

Spinal Manipulation, Medication, or Home Exercise
With Advice for Acute and Subacute Neck Pain:
A Randomized TrialThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Annals of Internal Medicine 2012 (Jan 3); 156 (1 Pt 1): 1–10 ~ FULL TEXT
OPEN ACCESS Gert Bronfort, DC, PhD; Roni Evans, DC, MS; Alfred V. Anderson, DC, MD;
Kenneth H. Svendsen, MS; Yiscah Bracha, MS; and Richard H. Grimm, MD, MPH, PhD
Northwestern Health Sciences University,
Pain Management and Rehabilitation Center,
Minneapolis, Minnesota, USA.
Clinical Relevance
Health care practitioners who treat patients with musculoskeletal complaints should take note and may need to alter their approach to the care of neck pain. The doctor of chiropractic should take extra note as he/she may be in the best position to offer the best care by combining their skills of SMT with education as to how the patient can continue their treatment at home. Providing appropriate exercises and postural management, as well as education in-office plus take home materials should aid in overall patient education and positive outcome.
Journal of the Academy of Chiropractic Orthopedists Editorial Summary:
SMT was more effective for acute and sub-acute neck pain than medication.
However, a few instructional sessions of HEA resulted in similar outcomes at most time points
Participants were excluded who had any of the following conditions: cervical spine instability, fracture, referred pain, neurologic deficits, existing cardiac disease, blood clotting disorders, DISH, infectious disease, substance abuse, pregnancy, previous cervical spine surgery or involved in litigation.
Overall, the greatest changes in cervical spine motion were observed in the HEA group.
Participant satisfaction was greatest in the SMT group and lowest in the medication group.
No serious adverse events were reported in the study. Expected, non-serious adverse events that are typical to these treatments did occur and were all transient in nature.
This study was intended to be pragmatic in nature and to answer clinical questions regarding commonly used treatment approaches by approximating how they are delivered in practice
Summary
In light of the current legislation regarding national health care, health care providers who treat neck pain should pay attention to this study and take note as to how they can best position their practice to not only provide consistent and beneficial outcomes but also at a reasonable cost. The doctor of chiropractic is in a unique position to do this. Failure to do so, may not only result in their exclusion from participation at a personal/local level but as a profession as a whole.BACKGROUND: Mechanical neck pain is a common condition that affects an estimated 70% of persons at some point in their lives. Little research exists to guide the choice of therapy for acute and subacute neck pain.
OBJECTIVE: To determine the relative efficacy of spinal manipulation therapy (SMT), medication, and home exercise with advice (HEA) for acute and subacute neck pain in both the short and long term.
DESIGN: Randomized, controlled trial. (ClinicalTrials.gov registration number: NCT00029770 )
SETTING: 1 university research center and 1 pain management clinic in Minnesota.
PARTICIPANTS: 272 persons aged 18 to 65 years who had nonspecific neck pain for 2 to 12 weeks.
INTERVENTION: 12 weeks of SMT, medication, or HEA.
MEASUREMENTS: The primary outcome was participant-rated pain, measured at 2, 4, 8, 12, 26, and 52 weeks after randomization. Secondary measures were self-reported disability, global improvement, medication use, satisfaction, general health status (Short Form-36 Health Survey physical and mental health scales), and adverse events. Blinded evaluation of neck motion was performed at 4 and 12 weeks.
RESULTS: For pain, SMT had a statistically significant advantage over medication after 8, 12, 26, and 52 weeks (P = 0.010), and HEA was superior to medication at 26 weeks (P = 0.02). No important differences in pain were found between SMT and HEA at any time point. Results for most of the secondary outcomes were similar to those of the primary outcome.
LIMITATIONS: Participants and providers could not be blinded. No specific criteria for defining clinically important group differences were prespecified or available from the literature.
CONCLUSION: For participants with acute and subacute neck pain, SMT was more effective than medication in both the short and long term. However, a few instructional sessions of HEA resulted in similar outcomes at most time points.
From the FULL TEXT Article:
Introduction
Neck pain is a prevalent condition that nearly three quarters of persons experience at some point in their lives [1–2]. One of the most commonly reported symptoms in primary care settings [3–4], neck pain results in millions of ambulatory health care visits each year and increasing health care costs [5–8]. Although it is not life-threatening, neck pain can have a negative effect on productivity and overall quality of life [1, 9–11].
Chiropractors, physical therapists, osteopaths, and other health care providers commonly apply spinal manipulation, a manual therapy, for neck pain conditions [12], and home exercise programs and medications are also widely used [13]. Recent Cochrane reviews [13, 14] report insufficient evidence to assess the effectiveness of commonly used medications or home exercise programs for the treatment of acute neck pain. The evidence for spinal manipulation is similarly limited, with only low-quality evidence supporting its use for neck pain of short duration [15].
Our goal was to test the hypothesis that spinal manipulation therapy (SMT) is more effective than medication or home exercise with advice (HEA) for acute and subacute neck pain.
Methods
Setting
The trial was conducted from 2001 to 2007 in Minneapolis, Minnesota. Eligibility screening, randomization, and short-term data collection occurred at a university-affiliated research center; long-term data collection took place by mail. A university-affiliated outpatient clinic provided SMT and instruction for home exercise. Medical treatment was provided at a pain management clinic. The institutional review boards of Northwestern Health Sciences University and Hennepin County Medical Center approved our study, and all participants gave written informed consent.
Participants
Participants were recruited by using mailings targeted to persons with neck pain who were registered with Blue Cross/Blue Shield Minnesota and through newspaper and radio advertisements. Interested persons were screened for eligibility at 2 baseline appointments by clinicians who were blinded to the randomization schedule. Inclusion criteria were age 18 to 65 years; primary symptom of mechanical, nonspecific neck pain equivalent to grades I or II according to the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders classification [16, 17]; current neck pain of 2 to 12 weeks’ duration; and a neck pain score of 3 or greater on a scale of 0 to 10. Participants were asked to refrain from seeking additional treatment for neck pain from nonstudy health care providers during the 12–week intervention.
Exclusion criteria were cervical spine instability, fracture, neck pain referred from peripheral joints or viscera, progressive neurologic deficits, existing cardiac disease requiring medical treatment, blood clotting disorders, diffuse idiopathic hyperostosis, inflammatory or destructive tissue changes of the cervical spine, infectious disease or other severe disabling health problems, substance abuse, pregnancy or breastfeeding, previous cervical spine surgery, and pending or current litigation. In addition, participants were excluded if they had received any of the study treatments in the past 3 months.
Randomization and Interventions
Participants were randomly assigned at the second baseline appointment by using permutated blocks of different sizes.[18] The randomization schedule was prepared off-site by the study statistician before enrollment and was concealed from the investigators, treatment providers, and research staff by using consecutively numbered, sealed, opaque envelopes. As participants became eligible, envelopes were opened in consecutive order by a research staff member in the presence of the participant.
The intervention protocol was tested in a pilot study by our research team. [19] Maximum treatment duration was 12 weeks. Treatment providers were trained in the study intervention protocols and were required to document treatment activities in standardized clinical records, which were routinely monitored by research staff to ensure protocol adherence.
SMT Group
Six chiropractors with a minimum of 5 years’ experience served as the primary providers of treatment. Visits lasted 15 to 20 minutes and included a brief history and examination of the cervical and thoracic spine. The primary focus of treatment was manipulation of areas of the spine with segmental hypomobility by using diversified techniques, including low-amplitude spinal adjustments (a high-velocity type of joint thrust manipulation) and mobilization (a low-velocity type of joint oscillation). [20] The specific spinal level to be treated and the number of treatment sessions over the 12 weeks was left to the discretion of the provider, based on manual palpation of the spine and associated musculature and the participant’s response to treatment. [21] Adjunct therapy common to clinical practice included limited light soft-tissue massage, assisted stretching, and hot and cold packs to facilitate the manipulation treatment. Advice to stay active or modify activity was recommended as needed.
Medication Group
A licensed medical physician provided care to participants, with the focus of treatment on prescription medication. Visits lasted 15 to 20 minutes and included a brief history and examination. The first line of therapy was nonsteroidal anti-inflammatory drugs, acetaminophen, or both. [22, 23] Participants who did not respond to or could not tolerate first-line therapy received narcotic medications. Muscle relaxants were also used. Advice to stay active or modify activity was issued as needed. The choice of medications and number of visits was made by the physician on the basis of the participant’s history and response to treatment.
HEA Group
Home exercise with advice was provided in two 1–hour sessions, 1 to 2 weeks apart, at the universityaffiliated outpatient clinic. Six therapists provided instruction to participants. The primary focus was simple selfmobilization exercise (gentle controlled movement) of the neck and shoulder joints, including neck retraction, extension, flexion, rotation, lateral bending motions, and scapular retraction, with no resistance (Supplement, available at www.annals.org). The delivery method was 1–on–1, and the program was individualized to each participant’s abilities, tolerance, and activities of daily living. Participants were instructed to do 5 to 10 repetitions of each exercise up to 6 to 8 times per day. A booklet [24] and laminated cards of prescribed exercises were provided. Sessions were supplemented with information about the basic anatomy of the cervical spine and advice, including postural instructions and practical demonstrations of lifting, pushing, pulling, and other daily actions.
Outcomes and Measurements
We collected participant demographic and clinical characteristics at the initial baseline appointment by using self-report questionnaires, clinical history, and physical examinations. Self-reported outcomes (such as pain) were measured 6 times during the 12–week treatment period (at the 2 baseline appointments and 2, 4, 8, and 12 weeks after randomization). Outcomes were also collected twice during the posttreatment period (at weeks 26 and 52) by using a mailed questionnaire. All self-report questionnaires were completed by participants independent of influence from investigator, study staff, or treatment provider. Participants were asked in each questionnaire if anyone had attempted to influence their responses. Objective measures of cervical spine motion were measured at 4 and 12 weeks by 7 trained examiners who were masked to treatment assignment. [25] Blinding was maintained by systematically instructing participants not to reveal treatment information and by ensuring that examiners had no exposure to activities in the outpatient clinics.
We chose participant-rated pain as the primary outcome measure a priori and used an 11–box numerical rating scale (range, 0 [no symptoms] to 10 [highest severity of pain]) (We chose participant-rated pain as the primary outcome measure a priori and used an 11–box numerical rating scale (range, 0 [no symptoms] to 10 [highest severity of pain]) [26–29]. Secondary outcomes included the Neck Disability Index [30], global improvement [31–33], medication use [34], satisfaction with care [25, 34], the Short Form-36 Health Survey (SF-36) [35], and cervical spine motion (measured with a CA 6000 Spine Motion Analyzer [Orthopedic Systems, Union City, California]) [36, 37].
Before random assignment, participants were asked in the self-report questionnaire how they expected their neck pain to change in response to treatment, with choices of much better, better, no change, worse, and much worse. Participants were also asked to report additional health care use visits to nonstudy providers in the self-report questionnaires at all time points.
Participants were asked standardized questions at each treatment visit to assess side effects since the last visit, and responses were documented in the clinical record.
Statistical Analysis
Our sample size calculation was based on an ability to detect a 0.8–point difference between the highest and lowest group means in participant-rated neck pain (the primary outcome) at the end of 12 weeks of treatment. This difference was informed by previous neck pain trials conducted by our group (Our sample size calculation was based on an ability to detect a 0.8–point difference between the highest and lowest group means in participant-rated neck pain (the primary outcome) at the end of 12 weeks of treatment. This difference was informed by previous neck pain trials conducted by our group [19, 25] and the ability to detect a small to medium effect size. We used an SD of 1.8 for our pain scale on the basis of our pilot study and estimates from the literature [25, 38]. With a power of 0.90 and a 3–group design tested at an ? level of 0.05 (2–tailed test), 75 participants per group were required (SPSS SamplePower 1.0, International Business Machines, Armonk, New York). To allow for a loss to follow-up rate of up to 15%, we aimed to recruit 90 participants per group for a total of 270 participants.
In primary analyses, we evaluated changes in neck pain between baseline and week 12 and performed longitudinal analyses by using data from weeks 2, 4, 8, and 12 (shortterm outcome). In secondary (exploratory) analyses of both primary and secondary outcomes, we evaluated changes in participant-rated outcomes between baseline and weeks 2, 4, 8, 12, 26, and 52 and performed longitudinal analyses by using data from weeks 2, 4, 8, 12, 26, and 52 (longterm outcome). Both analyses were conducted by using linear mixed-model analysis with the MIXED procedure in SAS, version 9.1 (SAS Institute, Cary, North Carolina), with baseline values as outcomes.[39–42] Clinical and demographic variables showing group differences at baseline were used as covariates in the analysis if they were at least moderately correlated with changes in outcomes[43, 44].
Clinical and demographic variables showing group differences at baseline were used as covariates in the analysis if they were at least moderately correlated with changes in outcomes (In primary analyses, we evaluated changes in neck pain between baseline and week 12 and performed longitudinal analyses by using data from weeks 2, 4, 8, and 12 (short-term outcome). In secondary (exploratory) analyses of both primary and secondary outcomes, we evaluated changes in participant-rated outcomes between baseline and weeks 2, 4, 8, 12, 26, and 52 and performed longitudinal analyses by using data from weeks 2, 4, 8, 12, 26, and 52 (long-term outcome). Both analyses were conducted by using linear mixed-model analysis with the MIXED procedure in SAS, version 9.1 (SAS Institute, Cary, North Carolina), with baseline values as outcomes [39–42]. Clinical and demographic variables showing group differences at baseline were used as covariates in the analysis if they were at least moderately correlated with changes in outcomes [43, 44].
The study database was prepared by data managers who were blinded to study allocation. The intention-totreat principle was adhered to by including all participants with baseline data in the analyses, regardless of loss to follow-up. To protect against increased risk for type I errors, we used the Fisher (protected) least-significantdifference test[45, 46]. The mixed-model analysis included all participants who had at least baseline assessments. In the event of missing data, the reasons were explored and the pattern of the missing data was determined to select the best method of data imputation. The original analyses were then repeated as sensitivity analyses with fully imputed data by using the MI procedure in SAS, to assess the effect of the missing data (47–51). No prespecified thresholds for clinically important group differences were set because none has been established in the literature. To facilitate interpretation of the magnitude of group differences, responder analyses were conducted by group for pain reduction (absolute risk reduction) of 50%, 75%, and 100% (including 95% CIs) at the end of treatment and at 26– and 52–week follow-up [52–55].
Role of the Funding Source
Our trial was funded by the National Center for Complementary and Alternative Medicine, National Institutes of Health. The funding source had no role in the study design, collection, analysis, data interpretation, or writing of this article.
Results
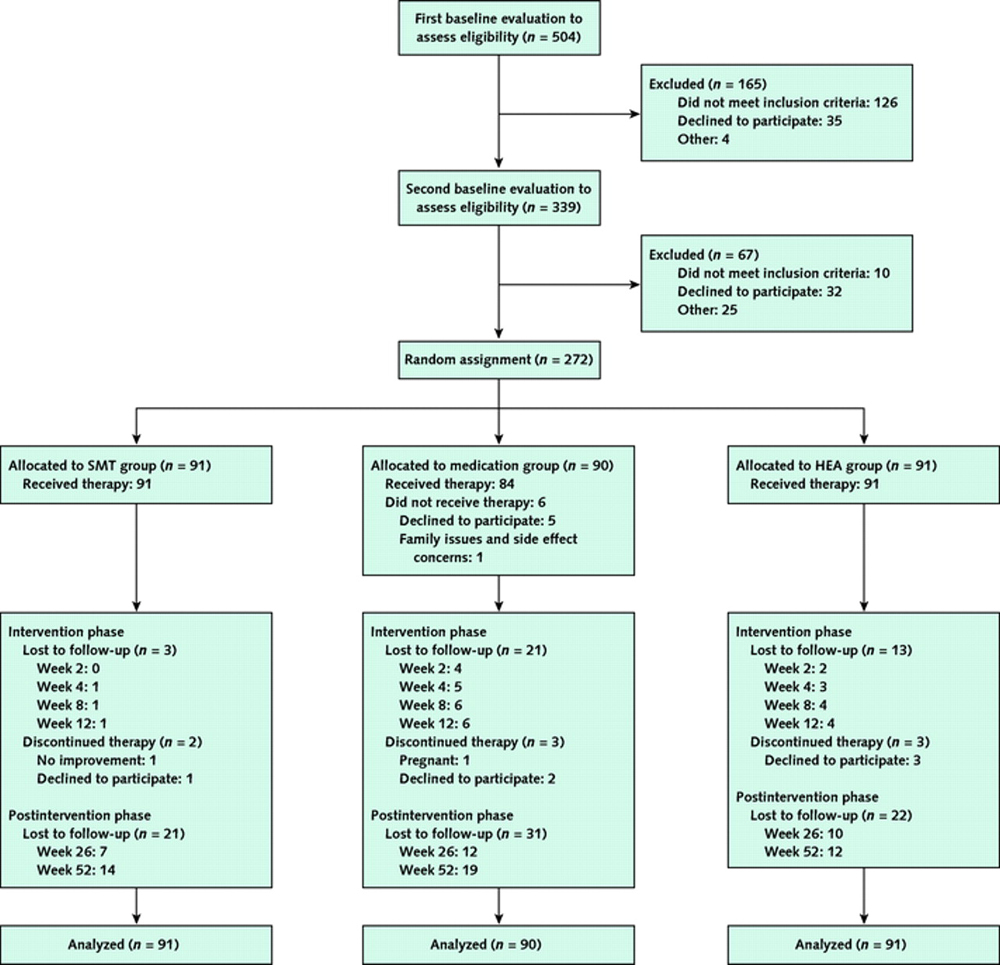
Figure 1

Table 1

Table 2

Table 3 We evaluated 504 persons for eligibility, of whom 272 were randomly assigned: 90 to the medication group, 91 to the SMT group, and 91 to the HEA group. The Figure summarizes recruitment, participation, and attrition.
Table 1 summarizes the demographic and clinical characteristics of the randomly assigned participants. Potentially important between-group differences were noted for sex, duration of neck pain, pain during the night, and expectation of change in neck pain. Table 2 provides details of the 3 study interventions.
Primary Outcomes
Improvement in participant-rated pain significantly differed with SMT compared with medication at 12 weeks (0.94 greater reduction in pain [95% CI, 0.37 to 1.51]; P = 0.001) and in longitudinal analyses that incorporated pain ratings every 2 weeks from baseline to 12 weeks (0.55 greater reduction in pain [CI, 0.10 to 1.00]; P = 0.017). At 12 weeks, a significantly higher absolute proportion of the SMT group experienced reductions of pain of at least 50% (Table 3). Differences in participant-rated pain improvement between the SMT and HEA groups were smaller and not statistically significant. Differences between the HEA and medication groups were also not statistically significant, although a higher absolute proportion of the HEA group experienced reductions in pain of at least 75% at 12 weeks compared with the medication group.
Longer-term analyses showed similar findings. At 26 and 52 weeks, participant-rated pain improvement favored SMT over medication, but not SMT over HEA or HEA over medication, compared with baseline. A higher absolute proportion in the SMT group than in the medication group experienced reductions of pain of at least 50% at 26 but not 52 weeks. Those proportions did not differ at any time in comparisons of SMT and HEA, and a higher absolute proportion in the HEA group than in the medication group experienced reductions of pain of at least 75% at 26 but not 52 weeks.
Adjustment for baseline imbalances in sex, cause of pain, and depression did not change the group differences in pain outcomes.
Secondary Outcomes

Appendix Table 1

Appendix Table 2

Appendix Table 3

Appendix Table 4 Group differences in most secondary outcomes were similar to those of the primary outcomes (Appendix Tables 1, 2, 3, and 4). Spinal manipulation therapy was superior to medication at the end of treatment and during follow-up in terms of global improvement, participant satisfaction, and SF-36–assessed physical but not mental function; SMT was also superior to medication in measures of long-term medication use (1.26 fewer days per week of use at week 52 [CI, 0.53 to 1.99 days]; P < 0.001).
The SMT and HEA groups performed similarly on most of the secondary outcomes, although SMT performed better than HEA for satisfaction with care in both the short and long term. Home exercise with advice was superior to medication in both the short and long term for satisfaction with care and for long-term medication use (1.00 fewer days per week of use at week 52 [CI, 0.27 to 1.73 days]; P = 0.008).
Appendix Table 4 shows changes in cervical spine motion after 4 and 12 weeks. Overall, the greatest changes in cervical spine motion were observed in the HEA group. Results of the group differences in 3–dimensional cervical spine motion patterns will be reported elsewhere.
One of the participants indicated that someone tried to influence his responses. Because this was a week–52 questionnaire collected by mail independent of study staff, it was probably not of consequence.
Missing Data Analysis
Among the 272 participants, 219 (80.5%) provided data on neck pain at every visit. We considered loss to follow-up to be nonrandom for 12 participants, 6 of whom never commenced treatment (all in the medication group) and 6 of whom stopped participating in the study after they received treatment (2 in the medication group, 1 in the SMT group, and 3 in the HEA group). We first imputed values to the missing responses of these 12 participants by using the mean percentage reduction from baseline at all time points specific to the group to which they belonged. Then, we imputed the rest of the missing data during treatment and the 2 posttreatment follow-up time points by using the SAS multiple imputation strategy, on the assumption that the data were missing at random. The results of the analyses with imputed values changed the estimates of group differences very little, and all statistically significant differences remained the same.
Nonstudy Treatments
During the 12–week intervention, 4 participants (3 in the medication group and 1 in the HEA group) reported visits to other health care providers for their neck pain. By week 52, about equal numbers of persons in each treatment group sought additional health care after completing the treatment phase (18 in the SMT group, 14 in the medication group, and 17 in the HEA group).
Adverse Events

Appendix Table 5 No serious adverse events were reported in the study. Expected, nonserious adverse events that are typical to these treatments did occur and were all transient in nature, requiring little or no change to activity levels. Forty percent of the SMT group and 46% of the HEA group reported adverse events, primarily musculoskeletal pain. Paresthesia, stiffness, headache, and crepitus were less frequent (Appendix Table 5). Sixty percent of participants in the medication group reported side effects, the most common being gastrointestinal symptoms and drowsiness. Dry mouth, cognitive disturbances, rash, congestion, and disturbed sleep were less commonly reported.
DISCUSSION
In the absence of available criteria for what constitute clinically important group differences, several factors should be considered in aggregate. This includes the statistical significance of the results of our primary efficacy analysis, as well as those of the responder and secondary outcomes analyses. The durability of the treatment effect, the safety and tolerability of the interventions, and the participant's ability and willingness to adhere to treatment should also be taken into account [56].
In this trial of SMT versus medication or HEA for the treatment of acute and subacute neck pain, SMT seemed more effective than medication according to various measures of neck pain and function. However, SMT demonstrated no apparent benefits over HEA. Spinal manipulation therapy and HEA led to similar short- and long-term outcomes, but participants who received medication seemed to fare worse, with a consistently higher use of pain medication for neck pain throughout the trial's observation period. The performance of the HEA group, which has the potential for cost savings over both SMT and medication interventions, is noteworthy.
Participants and clinicians consider the potential for side effects when making treatment decisions. Although the frequency of reported side effects was similar among the 3 groups (41% to 58%), the nature of the side effects differed, with participants in the SMT and HEA groups reporting predominantly musculoskeletal events and those in the medication group reporting side effects that were more systemic in nature. Of note, participants in the medication group reported higher levels of medication use after the intervention.
Most participants had subacute neck pain that lasted more than 4 weeks, beyond the time when pain will probably resolve spontaneously, and evidence suggests that one half of persons with nonspecific neck pain continue to have neck pain 1 year after the original report [57]. Although our trial did not have a placebo group, the observed results are unlikely to be due to natural history alone.
To date, few clinical trials have assessed the effectiveness of noninvasive interventions for acute and subacute neck pain not associated with whiplash; therefore, no evidence-informed first-line therapy for this type of neck pain has been established [12, 13].
We searched MEDLINE, EMBASE, CINAHL, and the Cochrane Library, using the terms spinal manipulation and neck pain, to identify all randomized trials published from 1960 to 2011 that evaluated SMT for acute or subacute neck pain. We found 3 trials [58–61]. Our trial is most similar to that of Hoving and colleagues [58, 59], in which 75% of patients had neck pain of less than 12 weeks' duration. Six weeks of manual therapy (mainly spinal mobilization) was compared with usual medical care (advice, home exercise, and medication). The investigators found manual therapy to be superior to medical care, with reductions in pain and disability similar to what we observed at 8 weeks but less than what we observed at 12 weeks. Pool and colleagues [60] compared 6 weeks of manual therapy (up to 6 sessions) with 6 weeks of a behavioral-graded activity program (maximum of 18 sessions of 30 minutes each). At 3 months, the behavioral-graded activity program demonstrated slightly larger reductions in pain and disability than manual therapy; however, the magnitude of improvements in the behavioral program was similar to that found for SMT in our trial. Finally, Cleland and colleagues [61] found thrust mobilization and manipulation to be more effective than nonthrust manual treatment in patients with subacute neck pain. When considered in the context of the existing evidence, our results suggest that SMT and HEA both constitute viable treatment options for managing acute and subacute mechanical neck pain.
Our study has several strengths, including a rigorous concealed randomization procedure, use of recommended reliable outcome measures, masked objective outcomes assessors, and long-term postrandomization follow-up (6 and 12 months.) It also has limitations. First, participants and providers could not be blinded because of the nature of the treatments received and delivered. Second, no criteria are available to define clinically important group differences for the different outcomes. Finally, our study does not differentiate between the specific effects of treatment and the contextual (nonspecific) effects, including participant–provider interactions and expectations. This study was intended to be pragmatic in nature and to answer clinical questions regarding commonly used treatment approaches by approximating how they are delivered in practice.
For participants with acute and subacute neck pain, SMT was more effective than management with medication in both the short and long term; however, a few sessions of supervised instruction in HEA resulted in similar outcomes at most time points.
Acknowledgment:
The authors thank the study staff for dedicating substantial time and energy to ensure successful completion of the trial, as well as Brent Leininger, DC, and Jennifer Hart, MS, for their technical assistance in preparing this manuscript.
Grant Support:
By the National Institutes of Health’s National Center for Complementary and Alternative Medicine (grant R01 AT000707).
References:
Cote P, Cassidy JD, Carroll L.
The Saskatchewan Health and Back Pain Survey.
The Prevalence of Neck Pain and Related Disability in Saskatchewan Adults
Spine (Phila Pa 1976). 1998 (Aug 1); 23 (15): 1689–1698Fejer R, Kyvik KO, Hartvigsen J.
The prevalence of neck pain in the world population: a systematic critical review of the literature.
Eur Spine J. 2006;15: 834-48Hogg-Johnson S, van der Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, et al;
The Burden and Determinants of Neck Pain in the General Population:
Results of the Bone and Joint Decade 2000–2010 Task Force
on Neck Pain and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S39–51Guzman J, Haldeman S, Carroll LJ, et al.
Clinical Practice Implications of the Bone and Joint Decade 2000-2010
Task Force on Neck Pain and Its Associated Disorders:
From Concepts and Findings to Recommendations
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S199–S212Riddle DL, Schappert SM.
Volume and characteristics of inpatient and ambulatory medical care for neck pain in the United States: data from three national surveys.
Spine (Phila Pa 1976). 2007;32:132-40Barnes PM , Powell-Griner E , McFann K , Nahin RL:
Complementary and Alternative Medicine Use Among Adults:
United States, 2002
Advance Data 2004 (May 27); 343: 1–19Coulter ID, Hurwitz EL, Adams AH, Genovese BJ, Hays R, Shekelle PG.
Patients Using Chiropractors in North America:
Who Are They, and Why Are They in Chiropractic Care?
Spine (Phila Pa 1976) 2002; 27 (3) Feb 1: 291–298Martin, BI, Deyo, RA, Mirza, SK et al.
Expenditures and Health Status Among Adults With Back and Neck Problems
JAMA 2008 (Feb 13); 299 (6): 656–664Cote P, van der Velde G, Cassidy JD, Carroll LJ, Hogg-Johnson S, Holm LW, et al.
The Burden and Determinants of Neck Pain in Workers: Results of the Bone and Joint Decade
2000–2010 Task Force on Neck Pain and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S60-74Cote P, Kristman V, Vidmar M, Van Eerd D, Hogg-Johnson S, Beaton D, et al.
The prevalence and incidence of work absenteeism involving neck pain: a cohort of Ontario lost-time claimants.
Spine (Phila Pa 1976). 2008;33:S192-8Linton SJ, Hellsing AL, Hallde´n K.
A population-based study of spinal pain among 35-45-year-old individuals. Prevalence, sick leave, and health care use.
Spine (Phila Pa 1976). 1998;23:1457-63Gross AR, Hoving JL, Haines TA, Goldsmith CH, Kay T, Aker P, et al;
Cervical overview group. Manipulation and mobilisation for mechanical neck disorders.
Cochrane Database Syst Rev. 2004:CD004249Kay TM, Gross A, Goldsmith C, Santaguida PL, Hoving J, Bronfort G; Cervical Overview Group.
Exercises for mechanical neck disorders.
Cochrane Database Syst Rev. 2005:CD004250Peloso P, Gross A, Haines T, Trinh K, Goldsmith CH, Burnie S;
Cervical Overview Group. Medicinal and injection therapies for mechanical neck disorders.
Cochrane Database Syst Rev. 2007:CD000319Gross A, Miller J, D’Sylva J, Burnie S, Goldsmith G, Graham N et al..
Manipulation or Mobilisation For Neck Pain: A Cochrane Review
Manual Therapy 2010 (Aug); 15 (4): 315–333Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, Zeiss E.
Scientific Monograph of the Quebec Task Force on Whiplash-Associated Disorders
Redefining Whiplash and its Management
Spine (Phila Pa 1976). 1995 (Apr 15); 20 (8 Suppl): S1-S73Guzman J, Hurwitz EL, Carroll LJ, Haldeman S, Coˆte´ P, Carragee EJ, et al;
A New Conceptual Model Of Neck Pain: Linking Onset, Course, And Care
Results of the Bone and Joint Decade 2000–2010 Task Force
on Neck Pain and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S14–23Pocock SJ. Clinical Trials.
A Practical Approach.
Chichester, United Kingdom: J Wiley; 1986Evans R, Bronfort G, Bittell S, Anderson AV.
A pilot study for a randomized clinical trial assessing chiropractic care, medical care,
and self-care education for acute and subacute neck pain patients.
J Manipulative Physiol Ther. 2003;26: 403-11Bergmann TF, Peterson DH.
Chiropractic Technique: Principles and Procedures.3rd ed.
St. Louis: Mosby; 2011Seffinger MA, Najm WI, Mishra SI, Adams A, Dickerson VM, Murphy LS, et al.
Reliability of spinal palpation for diagnosis of back and neck pain:
a systematic review of the literature.
Spine (Phila Pa 1976). 2004;29:E413-25Tierney LM, McPhee SJ, Papadakis MA.
Current Medical Diagnosis and Treatment. 36th ed.
Stamford, CT: Appleton & Lange; 1997.Scholten-Peeters GG, Bekkering GE, Verhagen AP, van Der Windt DA, Lanser K, Hendriks EJ, et al.
Clinical practice guideline for the physiotherapy of patients with whiplash-associated disorders.
Spine (Phila Pa 1976). 2002;27:412-22McKenzie R.
Treat Your Own Neck. 3rd ed.
Waikanae, New Zealand: Spinal Publications; 2002.Bronfort G, Evans R, Nelson B, Aker PD, Goldsmith CH, Vernon H.
A Randomized Clinical Trial of Exercise and Spinal Manipulation
for Patients with Chronic Neck Pain
Spine (Phila Pa 1976). 2001 (Apr 1); 26 (7): 788–797Jaeschke R, Singer J, Guyatt GH.
A comparison of seven-point and visual analogue scales. Data from a randomized trial.
Control Clin Trials. 1990;11:43-51Jensen MP, Karoly P, Braver S.
The measurement of clinical pain intensity: a comparison of six methods.
Pain. 1986;27:117-26Huskisson EC.
Measurement of pain.
Lancet. 1974;2:1127-31Carlsson AM.
Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale.
Pain. 1983;16:87-101Vernon H, Mior S.
The Neck Disability Index: A Study of Reliability and Validity
J Manipulative Physiol Ther 1991 (Sep); 14 (7): 409–415Koes BW, Bouter LM, van Mameren H, Essers AH, Verstegen GM, Hofhuizen DM, et al.
A blinded randomized clinical trial of manual therapy and physiotherapy for chronic back and neck complaints:
physical outcome measures.
J Manipulative Physiol Ther. 1992;15:16-23Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S.
A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain.
N Engl J Med. 1990;322:1627-34Hansen FR, Bendix T, Skov P, Jensen CV, Kristensen JH, Krohn L, et al.
Intensive, dynamic back-muscle exercises, conventional physiotherapy, or placebo-control treatment of low-back pain.
A randomized, observer-blind trial.
Spine (Phila Pa 1976). 1993;18:98-108Bronfort G, Goldsmith CH, Nelson CF, Boline PD, Anderson AV.
Trunk Exercise Combined with Spinal Manipulative or NSAID Therapy
for Chronic Low Back Pain: A Randomized, Observer-blinded Clinical Trial
J Manipulative Physiol Ther. 1996 (Nov); 19 (9): 570–582Daffner SD, Hilibrand AS, Hanscom BS, Brislin BT, Vaccaro AR, Albert TJ.
Impact of neck and arm pain on overall health status.
Spine (Phila Pa 1976). 2003;28:2030-5Dvorak J, Antinnes JA, Panjabi M, Loustalot D, Bonomo M.
Age and gender related normal motion of the cervical spine.
Spine (Phila Pa 1976). 1992; 17:S393-8Petersen CM, Johnson RD, Schuit D.
Reliability of cervical range of motion using the OSI CA 6000 spine motion analyser on asymptomatic
and symptomatic subjects.
Man Ther. 2000;5:82-8Evans R, Bronfort G, Nelson B, Goldsmith CH.
Two-year Follow-up of a Randomized Clinical Trial of Spinal Manipulation
and Two Types of Exercise for Patients With Chronic Neck Pain
Spine (Phila Pa 1976). 2002 (Nov 1); 27 (21): 2383–2389Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS
System for Mixed Models.
Cary, NC: SAS Publications; 1996.Verbeke G, Molenberghs G, eds.
Linear Mixed Models in Practice: A SASOriented Approach.
New York: Springer; 1997.Brown H, Prescott R.
Applied Mixed Models in Medicine.
New York: J Wiley; 1999.Jennrich RI, Schluchter MD.
Unbalanced repeated-measures models with structured covariance matrices.
Biometrics. 1986;42:805-20Pocock SJ, Assmann SE, Enos LE, Kasten LE.
Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems.
Stat Med. 2002;21:2917-30Yu LM, Chan AW, Hopewell S, Deeks JJ, Altman DG.
Reporting on covariate adjustment in randomised controlled trials before and after revision of the 2001
CONSORT statement: a literature review.
Trials. 2010;11:59Sherman KJ, Cherkin DC, Erro J, Miglioretti DL, Deyo RA.
Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial.
Ann Intern Med. 2005;143:849-56Levin J, Serlin R, Seaman M.
A controlled, powerful multiple-comparison strategy for several situations.
Psychol Bull. 1994;115:153-9.Little RJ, Rubin DB.
Statistical Analysis with Missing Data. 2nd ed.
New York: J Wiley; 2002.Rubin DB.
Inference and missing data.
Biometrika 1976;63:581-92.Ostelo RW, de Vet HC.
Clinically important outcomes in low back pain.
Best Pract Res Clin Rheumatol. 2005;19:593-607Pool JJ, Ostelo RW, Hoving JL, Bouter LM, de Vet HC.
Minimal clinically important change of the Neck Disability Index and the Numerical Rating Scale
for patients with neck pain.
Spine (Phila Pa 1976). 2007;32:3047-51Sherman KJ, Cherkin DC, Hawkes RJ, Miglioretti DL, Deyo RA.
Randomized trial of therapeutic massage for chronic neck pain.
Clin J Pain. 2009; 25:233-8Fritz JM, Hebert J, Koppenhaver S, Parent E.
Beyond minimally important change: defining a successful outcome of physical therapy for patients with low back pain.
Spine (Phila Pa 1976). 2009;34:2803-9Bendtsen L, Bigal ME, Cerbo R, Diener HC, Holroyd K, Lampl C, et al;
International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs
in tension-type headache: second edition.
Cephalalgia. 2010;30:1-16Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, et al.
Interpreting change scores for pain and functional status in low back pain: towards international consensus
regarding minimal important change.
Spine (Phila Pa 1976). 2008;33:90-4Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS.
Interpreting treatment effects in randomised trials.
BMJ. 1998;316:690-3Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al.
Interpreting the clinical importance of group differences in chronic pain clinical trials:
IMMPACT recommendations.
Pain. 2009;146:238-44Hill J, Lewis M, Papageorgiou AC, Dziedzic K, Croft P.
Predicting persistent neck pain: a 1-year follow-up of a population cohort.
Spine (Phila Pa 1976). 2004;29:1648-54Hoving JL, Koes BW, de Vet HC, van der Windt DA, Assendelft WJ, van Mameren H, et al.
Manual Therapy, Physical Therapy, or Continued Care by a General Practitioner
for Patients with Neck Pain. A Randomized, Controlled Trial
Annals of Internal Medicine 2002 (May 21); 136 (10): 713–722Hoving JL, de Vet HC, Koes BW, Mameren H, Deville´ WL, van der Windt DA, et al.
Manual therapy, physical therapy, or continued care by the general practitioner for patients with neck pain:
long-term results from a pragmatic randomized clinical trial.
Clin J Pain. 2006;22:370-7Pool JJ, Ostelo RW, Ko¨ke AJ, Bouter LM, de Vet HC.
Comparison of the effectiveness of a behavioural graded activity program and manual therapy in patients
with sub-acute neck pain: design of a randomized clinical trial.
Man Ther. 2006;11:297-305Cleland JA, Glynn P, Whitman JM, Eberhart SL, MacDonald C, Childs JD.
Short-term effects of thrust versus nonthrust mobilization/manipulation directed at the thoracic spine
in patients with neck pain: a randomized clinical trial.
Phys Ther. 2007;87:431-40
Return to CHRONIC NECK PAIN
Return to EXERCISE AND CHIROPRACTIC
Since 1-15-2012


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |