supraspinal suppression of the spontaneous activity of thalamic nociceptive neurons has been recently reported after a single SMT in Wistar rats. [49] Instrument-assisted SMT ameliorated NP symptoms through a mechanism mediated by interleukin-10 decreasing inflammatory factors and leading to DRG neuron excitability recovery. [14, 29] The interleukin-10/miR-155 pathway appears to have an important role in regulating NOX2, a critical inducer of both extracellular and intracellular ROS in microglia/macrophages. [50] Thus, the ameliorative effect of ISMT on pain outcomes may be related to changes in interleukin-10, which might be contributing to a decrease in pro-oxidant biomarkers in nervous tissues after SMT.
Oxidative stress can activate a variety of transcription factors, leading to the activation of several inflammatory genes. [51] In chemotherapy-induced neuropathy, it has been observed that a complex cascade of events involving endothelial cell activation and infiltration of monocytes/macrophages expressing CX3C chemokine receptor 1 at the sciatic nerve leads to increased ROS and sensitization of sensory neurons expressing TRPA1 channels, thus evoking a pain response. [52] Activation of Schwann cell TRPA1 channels may generate a spatially constrained gradient of oxidative stress that maintains macrophage infiltration to the injured sciatic nerve and increases the traffic of paracrine signals to activate TRPA1 of unsheathed nociceptors, sustaining mechanical allodynia. [25] Thus, the beneficial effect of ISMT could involve a complex interplay between ROS and multiple cell types (immune cells, endothelial cells, and/or neural cells) in both the peripheral and central nervous system; further studies are required to address the impact of ISMT on these mechanisms and their role in NP.
There are some limitations of our present study. First, we used the whole lumbosacral spinal cord for oxidative stress analysis. It has been reported that functional changes in the gene expression of factors related to pain inhibition between the ipsilateral and contralateral spinal cord, as well as between the dorsal and ventral horns of the spinal cord, may exist after unilateral neuropathy. [53] Thus, we need to consider that the oxidative changes of the ipsilateral side of the spinal cord may be diluted by the contralateral side.
Second, as TAC results may vary depending on the assay performed [45], further studies should use a combination of TAC assays and analysis of other specific antioxidants. Third, there are several sources of ROS that were not examined. For example, it has been shown that NOX4, an enzymatic source of ROS, is involved in the development of NP states by producing oxidative stress and subsequent cytokine dysregulation at the lesion site. [42] Future studies may want to examine this as a source of ROS production, the impact on pain, and the effect of SMT.
Fourth, although the peak force developed by the spinal manipulation device after manufacturer adjustment was reduced to ~20 N to suit the rat model, preclinical setup peak force was not fully scaled to the rat body weight. Given the rat body mass (250 g) and the peak force applied, animals received approximately 100 times greater force than their body weight. Although previous studies have shown the validity of scaling the peak force to animal body mass, the determination of the ideal scaling parameters, the effects of tissue compliance (stiffness), and the response to input force (impedance) in rats are not clear. [54, 55]
Fifth, because ISMT was applied to the target area with its original probe and rubber end tip, we need to consider that this interface (probe end + treatment location) of contact with the rodent’s spine may be large for such a small animal compared with humans. Further studies are necessary to clarify this aspect. Sixth, the shockwave profile of the adapted device was not assessed. Although the approximation of a half sine wave with the thrust curves was 83% for Impulse®, and this wave was consistent across settings and tissue compliance [10], further studies are necessary to show the shockwave profile of animal model–adapted Impulse®.
Seventh, the oxidative stress markers were assessed only 15 days after ISMT. There is a need to assess these markers at earlier and longer time points to determine the temporal effect of ISMT. Eighth, we did not assess the types of afferent fibers activated by ISMT. It has been proposed that SMT activates peripheral afferent fibers, which initiate a cascade of peripheral and central neurophysiological effects. [56]
In acupuncture, the intensity, frequency, duration, and interval between stimuli directly influence the kind of afferent nerve fibers activated; excitation of Aβ (group II) and some Aδ fibers (group III) is involved in electroacupuncture-induced analgesia. [57] Aβ-fiber electrical stimulation modulated excitatory and inhibitory populations of superficial dorsal horn neurons, suggesting that Aβ stimulation at a segment adjacent to where the pain is located may improve analgesic efficacy. [58] Therefore, it is important to consider that ISMT-induced antinociception may be related to activation of afferent nerve fibers, specifically Aβ (group II) fibers, that may likely converge from various tissues and may likely be contributing to the antinociceptive effect seen in the present study. Despite these limitations, our results are important because they are the first to demonstrate the effect of ISMT on nervous tissue oxidative stress and thermal and mechanical hypersensitivity in a model of NP.
Conclusions
The present study demonstrates that Impulse®-assisted SMT (ISMT) induces antinociception in parallel with changes in oxidative stress markers in the spinal cords and the sciatic nerves of rats with CCI-induced NP. Given that ROS plays an important role in NP, modulation of the spinal cord and sciatic nerve oxidative stress markers may be physiological mechanisms underlying the effects of SMT in NP disorders. Overall, our findings add to the evidence supporting the potential role of SMT as a nonpharmacological therapeutic strategy to manage or treat NP.
Acknowledgments The authors thank Dr. Christopher J. Colloca for kindly providing the Impulse® (Neuromechanical Innovations, Phoenix, AZ, USA). We are also grateful to Dr. Daniel West for his valuable contribution in proofreading the manuscript.
Declarations
Funding sources: The study was supported by grants from the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Conflicts of interest: The authors declare that they have no conflicts of interest concerning this work.
References:
Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers 2017;3(1):17002.
Treed R-D, Rief W, Barke A, et al. Chronic pain as symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019;160(1):19–27.
Fernandes V, Sharma D, Vaidya S, et al. Cellular and molecular mechanisms driving neuropathic pain: Recent advancements and challenges. Expert Opin Ther Targets 2018;22:131–42.
Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19.
Rubinstein SM, de Zoeta A, van Middelkoop M, et al. Benefits and harms of spinal manipulative therapy for the treatment of chronic low back pain: Systematic review and meta-analysis of randomized controlled trials. BMJ 2019;364:1689.
Aspinall SL, Leboeuf-Yde C, Etherington SJ, Walker BF. Manipulation-induced hypoalgesia in musculoskeletal pain populations: A systematic critical review and meta-analysis. Chiropr Man Therap 2019;27:7.
Hawk C, Whalen W, Farabaugh R, et al. Best practices for chiropractic management of patients with chronic musculoskeletal pain: A clinical practice guideline. J Altern Complement Med 2020;26:884–901.
Bussières A, Stewart G, Al-Zoubi F, et al. Spinal manipulative therapy and other conservative treatments for low back pain: A guideline from the Canadian Chiropractic Guideline Initiative. J Manipulative Physiol Ther 2018;41:265–93.
Beliveau PJH, Wong JJ, Sutton DA, et al. The chiropractic profession: A scoping review of utilization rates, reasons for seeking care, patient profiles, and care provided. Chiropr Man Therap 2017;25:35.
Liebschner MA, Chun K, Kim N, et al.
In Vitro Biomechanical Evaluation of Single Impulse and
Repetitive Mechanical Shockwave Devices Utilized for
Spinal Manipulative Therapy
Annals of Biomedical Engineering 2014 (Dec); 42 (12): 2524–2536Neff SM, Okamoto CS. Chiropractic management of a patient with thoracic pain and a stable thoracic aortic aneurysm: A case report. J Chiropr Med 2017;16:78–82.
Keller TS, Colloca CJ, Moore RJ, Gunzburg R, Harrison DE. Increased multiaxial lumbar motion response during multiple-impulse mechanical force manually assisted spinal manipulation. Chriropr Osteopat 2006;14(1):6.
Colloca CJ, Keller TS, Black P, et al. Comparison of mechanical force of manually assisted chiropractic adjusting instruments. J Manipulative Physiol Ther 2005;28:414–22.
Song XJ, Huang ZJ, Song WB, et al. Attenuation effect of spinal manipulation on neuropathic and postoperative pain through activating endogenous anti-inflammatory cytokine interleukin 10 in rat spinal cord. J Manipulative Physiol Ther 2016;39:42–53.
Bishop MD, Beneciuk JM, George SZ. Immediate reduction in temporal sensory summation after thoracic spinal manipulation. Spine 2011;11:440–6.
Conesa-Buendía FM, Mediero A, Fujikawa R, et al. Beneficial effects of manually assisted chiropractic adjusting instrument in a rabbit model of osteoarthritis. Sci Rep 2020;10:13237.
Duarte FCK, Kolberg C, Riffel APK, et al. Spinal manipulation therapy improves tactile allodynia and peripheral nerve functionality and modulates blood oxidative stress marker in rats exposed to knee-joint immobilization. J Manipulative Physiol Ther 2019;42:385–98.
Poljsak B, Suput D, Milisav I. Achieving the balance between ROS and antioxidants: When use the synthetic antioxidants. Oxid Med Cell Longev 2013;2013:956792.
Bittar A, Jun J, La JH, et al. Reactive oxygen species affect spinal cell type-specific synaptic plasticity in a model of neuropathic pain. Pain 2017;158(11):2137–46.
Sözbir E, Naziroglu M. Diabetes enhances oxidative stress-induced TRPM2 channel activity and its control by N-acetylcysteine in rat dorsal root ganglion and brain. Metab Brain Dis 2016;31:385–93.
Binu P, Gifty K, Vineetha RC, et al. Eugenol, a plant-derived phenolic nutraceutical, protects thiol (SH) group in myocardium from ROS-mediated oxidation under chemotherapeutic stress induced by arsenic trioxide—a in vivo model study. Drug Chem Toxicol 2018;41:352–7.
Riffel APK, Santos MCQ, de Souza JA, et al. Treatment with ascorbic acid and α-tocopherol modulates oxidative-stress markers in the spinal cord of rats with neuropathic pain. Braz J Med Biol Res 2018;51:e7097.
Naik AK, Tandan SK, Dudhgaonka SP, et al. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur J Pain 2006;10:573–9.
Abbaszadeh A, Darabi S, Hasanvand A, et al. Minocycline through attenuation of oxidative stress and inflammatory response reduces the neuropathic pain in a rat model of chronic constriction injury. Iran J Basic Med Sci 2018;21:138–44.
De Logu F, Nassini R, Materazzi S, et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat Commun 2017;8:1887.
Kumar A, Kaur H, Singh A. Neuropathic pain models caused by damage to central or peripheral nervous system. Pharmacol Rep 2018;70(2):206–16.
Song, XJ, Gan, Q, Cao, J-L, Wang, Z-B, and Rupert, RL.
Spinal Manipulation Reduces Pain and Hyperalgesia After
Lumbar Intervertebral Foramen Inflammation in the Rat
J Manipulative Physiol Ther. 2006 (Jan); 29 (1): 5–13Teodorczyk-Injeyan JA, McGregor M, Triano JJ, Injeyan SH.
Elevated Production of Nociceptive CC-chemokines and sE-selectin
in Patients with Low Back Pain and the Effects of Spinal Manipulation:
A Non-randomized Clinical Trial
Clin J Pain. 2018 (Jan); 34 (1): 68–75Duarte FCK, Kolberg C, Barros RR. Evaluation of peak force of a manually operated chiropractic adjusting instrument with an adapter for use in animals. J Manipulative Physiol Ther 2014;37:236–41.
Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988;33(1):87–107.
Mills C, LeBlond D, Joshi S, et al. Estimating efficacy and drug ED50’s using von Frey thresholds: Impact of Weber’s law and log transformation. J Pain 2012;13:519–23.
Riffel AP, de Souza JA, Santos MC, et al. Systemic administration of vitamins C and E attenuates nociception induced by chronic constriction injury of the sciatic nerve in rats. Brain Res Bull 2016;121:169–77.
Wang HD, Pagano PJ, Du Y, et al. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res 1998;82:810–8.
Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods 1980;38:161–70.
Scheid T, Moraes MS, Henriques TP, et al. Effects of methanol fraction from leaves of Schinus terebinthifolius Raddi on nociception and spinal-cord oxidative biomarkers in rats with neuropathic pain. Evid Based Complement Alternat Med 2018;2018:5783412.
Jiang ZY, Woollard ACS, Wolff SP. Lipid hydroperoxide measurement by oxidation of Fe+2 in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 1991;26:853–6.
Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 2004;37(4):277–85.
Aksenov MY, Markesbery WR. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer's disease. Neurosci Lett 2001;302:141–5.
Gregory NS, Harris AL, Robinson CR, et al. An overview of animal models of pain: Disease models and outcome measures. J Pain 2013;14:1255–69.
Chen Q-Y, Tan C-Y, Wang Y, et al. Mechanism of persistent hyperalgesia in neuropathic pain caused by chronic constriction injury. Neural Regen Res 2019;14:1091–8.
Grace PM, Gaudet AD, Staikopoulos V, et al. Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci 2016;39:862–79.
Geis C, Geuss E, Sommer C, Schmidt HH, Kleinschnitz C. NOX4 is an early initiator of neuropathic pain. Exp Neurol 2017;288:94–103.
Dexter DT, Holley AE, Flitter WD, et al. Increased levels of lipid hydroperoxides in the Parkinsonian substantia nigra: An HPLC and ESR study. Mov Disord 1994;9:92–7.
Zhang G, Liu N, Zhu C, et al. Antinociceptive effect of isoorientin against neuropathic pain induced by the chronic constriction injury of the sciatic nerve in mice. Int Immunopharmacol 2019;75:105753.
Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Ceron JJ. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet Res 2016;12:166.
Kolberg C, Horst A, Moraes MS, et al. Peripheral oxidative stress blood markers in patients with chronic back or neck pain treated with high-velocity, low-amplitude manipulation. J Manipulative Physiol Ther 2015;38(2):119–29.
Skyba DA, Radhakrishnan R, Rohlwing JJ, Wright A, Sluka KA.
Joint Manipulation Reduces Hyperalgesia By Activation of Monoamine
Receptors But Not Opioid or GABA Receptors in the Spinal Cord
Pain. 2003 (Nov); 106 (1-2): 159–168Bolton P, Budgell BS. Spinal manipulation and spinal mobilization influence different axial sensory beds. Med Hypotheses 2006;66:258–62.
Reed WR, Pickar JG, Sozio RS, et al. Decreased spontaneous activity and altered evoked nociceptive response of rat thalamic submedius neurons to lumbar vertebra thrust. Exp Brain Res 2017;235(9):2883–92.
Sabirzhanov B, Li Y, Coll-Miro M, et al. Inhibition of NOX2 signaling limits pain-related behavior and improves motor function in male mice after spinal cord injury: Participation of IL-10/miR155 pathways. Brain Behav Immun 2019;80:73–87.
Hussain T, Tan B, Yin Y, et al. Oxidative stress and inflammation: What polyphenols can do for us? Oxid Med Cell Longev 2016;2016:7432797.
Malcangio M.
Role of the immune system in neuropathic pain.
Scand J Pain 2019;20:33–7.Kononenko O, Mityakina I, Galatenko V, et al.
Differential effects of left and right neuropathy on
opioid gene expression in lumbar spinal cord.
Brain Res 2018;1695:78–83.Reed WR, Pickar JG, Sozio RS, Liebschner MAK, Little JW, Gudavalli MR.
Characteristics of Paraspinal Muscle Spindle Response to
Mechanically Assisted Spinal Manipulation:
A Preliminary Report
J Manipulative Physiol Ther. 2017 (Jul); 40 (6): 371–380Reed, W. R., Cao, D. Y., Long, C. R., Kawchuk, G. N., & Pickar, J. G.
Relationship between Biomechanical Characteristics of Spinal Manipulation
and Neural Responses in an Animal Model: Effect of Linear Control
of Thrust Displacement versus Force, Thrust Amplitude,
Thrust Duration, and Thrust Rate
Evidence-Based Complementary and Alternative Medicine 2013 (Jan 20); 492039Pickar JG, Bolton PS.
Spinal Manipulative Therapy and Somatosensory Activation
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 785–794Zhao Z-Q.
Neural mechanism underlying acupuncture analgesia.
Prog Neurobiol 2008;85:355–75.Fan W, Sdrulla AD.
Differential modulation of excitatory and inhibitory populations of superficial
dorsal horn neurons in lumbar spinal cord by Aβ-fiber electrical stimulation.
Pain 2020;161:1650–60.
Return to INSTRUMENT ADJUSTING
Since 5-18-2021


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |