

Spinal Manipulation Epidemiology:
Systematic Review of Cost Effectiveness StudiesThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 655–662 ~ FULL TEXT
Michaleff ZA, Lin CW, Maher CG, van Tulder MW.
The George Institute for Global Health,
The University of Sydney,
Missenden Road, Sydney, NSW 2050, Australia.
zmichaleff@georgeinstitute.org.au
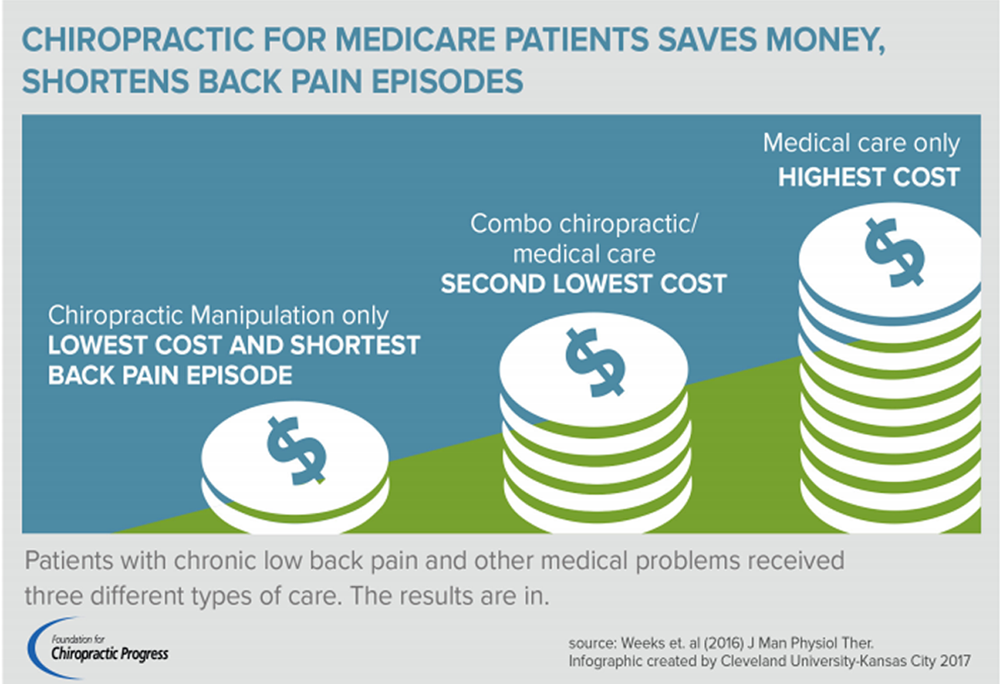
FROM: Weeks ~ JMPT 2016 (Feb) Hurwitz ~ JMPT 2016 (May)BACKGROUND: Spinal manipulative therapy (SMT) is frequently used by health professionals to manage spinal pain. With many treatments having comparable outcomes to SMT, determining the cost-effectiveness of these treatments has been identified as a high research priority.
OBJECTIVE: To investigate the cost-effectiveness of SMT compared to other treatment options for people with spinal pain of any duration.
METHODS: We searched eight clinical and economic databases and the reference lists of relevant systematic reviews. Full economic evaluations conducted alongside randomised controlled trials with at least one SMT arm were eligible for inclusion. Two authors independently screened search results, extracted data and assessed risk of bias using the CHEC-list.
RESULTS: Six cost-effectiveness and cost-utility analysis were included. All included studies had a low risk of bias scoring =16/19 on the CHEC-List. SMT was found to be a cost-effective treatment to manage neck and back pain when used alone or in combination with other techniques compared to GP care, exercise and physiotherapy.
CONCLUSIONS: This review supports the use of SMT in clinical practice as a cost-effective treatment when used alone or in combination with other treatment approaches. However, as this conclusion is primarily based on single studies more high quality research is needed to identify whether these findings are applicable in other settings.
From the Full-Text Article:
Introduction
Spinal pain, including neck pain and back pain, is a common condition in modern society (Woolf and Pfleger, 2003; Côté et al., 2003 ). It presents major social and economic burdens due to the high levels of chronicity and resultant long term disability which are associated with high costs in health care and losses of productivity (e.g. sick leave) (Woolf and Pfleger, 2003). While existing practice guidelines inform the individual, clinicians and policy makers on the effectiveness of a range of interventions, few provide information on the cost-effectiveness of treatments. It is arguable that cost-effectiveness of treatment is an equally important consideration as effectiveness, as all health administrators need to make decisions about how they allocate scarce health resources.
Economic evaluations are frequently conducted alongside randomised controlled trials of treatment effectiveness and involve the identification, measurement, valuation and then comparison of the costs and consequences (benefits) of two or more alternatives (Drummond et al., 2005). Economic evaluations are most useful when the treatments under question have been evaluated in terms of efficacy (can the treatment work in those who comply with the recommendations), effectiveness (is the treatment acceptable and does the treatment work in those who the treatment is offered) and availability (is the treatment accessible to all who would benefit from it). The result of an economic evaluation supplements the evidence base on treatment effectiveness by providing information on the efficiency or ‘‘value for money’’ of treatment alternatives (Drummond et al., 2005). This information can be used to inform consumers, insurers, governments and policy makers where the health budget should be spent.
Spinal manipulative therapy (SMT), including both manipulation (a high velocity thrust technique) and mobilisation (low velocity technique), is frequently used by a number of health professions, including physiotherapists, chiropractors and osteopaths, to manage people with neck pain and back pain (Gross et al., 2010; Assendelft et al., 2004). The effectiveness of SMT to treat spinal pain has been summarised in recent Cochrane Reviews (Gross et al., 2010; Assendelft et al., 2004; Rubinstein et al., 2011). Overall the evidence suggests that SMT provides greater improvements for pain and function than a placebo or no treatment but similar improvements to many competing treatments such as general practitioner management, medication and exercise. With many treatments for spinal pain having comparable outcomes to SMT, determining the cost effectiveness of these treatment alternatives has been identified as a high priority (Rubinstein et al., 2011). The purpose of this systematic review is to investigate the costeffectiveness of SMT compared to other treatment options for people with spinal pain of any duration.
Methods
We followed the method guidelines of the Cochrane Back Review Group (van Tulder et al., 2003; Furlan et al., 2009), Campbell and Cochrane Economic Methods Group (http://www.med.uea.ac.uk/ research/research_econ/cochrane/cochrane_home.htm), and the NHS Economic Evaluation Database Handbook (Craig and Rice, 2007). Full economic evaluations (i.e. cost-effectiveness, cost–utility or cost–benefit analysis) undertaken from any perspective conducted alongside randomised controlled trials were included in this review (Drummond et al., 2005). Studies which collected data on costs and/or utilisation but did not relate this information to a measure of benefit (e.g. cost minimisation analysis), or did not make inferences about the relative efficiency of the treatment alternatives, were excluded (Drummond et al., 2005; Briggs and O’Brien, 2001; Dakin and Wordsworth, 2011). Studies that recruited adults with non-specific spinal (neck or back) pain (i.e. pain is not the result of an accident, trauma or specific spinal pathology) of any duration, reported costs and effects of the interventions and included SMT in at least one intervention group were eligible for this review. We included studies where SMT was administered as the only intervention or as a mandatory component of a combined intervention. No restrictions were placed on the type of health professional performing the interventions or the comparison group used. Studies that recruited multiple musculoskeletal conditions (e.g. neck and shoulder pain), or investigated interventions implemented after spinal surgery were excluded. There was no language restriction.
Data sources and searches
Studies evaluating the cost-effectiveness of SMT for spinal pain were identified from the reference lists of three systematic reviews on the cost-effectiveness of neck pain and back pain treatments (Lin et al., 2011a,b; Driessen et al., accepted for publication). Because these reviews were conducted exclusively for neck or back pain, we also screened the articles which were excluded after full-text screening, in case any study was excluded due to including both neck and back pain. An electronic database search was also conducted on MEDLINE (via OvidSP), EMBASE (via OvidSP), CINAHL (via EBSCO), the American Economic Association’s electronic bibliography (EconLit), National Health System Economic Evaluation Database (NHS EED), and European Network of Health Economic Evaluation Databases (EURONHEED) to identify studies published since the previous literature searches were completed (June 2010–July 2011). Economic search terms (e.g. economics, costs and cost analysis) were developed from search strategies used by the NHS EED (http://www.york.ac.uk/inst/crd/nhseedfaq02.htm) and combined with the Cochrane Back Review Group’s search strategy to identify randomised controlled trials in neck pain and back pain (Bombardier et al., 2011). See Appendix 1 for an example of a full search strategy.
Study selection, risk of bias assessment and data extraction
Two reviewers independently screened first the titles and abstracts (if available), and then full papers. Risk of bias was assessed using the 19-item Consensus on Health Economic Criteria (CHEClist) Evers et al., 2005. Studies were included in the analysis regardless of their risk of bias. Data were extracted using a standardised data extraction sheet which was piloted on a cost-effectiveness study (Manca et al., 2006) before use. The risk of bias rating and data extraction were conducted by two reviewers. Publications related to the included studies (e.g. published protocol or clinical outcomes paper, listed in Appendix 2) were used to assist these processes. Throughout the review, disagreements between the two reviewers were resolved first in discussion, and then by an independent third reviewer if necessary.
Data extracted from each study included:(i) the type and perspective of the economic evaluation,
(ii) characteristics of participants,
(iii) treatment comparators,
(iv) year, study duration, country and currency of the study, and
(v) identification, measurement and valuation of costs and outcomes
used in the economic evaluation, and
(vi) results of the study.The primary outcome used was the relative cost-effectiveness of the interventions, usually reported as an incremental cost-effectiveness or cost–utility ratio (ICER). The ICER indicates the incremental difference in costs between the competing treatment alternatives relative to the incremental difference in effects, and can be interpreted as the additional monetary investment needed for an intervention to gain one extra unit of effect compared to the alternative treatment (Drummond et al., 2005). Whether the effects are worth the costs (value for money), is the key question in economic evaluations, which often triggers intense debate. In some countries consensus exists about thresholds for cost-effectiveness. For example, the British National Institute for Health and Clinical Excellence (NICE) uses a cost-effectiveness threshold of GBP20,000–GBP30,000 per QALY gained as an indicator of cost-effectiveness (Appleby et al., 2007; Towse, 2009). In other countries no threshold exists leading to difficult discussions about whether an intervention is cost-effective or not. When one treatment incurs lower costs and generates higher benefits compared to the alternative treatment, the treatment is said to be dominant. In these cases there will not be a big debate about the interpretation of the results and the choice will be in favour of the dominant treatment.
For data analysis and presentation, studies were grouped by the intervention that SMT was compared to and then the affected region (neck or back). Studies reporting ICER using generic outcomes (e.g. cost per quality-adjusted life-years (QALYs) gained) from the same perspective were compared as able. We used the cost-effectiveness threshold of NICE (see above) as an indicator of cost-effectiveness (Appleby et al., 2007; Towse, 2009). That is, if a treatment has an ICER lower than the NICE threshold when compared to an alternative, the treatment is said to be cost-effective compared with the alternative.
Results

Figure 1

Table 1 The search yielded 95 references; 48 references were identified through the electronic database search and 55 references from the three previous systematic reviews (Lin et al., 2011a,b; Driessen et al., accepted for publication). A total of six studies were included after screening (Figure 1). Most full papers were excluded because they did not evaluate SMT. All of the included studies were designed as a randomised controlled trial and published in English. The number of participants in each study ranged from 146 (Bosmans et al., in press) to 1334 (UK BEAM Trial Team, 2004). Characteristics of the included studies can be seen in Table 1.
Three studies reported on the cost-effectiveness of interventions for neck pain, two studies reported on LBP and one reported on a mixed neck and LBP population. The duration of neck and LBP symptoms varied between studies with the majority recruiting people with acute and sub-acute pain (P2–12 weeks). The duration of symptoms was not specified in one study (Lewis et al., 2007). All six studies conducted both cost-effectiveness and cost– utility analyses (Bosmans et al., in press; UK BEAM Trial Team, 2004; Lewis et al., 2007; Niemisto et al., 2005; Korthals-de Bos et al., 2003; Williams et al., 2004). The economic analysis was conducted from a societal perspective in three studies (Bosmans et al., in press; Niemisto et al., 2005; Korthals-de Bos et al., 2003), healthcare perspective in two studies (UK BEAM Trial Team, 2004; Williams et al., 2004), one study from both the societal and healthcare perspective (Lewis et al., 2007). SMT was delivered by physiotherapists, chiropractors and osteopaths with treatments often involving a combination of manipulation, mobilisation (active or passive) and advice. On average patients received one 20–40 min session once per week for 4–6 weeks.
Risk of bias of the economic evaluation (Table 2)

Table 2 All six studies scored 16 or more out of 19 on the CHEC-list. One study did not justify the perspective chosen (UK BEAM Trial Team, 2004). Discounting was not applicable in five studies and was not performed by one study which had a follow up duration of longer than 12 months (Niemisto et al., 2005). Two of six studies performed appropriate sensitivity analyses (UK BEAM Trial Team, 2004; Korthals-de Bos et al., 2003). An incremental cost-effectiveness analysis was conducted by all six studies, five studies presented cost-effectiveness planes (Bosmans et al., in press; Lewis et al., 2007; Niemisto et al., 2005; Korthals-de Bos et al., 2003; Williams et al., 2004) and four studies presented cost-effectiveness acceptability curves (Bosmans et al., in press; Lewis et al., 2007; Niemisto et al., 2005; Williams et al., 2004).
Cost-effectiveness of SMT
SMT compared to GP care

Table 3 One study investigated the cost-effectiveness of SMT versus general practitioner (GP) care (advice, education and drug prescription). In patients with neck pain, Korthals-de Bos et al. (2003) demonstrated SMT to be dominant over GP care, from a societal perspective, in terms of recovery and quality of life, as SMT was associated with lower total costs and higher rates of recovery. Interestingly, no difference was shown in the cost-effectiveness of SMT versus GP care for pain intensity and functional disability (Korthals-de Bos et al., 2003) which are the outcomes typically selected to judge effectiveness of SMT (Table 3). No studies were found to investigate the cost-effectiveness of SMT versus GP care for LBP.
SMT compared to exercise

Table 4 Two studies adopting a societal perspective reported on SMT versus exercise in patients with neck pain (Bosmans et al., in press; Korthals-de Bos et al., 2003). Both studies found SMT to be a costeffective treatment option compared to an exercise program in terms of pain, recovery and QALY gains (Table 4). The cost-effectiveness plane by Korthals-de Bos et al. (2003) showed 98% of the cost-effect pairs for pain located in the South East quadrant suggesting that SMT is dominant compared with exercise. No studies were found to investigate the cost-effectiveness of SMT versus exercise in patients with LBP.
SMT plus GP care compared to other

Table 5 Two studies reported on the cost-effectiveness of SMT plus GP care compared to GP care alone in LBP (UK BEAM Trial Team, 2004) or neck and LBP ( Williams et al., 2004); in addition one of the studies also compared SMT plus GP to GP care plus exercise (UK BEAM Trial Team, 2004). From a UK health care perspective both trials found SMT plus GP care to be a cost-effective treatment compared to GP care alone as both ICERs fell below the NICE threshold (GBP 20,000–GBP 30,000 per QALY gained) despite the different pain regions being under investigation (Williams et al.: ICER £3560 per QALY gained in 1999/2000 GBP; UK BEAM Trial Team: ICER = £4800 per QALY gained in 2000/2001 GBP). SMT plus GP care was also shown to be a cost-effective treatment when compared to GP care plus exercise with an ICER of £2300 per QALY gained in 2000/2001 GBP (Table 5).
SMT plus other treatment compared other

Table 6 Three studies investigated the cost-effectiveness of a combined treatment approach, which involved SMT plus advice (delivered by a physiotherapist or GP) and exercise (Table 6). Two studies, one from a societal perspective (Niemisto et al., 2005) and the other from a health sector perspective (UK BEAM Trial Team, 2004), compared the combined treatment approach to GP care alone for LBP (UK BEAM Trial Team, 2004; Niemisto et al., 2005). From a societal perspective, Niemisto et al. (2005) found no difference between groups in terms of quality of life however the data suggest that the combined treatment incurred lower annual costs compared to GP care alone. With respect to pain and disability outcomes the data suggested the combined treatment was dominant over GP care alone however this was not supported by the conclusions made by the authors. From a healthcare perspective the UK BEAM Trial found the combined treatment to be cost-effective over GP care alone with a low ICER (£3800 per QALY gained in 2000/2001 GBP) (UK BEAM Trial Team, 2004).
For people with neck pain the most cost-effective treatment was dependant on the perspective, societal or health sector, and of the threshold for willingness to pay. Advice and exercise was generally more cost-effective in terms of changes to neck disability scores from both a societal and healthcare perspective. In terms of QALY gained the combined approach was more cost-effective from a societal perspective however from a healthcare perspective there was more uncertainty as to the most cost-effective treatment with SMT appearing slightly more advantageous (Lewis et al., 2007). versus exercise in patients with LBP.
Discussion
Six economic evaluations were included in this systematic review which evaluated the cost-effectiveness of SMT compared to other treatment options for people with neck and back pain. The studies which evaluated treatments for back pain were primarily UK studies conducted from a health sector perspective, while the studies of neck pain were Dutch and Finnish studies conducted from a societal perspective. Regardless of the perspective employed or the region of pain, SMT appears to be a cost-effective treatment when used alone or in combination with GP care or advice and exercise compared to GP care alone, exercise or any combination of these. However, as a result of only six studies being eligible for inclusion the majority of these conclusions are based on the findings of single studies.
The findings of this review have important clinical, research and policy implications. Clinically, SMT is a treatment technique frequently used by a number of health professionals to manage neck and back pain. Based on the available literature, this review supports the use of SMT in clinical practice as a cost-effective treatment when used alone or in combination with other treatment approaches. In some studies SMT was also shown to be less costly per unit gained when compared to education, exercise and GP care. These results are applicable to clinicians, who make recommendations about treatment options, as well to health consumer who wish to make informed decision about available health care options.
From a research perspective this review highlights the need for more high quality economic evaluations to be conducted alongside randomised controlled trials of treatment effectiveness. While this review summarised the available literature, due to a limited number of studies with heterogeneous populations, perspectives, settings and analyses it was not possible to pool the results of included studies. This resulted in a number of conclusions as to the cost-effectiveness of SMT for neck or back pain to be based on one study alone. Systematic reviews of randomized controlled trials have shown that results of one study are often not reliable and precise. There is no reason to believe that this would be different for economic evaluations, especially because sample sizes of economic evaluations are often (too) small, and that economic consequences differ across different health settings. Of the comparisons supported by two studies it is worth noting the agreement between the studies which increases the robustness of the conclusions which can be made. For example, a comparable ICER was reported by Williams et al. (2004) and (UK BEAM Trial Team, 2004) when SMT plus GP care was compared to GP care alone despite the different pain regions and price years reported.
A number of tools are available to assess the risk of bias of economic evaluations (e.g. CHEC-List Evers et al., 2005, BMJ Check-list (Drummond and Jefferson, 1996) and The Quality of Health Economic Studies instrument (Chiou et al., 2003). In our study we used the CHEC-List as it was generated through the consensus of international experts in a Delphi method (Evers et al., 2005). In general, the economic evaluations included in this study were of low risk of bias with all studies scoring P16 out of 19 on the CHEC-List. The key item identified for improvement in future studies is the inclusion of a sensitivity analysis (Item 15). While not an item in the CHEC-List, other methods of investigating the level of uncertainty in cost-effectiveness estimates include the cost-effectiveness acceptability curves and cost-effectiveness plane. These methods were presented in four of the six included studies (Bosmans et al., in press; Lewis et al., 2007; Niemisto et al., 2005; Williams et al., 2004) and five of the six included studies (Bosmans et al., in press; Lewis et al., 2007; Niemisto et al., 2005; Korthals-de Bos et al., 2003; Williams et al., 2004), respectively. Measures of uncertainty such as these provide a systematic way of dealing with the level of uncertainty around the results and should be an essential component in all economic evaluations to assess the robustness of the conclusions made (van der Roer et al., 2005; Briggs et al., 1994).
Economic evaluations are an essential consideration to inform and support health care and funding decision made by insurers, governments and policy developers. In treatments for spinal pain, as in the case of SMT, effectiveness studies are often able to demonstrate treatment effects when compared to no treatment, but fail to demonstrate which active treatment is more effective (Gross et al., 2010; Rubinstein et al., 2011). The findings of this review show that while the treatment effectiveness of SMT is comparable to other treatments, SMT is a cost-effective treatment option. Furthermore, this review found the most effective treatment or the least costly treatment to not always be the most cost-effective treatment. This demonstrates the valuable information that can be provided by economic evaluations beyond results of effectiveness alone and supports the need for more economic evaluation to be conducted.
This systematic review found SMT to be a cost-effective treatment to manage spinal pain when used alone or in combination with GP care or advice and exercise compared to GP care alone, exercise or any combination of these. The findings were primarily based on single studies conducted in the UK and the Netherlands. More high quality studies can support whether the findings of this review are applicable in other settings.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
We would like to thank Maurice Driessen for the assistance on this review. Z.A.M. hold an Australian Government funded Postgraduate Award, C.G.M. holds a research fellowship from the Australian Research Council, C.-W.C.L. holds a research fellowship from the National Health and Medical Research Council of Australia.
References:
Woolf AD, Pfleger B.
Burden of Major Musculoskeletal Conditions
Bull World Health Organ 2003 (Nov 14); 81: 646–656Côté P, Cassidy J, Carroll L.
The Epidemiology of Neck Pain:
What We Have Learned From Our Population–based Studies
J Can Chiropr Assoc 2003 (Dec); 47 (4): 284–290Drummond MF, Sculpher MJ, Torrance GW.
Methods for the economic evaluation of health care programmes. 3rd ed.
Oxford University Press; 2005.Gross A, Miller J, D’Sylva J, Burnie SJ, Goldsmith CH, Graham N, et al.
Manipulation or Mobilisation For Neck Pain: A Cochrane Review
Manual Therapy 2010 (Aug); 15 (4): 315–333Assendelft WJJ, Morton SC, Yu EI, Suttorp MJ, Shekelle PG.
Spinal manipulative therapy for low-back pain.
Cochrane Database Syst Rev 2004(1):CD000447.Rubinstein SM, van Middelkoop M, Assendelft WJJ, de Boer MR.
Spinal manipulative therapy for chronic low-back pain.
Cochrane Database Syst Rev 2011(2):CD008112.van Tulder M, Furlan A, Bombardier C, Bouter L.
Editorial Board of the Cochrane Collaboration Back Review Group.
Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group.
Spine 2003;28(12):1290–9.Furlan AD, Pennick V, Bombardier C, van Tulder M.
Editorial Board Cochrane Back Review Group.
2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group.
Spine 2009;34(18):1929–41.Craig D, Rice S.
CRD Report 6: NHS Economic Evaluation Database Handbook. 3rd ed.
York: Centre for Reviews and Dissemination, University of York; 2007.Briggs A, O’Brien B.
The death of cost–minimization analysis?
Health Econ 2001;10(2):179–84.Dakin H, Wordsworth S.
Cost minimisation analysis vs cost effectiveness analysis, revisited.
Health Econ 2011.Lin C, Haas M, Maher C, et al.
Cost-Effectiveness of General Practice Care for Low Back Pain: A Systematic Review
European Spine Journal 2011 (Jul); 20 (7): 1012–1023Lin C-WC, Haas M, Maher CG, Machado LAC, Tulder MW.
Cost-effectiveness of Guideline-endorsed Treatments for Low Back Pain: A Systematic Review
European Spine Journal 2011 (Jul); 20 (7): 1024–1038Driessen MT, Lin CW, van Tulder MW.
Cost-effectiveness of Conservative Treatments for Neck Pain:
A Systematic Review on Economic Evaluations
European Spine Journal 2012 (Aug); 21 (8): 1441–1450Bombardier C, van Tulder M, Brønfort G, Deyo R.
About The Cochrane Collaboration.
Cochrane Review Groups (CRGs) 2011 (issue 2).Evers S, Goossens M, de Vet H, van Tulder M, Ament A.
Criteria list for assessment of methodological quality of economic evaluations:
Consensus on Health Economic Criteria.
Int J Technol Assess Health Care 2005;21(2):240–5.Manca A, Epstein DM, Torgerson DJ, Klaber Moffett JA.
Randomized trial of a brief physiotherapy intervention compared with usual physiotherapy for neck pain patients: cost-effectiveness analysis.
Int J Technol Assess Health Care 2006;22(1):67–75.Appleby J, Devlin N, Parkin D.
NICE’s cost effectiveness threshold.
BMJ 2007;335(7616):358–9.Towse A.
Should NICE’s threshold range for cost per QALY be raised? Yes.
BMJ 2009;338:b181.Bosmans JE, Pool JJ, de Vet HC, van Tulder MW, Ostelo RW.
Is behavioral graded activity cost-effective in comparison with manual therapy for patients with sub-acute neck pain? An economic evaluation alongside a randomized clinical trial.
Spine 2011;36(18):E1179–86.United Kingdom Back Pain Exercise and Manipulation (UK BEAM) Randomised Trial
Cost Effectiveness of Physical Treatments for Back Pain in Primary Care
British Medical Journal 2004 (Dec 11); 329 (7479): 1381–1385Lewis M, James M, Stokes E, Hill J, Sim J, Hay E, et al.
An economic evaluation of three physiotherapy treatments for non-specific neck disorders alongside a randomized trial.
Rheumatology (Oxford) 2007;46(11):1701–8.Niemisto L, Rissanen P, Sarna S, Lahtinen-Suopanki T0.
Cost-effectiveness of combined manipulation, stabilizing exercises, and physician consultation compared to physician consultation alone for chronic low back pain: a prospective randomized trial with 2-year follow-up.
Spine 2005;30(10):1109–15.Korthals-deBos IBC, et al.
Cost Effectiveness of Physiotherapy, Manual Therapy, and General Practitioner Care
for Neck Pain: Economic Evaluation Alongside a Randomised Controlled Trial
British Medical Journal 2003 (Apr 26); 326 (7395): 911Williams N, Edwards R, Linck P, Muntz R, Hibbs R, Wilkinson C, et al.
Cost-utility analysis of osteopathy in primary care: results from a pragmatic randomized controlled trial.
Fam Pract 2004;21(6):643–50.Drummond M, Jefferson T.
Guidelines for authors and peer reviewers of economic submissions to the BMJ.
BMJ 1996;313:275–83.Chiou C, Hay J, Wallace J, Bloom B, Neumann P, Sullivan S, et al.
Development and validation of a grading system for the quality of cost-effectiveness studies.
Med Care 2003;41:32–44.van der Roer N, Goossens ME, Evers SM, van Tulder MW.
What is the most costeffective treatment for patients with low back pain? A systematic review.
Best Pract Res Clin Rheumatol 2005;19(4):671–84.Briggs A, Sculpher M, Buxton M.
Uncertainty in the economic evaluation of health care technologies: the role of sensitivity analysis.
Health Econ 1994;3(2):95–104


Return to COST-EFFECTIVENESS
Since 4-17-2013


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |
