

Can Manipulation of the Ratios of Essential Fatty Acids
Slow the Rapid Rate of Postmenopausal Bone Loss?This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Alternative Medicine Review 2001 (Feb); 6 (1): 6177 ~ FULL TEXT
Debra B. Kettler, MS, DCThe rapid rate of postmenopausal bone loss is mediated by the inflammatory cytokines interleukin-1, interleukin-6, and tumor necrosis factor alpha. Dietary supplementation with fish oil, flaxseeds, and flaxseed oil in animals and healthy humans significantly reduces cytokine production while concomitantly increasing calcium absorption, bone calcium, and bone density. Possibilities may exist for the therapeutic use of the omega-3 fatty acids, as supplements or in the diet, to blunt the increase of the inflammatory bone resorbing cytokines produced in the early postmenopausal years, in order to slow the rapid rate of postmenopausal bone loss. Evidence also points to the possible benefit of gamma-linolenic acid in preserving bone density.
From the FULL TEXT Article:
Introduction
The National Institutes of Health Consensus Development Conference Statement on Osteoporosis Prevention, Diagnosis and Therapy, published in March 2000 states:
"Osteoporosis, once thought to be a natural part of aging among women, is no longer considered age or gender-dependent. It is largely preventable due to the remarkable progress in the scientific understanding of its causes, diagnosis and treatment. Optimization of bone health is a process that must occur throughout the lifespan in both males and females. Factors that influence bone health at all ages are essential to prevent osteoporosis and its devastating consequences." [1]
In the United States today, eight million women have osteoporosis and 15 million more have osteopenia, placing them at increased risk for osteoporosis. One out of two women will have an osteoporosis-related fracture in their lifetime. Osteoporosis is responsible for more than 1.5 million fractures annually, with an associated cost for direct expenditures in 1995 (hospitals and nursing homes) of $13.8 billion. [2] The most typical sites of osteoporosis related fractures are the thoracic and lumbar vertebral bodies (T8 through L3), the proximal femur, distal radius, humerus, pelvis, and ribs. Of all osteoporotic fractures, those at the hip are associated with the highest risk of morbidity and mortality. [3]
Many factors contribute to the lifetime accumulation or decline in bone mineral density (BMD), including levels of the nutrients vitamin D, calcium, sodium, and protein, as well as lifestyle factors such as body mass index, exercise, drug and alcohol use, and smoking. [1, 2] Remodeling of bone takes place throughout adult life, with osteoclasts resorbing old bone and osteoblasts creating new bone. These cells continuously renew the skeleton while maintaining its strength and density. Normally, in the adult skeleton, three percent of cortical bone and 25 percent of trabecular bone is remodeled each year. The primary characteristic of osteoporosis is a reduction in bone mass due to an increase in bone resorption over bone formation. Postmenopausal osteoporosis is characterized by an accelerated loss of bone tissue (24% per year on average) that begins after natural or surgical menopause, and lasts 510 years in the absence of treatment. Fractures are most likely to occur within 1520 years after ovarian function ends. [4]
Postmenopausal bone loss is associated with an increase in both the number and activity of osteoclasts in trabecular bone. This rapid decline in BMD at menopause is often followed by a gradual decline in BMD, known as age-related osteoporosis (12% per year on average), which may persist indefinitely and may accelerate once more after the age of 70. [5] The rapid decline in BMD at menopause is the major factor contributing to the high rate of disabling bone fractures in postmenopausal women. [6]
Biology of Cytokines
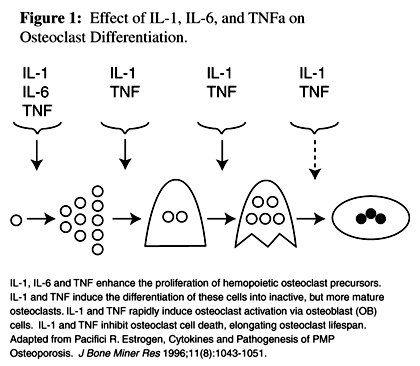
Figure 1 Bone cells and hematopoietic cells share the same progenitors, respond to some of the same cytokines (Figure 1), and enjoy a symbiotic relationship. Osteoclasts and osteoblasts are both formed in the bone marrow. The progenitors of osteoclasts are from the hematopoietic cell line and the osteoblasts originate from the marrow stroma. Osteoclasts only develop in the presence of stromal/osteoblast cells, which mediate the effects of cytokines and systemic hormones. [7]
Cytokines include interleukins (IL), interferons, colony stimulating factors, tumor necrosis factor alpha (TNFa), and transforming growth factors. Cytokines are secreted proteins that induce cells to proliferate and differentiate. They are produced by both lymphocytes and monocytes and vary tremendously in function and biochemical properties. However, cytokines do have several characteristics in common:(1) cytokines are all glycosylated proteins;
(2) cytokines act only on cells that express specific receptors for that cytokine; and
(3) cytokines may have several functions, acting on several different types of cells.Cytokines regulate hematopoiesis, the inflammatory response, and immunity. [8] Three cytokines, interleukin-1 (IL-1), interleukin-6 (IL-6), and TNFa are described as inflammatory cytokines; [9] they are active in the pathophysiology of osteoporosis, increasing osteoclast formation, activity, and lifespan. [7]
IL-1 and TNFa, produced primarily by monocytes and macrophages, stimulate their own and each other's synthesis. [10] IL-1 and TNFa stimulate stromal/osteoblast cells to produce IL-6. [11] IL-6 regulates osteoclast progenitor differentiation and stimulates the early stages of osteoclastogenesis in human and murine cultures, suggesting that it acts on osteoclast hemopoietic precursors, but does not activate mature osteoclasts. IL-1 and TNFa are powerful stimulators of bone resorption and inhibitors of bone formation. They cause bone resorption in vitro and hypercalcemia when infused in vivo. They activate mature osteoclasts indirectly through osteoblasts, inhibit osteoclast cell death, and stimulate osteoclast progenitor formation. [5]
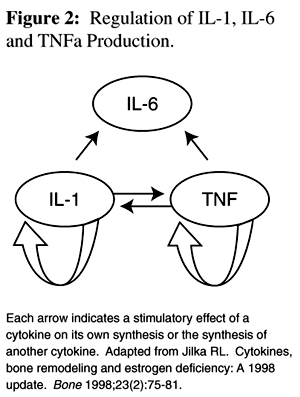
Figure 2 Due to feedback interactions of the cytokines (Figure 2), a small increase in IL-1 and TNFa formation leads to a significant increase in levels of all three cytokines. Conversely, the lack of any one of these cytokines will decease the levels of the others, possibly inhibiting osteoclast formation and bone loss. [10]
Estrogen replacement therapy is useful in the reduction of postmenopausal bone loss. [12] Studies suggest estrogen acts to reduce bone resorption by inhibiting the release of cytokines from bone marrow and bone cells. [1316]
Review of Fatty Acids
There are two classes of essential fatty acids (EFAs): omega-3 and omega-6. Humans (like all mammals) are unable to synthesize EFAs so they must be provided in the diet. [17] EFAs are required for membrane integrity, visual and neurological function, and their deficiency is associated with neurological and immunological disease. [18] Small changes in the fatty acid composition of the cell membrane can significantly alter cell function. [19]
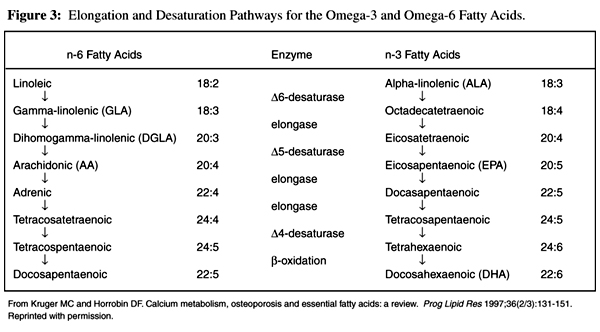
Figure 3 The parent compound in the omega-6 fatty acid family is linoleic acid (LA), while the parent compound of the omega-3 fatty acid family is a-linolenic acid (ALA). These parent compounds are metabolized to longer-chain fatty acids (which play other, more important roles in the body) by a series of elongation and desaturation steps (Figure 3). LA is first converted to gamma-linolenic acid (GLA), then to dihomogamma-linolenic acid (DGLA) and arachidonic acid (AA), while ALA is converted to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). [19]
Although the omega-3 and omega-6 fatty acids compete for the desaturation enzymes, the D 4 and D 6 desaturases favor the omega-3 fatty acids. [17] Generally, the desaturation steps are slow and rate limiting, while the elongation steps usually proceed rapidly. Factors known to inhibit fatty acid desaturation are aging, smoking, diabetes, high sodium intake, and biotin deficiency, whereas calcium deficiency can impair essential fatty acid elongation. [19]
Fatty fish are the major source of EPA and DHA in the U.S. diet, while vegetable oils, especially soybean and canola oils, are the primary sources of ALA. Although flaxseed oil contains approximately 57-percent ALA, it is not commonly used in food preparation. Nuts, seeds, vegetables, and some fruit, as well as egg yolk, poultry, and meat contribute small amounts of omega-3 fatty acids to the diet.
The typical American diet has a high ratio of omega-6:omega-3 fatty acids. [20] Studies show that the consumption of increased amounts of fish, [21] fish oil, [22-25] flaxseed oil, [25, 26] or canola oil [27] will result in the incorporation of the longer-chain omega-3 fatty acids EPA and DHA into the plasma and cell membranes of platelets, erythrocytes, neutrophils, monocytes, and liver cells. This leads to a change in the ratio of omega-6:omega-3 fatty acids in the membranes, [28, 29] a change in the function of the membranes, [29] and a decrease in the production of IL-1, IL-6 and TNFa. [2225]
Experimental and Clinical Evidence for the Involvement
of Essential Fatty Acids in Osteoporosis
Studies in rats have shown that EPA inhibits bone loss due to ovariectomy, [30] that fish oil can inhibit bone resorption, [31] and supplementation of essential fatty acids as GLA and EPA can increase calcium absorption, [32] and enhance bone calcium. [3133] A pilot study in humans, supplementing GLA and EPA, also showed an increase in BMD. [34]
In this review, the MEDLINE database was searched for research to support or refute the question: Do essential fatty acids (especially EPA, DHA, and GLA) slow the rapid loss of bone at menopause? The relationships between cytokines and menopause in humans, omega-3 fatty acids and cytokines in humans, and omega-3 and omega-6 fatty acids and bone in rats and humans are explored.
Clinical Studies of the Effect of Menopause on Cytokines

Table 1 A series of small cross-sectional and prospective studies (Table 1) performed by Pacifici et al, [13, 14, 35] on cultured peripheral blood mononuclear cells (PBMC), supports the idea that cytokines and loss of estrogen at menopause effect postmenopausal osteoporosis. Under normal conditions circulating cytokine levels in healthy humans are extremely low. Therefore, in most human studies, PBMCs are isolated, cultured for 24 hours and then stimulated in vitro to produce detectable cytokine concentrations. These tests measure the capacity for PBMCs to produce cytokines.
In a 1987 cross-sectional study, Pacifici et al [35] were the first to recognize that IL-1 was secreted in higher amounts from the PBMCs of patients with "high turnover" osteoporosis. "High turnover" osteoporosis is recognized as the hallmark of postmenopausal osteoporosis. Thirty-six individuals were enrolled from the Jewish Hospital of St. Louis, Missouri. The participants were healthy, ambulatory, and voluntarily sought treatment. Patients previously treated for osteoporosis or with secondary osteoporosis were excluded from the study.
There were 14 control subjects ages 44.0 ± 9.2 years (range 30-59 years). Their history was negative for back pain, fractures, or loss of height, and they had normal vertebral mineral density (137.0 ± 5.4 mg Ca/cm3) by quantitative CT scan. The twenty-two subjects with a mean age of 51.4 ± 12.8 years (range 29-77 years), had a positive history for at least one spontaneous spinal fracture and evidence of osteopenia on lateral lumbar spine X-rays. Their vertebral mineral density was significantly lower than controls (60 ± 7.4 mg Ca/cm3; p< 0.001). Monocytes from both the normal and osteoporotic subjects were cultured for 48 hours and found to secrete IL-1 spontaneously at all dilutions tested. The mean IL-1 secretion was significantly higher in the subjects (14.8 ± 3.0; p < 0.001) than the controls (3.1 ± 0.8).
The osteoporotic subjects could be further separated into two groups: those whose cultured monocytes secreted high amounts of IL-1 (26.5 ± 3.4; p< 0.05), and those whose cultured monocytes secreted low amounts of IL-1 (3.2 ± 0.4). Levels of immunoreactive bone 4-carboxyglutamic acid protein, a marker of bone formation, were positively correlated with high IL-1 levels, indicating an increased rate of bone formation in the high IL-1 group.
In a small cross-sectional study on 57 pre- and postmenopausal osteoporotic and non-osteoporotic women, Pacifici et al [13] reported that menopause without hormone replacement therapy (HRT) was positively correlated with a marked increase in peripheral blood monocyte IL-1 production (101.2 ± 42.1 units/mL) that was suppressed by estrogen therapy (1.2 ± 0.5 units/mL; p<0.01). Additionally, they found a significant negative correlation between IL-1 production and years since menopause; however, non-osteoporotic postmenopausal women showed a reduction in IL-1 to premenopausal levels within eight years postmenopause, while osteoporotic women continued to demonstrate high IL-1 levels. In a second prospective study within this report, HRT consisting of conjugated estrogen (0.625 mg/d for days 1-25 of the month) and medroxyprogesterone acetate (10 mg/d for days 15-25 of the month) was initiated in three non-osteoporotic and five osteoporotic postmenopausal women. HRT decreased PBMC secretion of IL-1 significantly from a mean of 79.1 ± 47.5 units/mL before treatment to a mean of 2.1 ± 1.0 units/mL (p< 0.01) within one month of treatment.
The effect of oophorectomy and subsequent estrogen replacement therapy (ERT) on the spontaneous secretion of IL-1 and TNFa from PBMCs was evaluated in a prospective study in 1991. [14] The study population consisted of 15 healthy Caucasian premenopausal women, 41.9 ± 2.4 years, undergoing total hysterectomy with bilateral oophorectomy, and nine healthy control premenopausal Caucasian women, 39.8 ± 2.3 years, undergoing hysterectomy without oophorectomy. Surgery was performed for uterine myomas or uncontrollable non-neoplastic bleeding. All women had normal BMD. The women who underwent hysterectomy without oophorectomy did not show any changes in estrogen levels, indices of bone turnover, or cytokine release. In the women who underwent oophorectomy, 17b-estradiol levels decreased significantly within one week and significant elevations in IL-1 and TNFa (p< 0.05), as well as urinary indices of bone resorption (p< 0.01), were seen within two weeks of surgery. Six women did not take ERT and their levels of IL-1, TNFa, and indices of bone turnover continued to increase throughout the eight weeks of the study. In the nine women who took ERT, estrogen levels increased to preoperative levels within one week of treatment (week 5 after surgery) and IL-1 and TNFa decreased significantly (p<0.05) after two weeks of ERT, reaching preoperative levels by the fourth week of treatment. This study appears to demonstrate that it is the change in ovarian hormone status that accounts for the postovariectomy cytokine increase, not the surgical stress, as no rise in cytokines was seen in the hysterectomy-without-oophorectomy group. The study would have been strengthened if the women had been followed for a longer period of time.
A cross-sectional study by Bismar et al [15] examined cytokine levels in bone marrow aspirates of patients with localized breast cancer without metastasis, inflammatory diseases, or intake of drugs known to affect bone metabolism (except HRT). Forty women participated in the study: 12 were premenopausal (41 ± 7 years, range 28-51); five were within five years of menopause (51 ± 5 years, range 4457 years, 2.3 ± 1.6 years since menopause); 18 were postmenopausal for over eight years (70 ± 6 years, range 6283, 18 ± 8 years since menopause); and five (61 ± 5 years, range 5569, 15 ± 15 years since menopause) had been receiving estrogen for 3, 5, 9, 18, or 38 years, respectively, and had discontinued estrogen within one month of surgery. Significantly higher levels of IL-1, IL-6 and TNFa were seen in the bone marrow cell cultures of women who had recently discontinued estrogen therapy than pre- or postmenopausal women. The highest cytokine levels were seen in the three women who had been receiving estrogen therapy for over eight years.
This study demonstrated that estrogen-associated changes in cytokine secretion that have been observed in PBMCs in culture also occur in human bone marrow. Bone marrow cells from early postmenopausal women or from women who have recently discontinued HRT have an increased potential for cytokine secretion. Long-term estrogen therapy does not prevent increased cytokine production on discontinuing estrogen. The increase in cytokine production after natural menopause appears to be self limiting, as bone marrow cultures from women more than eight years after menopause had slightly lower cytokine levels than the premenopausal women. This correlates with the rapid self-limiting increase in bone turnover that occurs after menopause. This study did not have a control group of non-breast cancer patients and therefore an effect of the breast cancer itself cannot be ruled out, although the results were in line with the results of other studies that have looked at cytokine levels in PBMCs.
A 1995 study by Cantatore et al [16] examined the effect of estrogen replacement on bone metabolism and serum cytokine levels in surgical menopause. No significant changes in IL-1 or IL-6 were observed in women without oophorectomy. Significant increases in IL-1 and IL-6, as well as parathyroid hormone (PTH) were seen six months post-surgical menopause in women with oophorectomy and without HRT. The long interim period (six months) rules out the possibility of inflammation causing the rise in cytokines. The rise in alkaline phosphatase, indicating increased bone remodeling, was positively correlated with the rise in PTH and cytokines. It is interesting that this study was conducted on serum cytokine levels, not on cultured PBMCs.
McKane et al [36] studied eighty normal, healthy women (2487 years). Cytokines were measured in fasting morning blood, and IL-6 was positively correlated with both an increase in age (increasing three-fold over the 2487 year range in age of the women (p< 0.001)) and with type I collagen carboxyl-terminal telopeptide (p< 0.05), a marker of bone breakdown. IL-1 levels did not appear to be associated with age, menopausal status, serum estra-diol, bone mineral density, or bone biochemical markers. The contradictory results of this study point to the possibility that an increased production of bone resorbing cytokines may occur only in the local environment of the bone or bone marrow, and may not easily be detected in the serum. Or, since the authors did not divide the postmenopausal women into early (510 years) and late (>10 years) postmenopause, the results may have been obscured, as the rise in cytokines occurs, as stated in the previous papers, in early postmenopause in most women, returning to normal levels after eight years postmenopause. [11, 13] Proper division of the women might have altered the results as cytokine values in late menopausal women can return to premenopausal levels.
A 1998 cross-sectional study by Rogers and Eastell [37] assessed the effects of ERT on the secretion of cytokines in the peripheral blood. The subjects were ten women ages 5659 years, between three and nine years postmenopause, who took ERT for at least two years. Ten age-matched women, age 5459 years, between four and ten years postmenopause, without ERT in the previous two years acted as controls. The authors did not mention ruling out illness or other medical treatments that could affect bone. The study showed a trend toward decreased levels of IL-1 in ERT women, but this was not significant. The difference in results in this study compared to the studies by Pacifici et al [13, 14, 35] can be attributed to the different methods of sample collection. This study used whole blood, as compared to PBMCs in the studies by Pacifici et al. The lack of increased serum cytokine levels in estrogen-deficient women is consistent with the idea that cytokine release requires the adherence to a solid substrate (bone); therefore, estrogen deficiency is unlikely to stimulate cytokine secretion from circulating cells. [5]
Clinical Trials of the Effect of Essential Fatty Acid Supplementation on Cytokines

Table 2 Omega-3 fatty acids have been investigated for their possible anti-inflammatory effects in rheumatoid arthritis, psoriasis, ulcerative colitis, and heart disease. Originally, the anti-inflammatory effects were thought to be modulated by the production of prostaglandins and leukotrienes. More recent studies point to a decrease in cytokine production as another potential mechanism for their anti-inflammatory effects. [38] Clinical trials on the effects of omega-3 fatty acid supplementation on cytokine production in humans are reviewed here (Table 2).
Two studies showed dramatic decreases in cytokine production following omega-3 fatty acid supplementation. Endres et al22 gave 18 g/d MaxEPA® fish oil containing 2.7 g EPA and 1.85 g DHA to nine healthy, young (2139 years) male volunteers for six weeks. Production of IL-1 and TNFa by stimulated PBMCs was assessed four times during the study: at baseline, after six weeks of supplementation, and 10 and 20 weeks after ending supplementation; PBMC fatty acid profiles were also analyzed. The results showed that dietary supplementation with omega-3 fatty acids reduced the inducible production of IL-1b (43%, p=0.048) at six weeks. Ten weeks after the end of supplementation there was a further decrease (61%, p=0.005). The production of IL-1b returned to pre-supplementation levels 20 weeks after supplementation ended. IL-1a and TNFa levels fell in a similar pattern. Although the decreases in IL-1a and TNFa were not significant at six weeks, they were significant 10 weeks after the end of supplementation (IL-1a decreased 39%, p=0.022; and TNFa decreased 40%, p=0.008). Twenty weeks after the end of supplementation the production of these cytokines had returned to pre-supplementation levels. The control group did not show any of these changes.
In the same study, the results from a sample of five subjects showed a significant increase in the omega-3 fatty acid composition of mononuclear-cell membranes (from a baseline value of 3.0 ± 0.3% to 7.1 ± 1.1%) after six weeks of supplementation, an increase of more than 100 percent (p< 0.03). The ratio of AA:EPA in the mononuclear-cell membranes was significantly changed after six weeks of supplementation when compared to baseline. The ratio of AA:EPA remained lower than baseline ten weeks after supplementation was discontinued (20.9 ± 2.2 at baseline; 2.4 ± 0.2 at six weeks; 12.0 ± 2.1 at ten weeks after supplementation).
In a second clinical trial, Meydani et al [23] measured the effect of dietary omega-3 fatty acids on cytokine production in young and older women. Six healthy young women (2333 years, mean age 26.7 ± 1.7 and non-menopausal) and six healthy older women (5168 years, mean age 60.7 ± 2.9 and naturally postmenopausal for at least two years) supplemented their typical American diet (3540% of energy from fat, 300400 mg cholesterol/d) with omega-3 fatty acids daily for 12 weeks. Each subject received 1.68 g EPA and 0.72 g DHA daily. Blood samples were collected at baseline and at one, two, and three months to measure IL-1, IL-2, IL-6 and TNFa. Compliance was confirmed by the significant increase in plasma EPA and DHA noted in both groups, with a ten-fold increase in EPA in older women and a five-fold increase in EPA in younger women. AA was significantly decreased only in the older women, but the AA:EPA ratio was significantly decreased in both groups (young women: p< 0.003 and older women: p< 0.001). The production of the pro-inflammatory cytokines, IL-1, IL-6, and TNFa, was not significantly different between young and older women prior to omega-3 fatty acid supplementation. Omega-3 fatty acid supplementation for a three-month period significantly suppressed the inducible production of IL-1, IL-6, and TNFa, as well as IL-2 in both young and older women. The synthesis of IL-1 and TNFa was reduced by more than 50 percent in an eight-week period and continued to decline at 12 weeks. While the decrease in inducible production of IL-1, IL-6, TNFa, and IL-2 was present in both younger and older women, the decrease was greater in older women, even though the baseline levels were similar. The authors noted that the decrease in IL-2 could negatively impact the immune response and lead to an increased risk of infections and tumors, particularly in the older women. However, Wu et al [39] demonstrated that in the presence of adequate vitamin E levels, increasing the intake of EPA and DHA could increase IL-2 production. All values returned to presupplementation levels at 20 weeks and no significant change was seen in cytokine production in the control group not taking fatty acid supplements.
In contrast to the results seen in the above studies, a 1997 study by Blok et al [40] found no difference in cytokine production between the placebo and omega-3 treatment groups at any point during the one-year random blinded intervention. Fifty-eight monks in good health ranging in age from 2187 years (56.2 ± 16.5 years) participated in the study. The study consisted of a two-week baseline period, a one-year intervention, and a six-month follow-up. The subjects were randomly and blindly divided into four groups: one group received no omega-3 (n = 14); a second group received 1.06 g omega-3/d (n = 15); a third group received 2.13 g omega-3/d (n = 15); and a fourth group 3.19 g omega-3/d (n = 14). The supplementation was in the form of fish oil capsules.
The production of IL-1 and TNFa was not significantly different among the four diet groups at 26 or 52 weeks of supplementation or 4, 8 or 24 weeks post-supplementation. Interestingly, in all three treatment groups as well as the placebo group, endotoxin-stimulated secretion of IL-1 was significantly higher during oil supplementation. The study found levels of EPA in erythrocyte membranes increased significantly in all groups except placebo. However, the baseline values of EPA in the membranes were almost one-percent of total fatty acids in all of the treatment groups, and approached one-percent even in the placebo group during the study. A study by Caughey [25] noted that as little as one-percent EPA in the membrane was necessary to inhibit IL-1 and TNFa production. It should also be noted the cytokines were measured ex vivo in whole blood, not from cultured PBMCs as in the other studies.
Dietary Effects on Cytokine Production
It is possible to influence cytokine production by dietary manipulation. The National Cholesterol Education Panel Step 2 diet (NCEP Step 2) for the reduction of cholesterol recommends a fat intake of <30 percent of calories (<7-percent calories from saturated fatty acids, 10-15 percent of calories from monounsaturated fatty acids, and £10 percent of calories from polyunsaturated fats (PUFA)) with a cholesterol intake of <200 mg/d.
Meydani et al [24] studied the effects of long-term (24 weeks) feeding of the NCEP Step 2 diet with or without fish-derived omega-3 fatty acids on in vitro and in vivo cytokine production. The 30-week clinical trial period was divided into two diet phases and all food was supplied by the study. Twenty-two healthy men and women volunteers over the age of 40 (range 5073 years) were initially fed a typical American diet for six weeks. For the following 24 weeks the group was divided in half; each half consumed low-fat, low-cholesterol, high PUFA diets based on the NCEP Step 2 recommendations. One diet was rich in omega-3 fatty acids (low-fat, high fish: 0.54% or 1.23 g/d EPA and DHA, equal to 121188 g fish/d), while the other was low in omega-3 fatty acids (low-fat, low-fish: 0.13% or 0.27 g/d EPA and DHA, equal to 33 g fish/d).
Inducible IL-1b (40%; p=0.03), IL-6 (34%; p< 0.05), and TNFa (35%; p=0.4) were all significantly decreased in the low-fat, high-fish diet group. The low-fat, low-fish diet caused a significant increase in inducible IL-1b (62%; p< 0.05) and TNFa production (47%; p<0.05). This dietary intervention shows that omega-3 fatty acids supplied as fish (121188 g/d (4.36.7 oz/d) from tuna, filet of sole and salmon) can have similar cytokine lowering effects as fish oil supplements.
Caughey et al [25] examined the effects of a flaxseed oil-based diet on IL-1 and TNFa levels in healthy male volunteers. A sunflower based diet was compared with a flaxseed oil based diet in parallel groups. The flaxseed oil group (n=15) was instructed to maintain a diet high in omega-3 fatty acids by using flaxseed oil and a flaxseed oil and butter spread (2:1) in place of their usual cooking oils and spreads. The flaxseed oil contained 56-percent ALA and 18percent LA, and the flaxseed oil and butter spread contained 23percent ALA and 8percent LA. The control group (n=15) was instructed to maintain a diet high in omega-6 fatty acids by using sunflower oil, and sunflower-based spreads and salad dressings. The diets were maintained for eight weeks. After the first four weeks, both groups supplemented their diets with 1.62 g/d EPA and 1.08 g/d DHA from fish oil.
The average dietary intake of ALA in the flaxseed group was 13.7 g/d and resulted in a membrane EPA content of 0.4-percent of total fatty acids. ALA inhibited the inducible production of IL-1b and TNFa by approximately 30 percent (p< 0.05) after four weeks. EPA ingestion of 1.6 g/d in the second four-week period resulted in a membrane EPA content of 1.6 and 1.7percent of total fatty acids in the sunflower and flaxseed groups, respectively, and inhibited the inducible production of IL-1b and TNFa by 7080 percent (p< 0.05) in both the sunflower and flaxseed oil groups. The suppression of both cytokines was maximal when the membrane EPA content reached approximately one percent. Further suppression of cytokine secretion was not seen with higher membrane levels of EPA, indicating that high doses of fish oils may not be necessary to provide maximal cytokine inhibition. This study demonstrated that free-living subjects could elevate their membrane EPA concentrations and decrease inducible cytokine production with the use of flaxseed oil in their own domestic food preparation. However, flaxseed oil is not suitable for all aspects of food preparation, such as frying, due to its high degree of unsaturation. The study further demonstrated a greater decrease in cytokine production with the addition of EPA supplementation.
Animal Studies of the Effect of EFAs on Bone
Sakaguchi [30] was the first to report on the interaction of estrogen deficiency, EPA, and bone activity in rats. Ovariectomy and low calcium diet caused a decrease in bone weight and bone strength (both p< 0.01). EPA prevented the loss of bone weight and bone strength in the ovariectomy and low calcium diet group, but it failed to show an increase in bone weight and strength in the normal calcium group.
Claassen et al [31] studied the effects of feeding different ratios of GLA and EPA on bone status and parameters of bone collagen breakdown by assessing free urinary pyridinium cross links (Pyd) in growing rats, age 5-12 weeks. Pyd excretion was significantly lower in all the groups receiving the diets containing GLA and EPA. No abnormal bone growth stimulation or restriction was seen in any of the supplemented groups. After six weeks of supplementation the 3:1 and 1:1 (GLA:EPA) diet groups showed significantly higher levels of bone calcium than controls (24.7% and 9.0%, respectively, p< 0.05), and bone calcium was significantly higher in the 3:1 diet group than in the 1:1 diet group (p< 0.05). The 1:3 diet group experienced a statistically insignificant decrease in bone calcium compared to the control group. Claassen et al [32] further explored the effect of GLA:EPA on calcium absorption in the same group of rats. Calcium absorption (calcium intake minus fecal excretion) after the six-week supplementation period was significantly higher in the 3:1 and 1:3 supplemented groups (41.5% and 21.4%, respectively) as compared to the control group (p< 0.001 and p< 0.05, respectively). This study shows that essential fatty acid supplementation may have a role in reducing the age-related decline in calcium absorption.
Kruger et al [33] used ovariectomized (OVX) female rats to study the relationship between EFAs, bone turnover, and bone calcium. The rats were supplemented from age 1218 weeks with a semi-synthetic diet containing different ratios of GLA:EPA+DHA (9:1, 3:1, 1:3, 1:9) added to the diet. LA:ALA (3:1) was used as a control in a sham-operated and OVX group (n=7 per group). DGLA (r=0.54; p=0.007), DHA (r=0.65; p=0.002) and EPA (r=0.59; p=0.003) were all significantly and positively correlated with calcium concentration in the femur. DGLA (r=0.61; p=0.002), DHA and EPA were negatively correlated with deoxypyridinoline (Dpyd), a marker of bone degradation but only DGLA reached significance. DGLA may have an anabolic effect on bone, indicated by the positive correlation with bone calcium and the negative correlation with Dpyd.
Schlemmer et al [41] tested the effect of GLA and EPA, in the form of a novel diester, in the prevention of bone loss in the ovariectomized rat. The ovariectomy + placebo (OVX/P) group showed lower femur calcium levels and increased Dpyd levels. The one-percent linoleic acid + estrogen (linoleic/E) and the diester + estrogen (diester/E) groups both showed significant increases in mg calcium/mm (12.6% and 17.5%, respectively; p=< 0.05) when compared to OVX/P. Additionally, linoleic/E and diester/E had significantly lower excretion of Dpyd compared to OVX/P and the effect of estrogen was enhanced in the diester/E group by the diester. In this study, only the groups with the estrogen implant showed significant increases in bone calcium and significant decreases in bone turnover as measured by Dpyd, although the diester alone did increase bone calcium toward baseline levels.
Clinical Studies of the Effect of EFAs on Bone
In a single-blind, randomized study, Kruger et al [34] studied 65 osteoporotic or osteopenic women, confirmed by bone densitometry, mean age 79.5 ± 5.6. All of the women were living in the same institution for the elderly and fed the same low-calcium, non-vitamin D enriched foods, and had similar amounts of sunlight. The study was conducted for 18 months and at the end of the study all of the women were offered the option of continuing treatment for another period of 18 months. A total of 21 women agreed to continue, including 11 women who had previously been on placebo. The subjects received a 6 g mixture of evening primrose oil and fish oil. Analysis of the capsules showed 60-percent LA, 8-percent GLA, 4-percent EPA, and 3-percent DHA. The placebo capsules contained 6 g coconut oil (97% saturated fat and 0.2% LA). The fatty acids were supplied as 500 mg capsules and four were taken three times daily with meals. In addition, all patients received 600 mg/d calcium, as calcium carbonate, which brought their daily calcium intake to 1253 ± 249 mg/d. Fatty acids and calcium were supplemented for 18 months.

Table 3 The marker of bone degradation, Dpyd, measured in urine, was decreased significantly in both the treatment and placebo groups (p< 0.05), perhaps indicating an effect due to the increase in calcium intake in both groups. A Lunar DPX-L densitometer was used to measure the lumbar spine BMD at baseline, and at 12 and 18 months. It was measured again at 36 months in those continuing treatment. During the first 18 months of the study lumbar spine BMD stayed the same in the treatment group, while it decreased 3.2 percent in the placebo group. Femoral bone density increased 1.3 percent in the treatment group and decreased 2.1 percent in the placebo group. The difference in risk for fracture at 18 months between the two groups was significant (p=0.037) with the treatment group having a lower risk. At 36 months the lumbar spine BMD of the group who had received continual treatment increased 3.1 percent, while the change 18 months earlier to active treatment from placebo increased lumbar spine BMD 2.3 percent. Femoral neck BMD remained the same in the treatment group but increased 4.7 percent in patients who changed from placebo to active treatment. The increases in BMD in the groups continuing treatment may possibly indicate a specific effect due to the EFAs, as the calcium was maintained in both groups throughout the length of the study. Table 3 summarizes the EFA and bone studies.
Conclusion
Although low peak bone mass contributes to postmenopausal osteoporosis, an ovarian hormone-dependent increase in bone remodeling and accelerated loss of bone in the early years postmenopause appear to be the main pathologic factors. The NIH Consensus Statement calls for HRT and consumption of recommended dietary intake levels of calcium and vitamin D as the most effective way to build bone mass at menopause. [1] However, a proper balance of the essential fatty acids, without the inclusion of HRT, may also play a role in minimizing bone loss at menopause. [3033, 42] Most women are very concerned with menopausal weight gain and may diet extensively to control their weight. A study by Salamone et al [43] demonstrated that this could have deleterious effects on BMD, as the intervention group of perimenopausal women (average age 46.7 ± 1.7 years), who modified their lifestyle to lose weight by lowering fat intake and increasing physical activity, had a two-fold greater rate of loss in hip BMD (p=0.015) compared to a non-dieting control group. The loss of BMD with dieting may be induced by alterations in the total body content of the essential fatty acids, such as by membrane depletion or preferential utilization and excretion. [44]
None of the studies reviewed can definitively conclude that increasing the level of omega-3 fatty acids or manipulating the ratio of GLA:EPA in the diet will slow the rapid loss of bone at menopause. However, there are interesting associations that deserve further attention. Inflammatory cytokines are produced in the local bone environment at menopause, [1315] and monocytes are the primary producers of IL-1 and TNFa in the local bone environment. [5] Supplementation of omega-3 fatty acids as fish oil, dietary fish, and flaxseed oil decreases the production of IL-1, IL-6, and TNFa in cultured PBMCs. [2225] The fatty acids GLA, EPA, and DHA in plasma and cell membranes are positively correlated to bone calcium. [32]
Incorporating higher amounts of the omega-3 fatty acids into the diet, thereby altering the ratio of omega-6:omega-3, while concurrently increasing vitamin E intake to inhibit lipid peroxidation, may have a positive effect on calcium absorption and bone density. There is also a need for additional study to further understand the relationships between fatty acids, calcium, and vitamin D. Such studies could supplement different ratios of the parent fatty acids LA:ALA, different ratios of GLA:EPA+DHA, or different ratios of GLA:ALA, while controlling for current LA:ALA levels in the diet, saturated and monounsaturated fat, vitamin D and calcium intake, and measuring BMD, Dpyd, serum 25(OH)D, and PTH in pre- and postmenopausal women.

Return to OMEGA-3 FATTY ACIDS
Return to GAMMA-LINOLENIC ACID
Since 4-01-2001


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |