

Effects of Manual Versus Instrumental Spinal Manipulation
on Blood Flow of the Vertebral and Internal Carotid
Arteries in Participants With Chronic Nonspecific
Neck Pain: A Single-Blind, Randomized StudyThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Chiropractic Medicine 2023 (Mar); 22 (1): 110 ~ FULL TEXT
OPEN ACCESS Burcu Kocabey, Dilber Karagozoglu Coskunsu, PhD, Koray Guven, Mustafa H. Agaoglu, DC, Selvi Yuce
Bahcesehir University Institute of Health Sciences,
Istanbul, Turkey
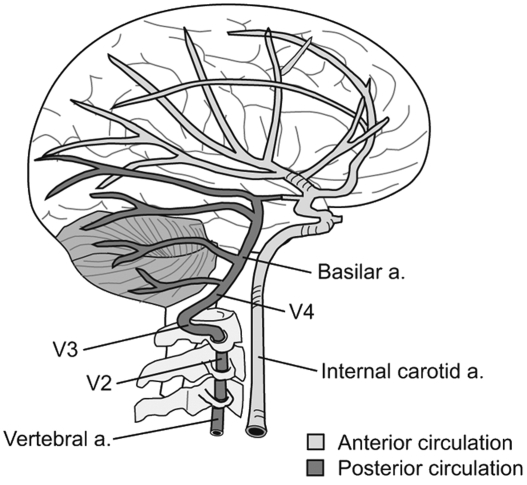
FROM: J Chiropractic Medicine 2007Objective The aim of this study was to compare the hemodynamic effects of manual spinal manipulation (MSM) and instrumental spinal manipulation (ISM) on the vertebral artery (VA) and internal carotid artery (ICA) in participants with chronic nonspecific neck pain (NNP).
Methods Thirty volunteers aged 20 to 40 years old with NNP over 3 months duration were included. Participants were randomly divided into the following 2 groups: (1) MSM group (n = 15) and (2) ISM group (n = 15). Ipsilateral (intervention side) and contralateral (opposite side of intervention) VAs and ICAs were evaluated using spectral color Doppler ultrasound before and immediately after manipulation. Measurements were recorded by visualizing the ICA carotid sinus (C4 level) and the VA at the V3 segment (C1C2 level). The blood flow parameters of peak systolic velocity (PSV), end-diastolic velocity, resistive index, and volume flow (only for VA) were evaluated. The spinal segment, in which biomechanical aberrant movement was detected by palpation in the upper cervical spine, was manually manipulated in the MSM group. The same methodology was performed for the ISM group using an Activator V instrument (Activator Methods).
Results Intragroup analysis exhibited no statistically significant difference between the manual spinal manipulation (MSM) and instrumental spinal manipulation (ISM) groups in terms of of peak systolic velocity (PSV), end-diastolic velocity, resistive index of ipsilateral and contralateral ICA and VA, in addition to volume flow of both VAs preintervention and postintervention (P > .05). Within the intergroup analysis, there was a significant difference in ipsilateral ICA PSV (P = .031) (preintervention vs postintervention difference was 7.9 ± 17.2 cm/s [95% confidence interval, 17.4 to 1.6] in the ISM group and 8.7 ± 22.5 cm/s [95% confidence interval, 3.6 to 21.2]) in the MSM group (P < .05). Other parameters did not show any significant difference (P > .05).
Conclusion Manual and instrumental spinal manipulations applied to the upper cervical spine in participants with chronic NNP did not appear to alter blood flow parameters of the VAs and ICAs.
Key Indexing Terms Blood Flow Velocity; Chiropractic; Carotid Artery, Internal; Manipulation, Spinal; Vertebral Artery
From the FULL TEXT Article:
Introduction
Neck pain is a commonly reported musculoskeletal disorder, with a mean lifetime prevalence of 50% and point prevalence of 10% in the adult population. [1] Neck pain can be caused by trauma, musculoskeletal conditions, systemic conditions, infection, inflammatory conditions, or neoplasm. [2] The underlying cause of neck pain is often nonspecific and cannot be related to a particular pathology as a cause of the presenting symptoms. [2, 3] Nonspecific neck pain (NNP) is pain in the posterior neck between the superior nuchal line and the spinous process of the first thoracic vertebra and is not associated with major disease or with neurologic signs of nerve compression. [24]
High-velocity low-amplitude cervical manipulation falls within clinical practice guidelines as an effective treatment method for NNP. [5] This manipulation can be applied manually or with use of an instrument. [6, 7] Manual manipulation is the manual application of a force to move the joint near the end of its physiological range of motion without exceeding the anatomical limit of motion. [6, 8] Conversely, the Activator V (Activator Methods) adjusting instrument is a manually assisted device [7, 9] that produces a high-velocity, low-amplitude mechanical force typically delivered when the joint is in neutral and within the physiological range of the joint. [6]
Hemodynamic parameters of blood flow volume and velocity are considered indicators of mechanical stress on vessels and are used to investigate mechanical stress on arteries. [10] It has been reported that especially rotational movements of the cervical spine create stress on the walls of the cervical arteries and change these hemodynamic parameters. [11] This stress mostly occurs in the suboccipital part (V3 segment) of the vertebral artery (VA) due to the anatomical and biomechanical properties of the upper cervical spine. [12] A change in VA blood flow, especially in the opposite direction of rotation, has been demonstrated in many studies. However, these results cause some inconsistency and are insufficient in terms of clinical relevance. [12] Although in vivo and in vitro studies investigating the effect of manual spine manipulation (MSM) on cervical arteries exist in the literature, [1219] no study investigating the effects of ISM on these arteries has been reported. Huggins et al emphasized the lack of evidence in the literature that the use of instrumental manipulation has fewer serious adverse events in patients with defined or undefined vascular risk factors. [19]
Therefore, our primary aim in this study was to investigate whether MSM and instrumental spinal manipulation (ISM) methods cause a change in the hemodynamics of the cervical arteries in participants with NNP. Our second aim was to compare the effects of MSM and ISM applications on cervical artery hemodynamics. We hypothesized that neither MSM or ISM applied to the upper cervical spine would be associated with significant changes in the VA and ICA hemodynamics in participants with NNP.
Methods
Study Design and Ethics
This single-blind, randomized study was performed at Acibadem Maslak Hospital (physical therapy and radiology departments) between February and April 2017, and in accordance with the Declaration of Helsinki and the Consolidated Standards of Reporting Trials extension for clinical trials. Ethical approval was obtained from Acibadem University Health Institutions Medical Research Ethics Committee (ATADEK) (2017-3/25). All participants gave consent to participate in this study. The study protocol was registered prospectively at ClinicalTrials.gov (NCT03435159).
Participants
A total of 33 health care professionals volunteered to participate in this study; 30 of them (22 women, 8 men) were recruited. All volunteers presented their informed consent before enrollment in the study. The study was announced by posting information to notice boards to health care professionals at the Acibadem Maslak Hospital in Turkey. Inclusion criteria were as follows: age 20 to 40 years; NNP duration of more than 3 months, with symptoms being provoked by neck postures, movements, and/or palpation; and a signed consent form.
Exclusion criteria included the following: signs of spinal root compression (radiculopathy); neurological symptoms, such as weakness and numbness in the extremities and/or face; uncontrolled movements; abnormal gait; dizziness; undefined nausea/vomiting; dysphagia and dysarthria; acute inflammatory disease; family history of spontaneous dissection; use of anticoagulant and antiaggregant medications; pregnancy; and pathological findings recorded during Doppler Ultrasonography (USG).
Demographic information (name, surname, age, occupation, sex) of the participants were taken. The onset, initial time, location, quality, intensity, pain radiation, and the provocative and palliative conditions of the neck pain were recorded. In the general assessment, previous trauma, ongoing conditions, current medication, past surgeries, pregnancy, smoking, and family history of cervical arterial dysfunction were also recorded. Participants were assessed with a cervical foraminal compression test [20] that was used to differentiate cervical root compression findings. Myotomes [20] were checked to rule out any neurologic deficits originating from the nerve roots in the cervical spine. The Turkish version of the Neck Disability Index (NDI) [21] was used to evaluate the functional neck status, while a numeric pain scale (NPS) [22] was used to measure the amount of pain of the participants. Cervical ranges of motion were assessed by a physiotherapist using a universal goniometer (Renhotec Group, Wuhan, China) with the participants in the sitting position. [23]
Doppler Ultrasonography
The primary outcome measure of the current study was the cerebrovascular hemodynamics of the ICA and VAs, which were measured using Doppler USG before and immediately after intervention. After baseline evaluation by the physiotherapist, participants were evaluated using Doppler USG by a radiologist with 13 years of experience. Participants were rested in a supine position for 5 minutes before Doppler USG evaluation to assure hemodynamic stabilization. [24, 25] Evaluations were performed with all participants in the supine position and the head in slight extension and rotation according to the angle of the artery displayed.
An ultrasound machine (General Electric LOGIQ S8) was used to measure blood flow parameters in the right and left ICAs and VAs. All vessels were examined in the axial plane through their traces in the B-mode using a C6-15 MHz curvilinear matrix probe. Flow patterns and directions of the vessels were examined with color Doppler, and the condition of stenosis was investigated. The common carotid, ICA, and VA were scanned and examined for pathology. The intimal thickness of the common carotid artery was measured using the spectral Doppler method. Measurements were recorded by visualizing the ICA carotid sinus (C4 level) and the VA at the V3 segment (C1-C2 level) at less than 60°. All measurements before and after the intervention were performed at the same level. Blood flow parameters of peak systolic velocity (PSV), end-diastolic velocity (EDV), resistive index (RI), and volume flow (VF) (for VA only) were evaluated.
Interventions
Manipulation procedures were applied to the C1 or C2 (atlas or axis) vertebra once per participant. First, the physiotherapist performed the palpation method to determine the dysfunctional vertebra. Spinal manipulation of the vertebra was used to correct the mechanical dysfunction, either manually or using Activator V. Interventions were performed by the same physiotherapist with 14 years of experience in clinical practice.
Manual Spinal Manipulation Manual spinal manipulation was performed only on 1 of the C1 or C2, from 1 direction (left or right) for each participant according to palpation findings. MSM for C1 was performed with the participant in the sitting position. The clinician stood in front of the participant, with the participant's head in lateral flexion and rotation posture, placed her index finger on the transverse process, and applied a pulling force in the direction of rotation. For the C2, the MSM was performed with the participant placed in the supine position. Standing at the head of the table, the clinician positioned the participant's head in lateral flexion and rotation posture, placed her index finger on the facet joint of the relevant vertebra, and applied a push technique in the direction of rotation. [7]
Instrumental Spinal Manipulation Instrumental spinal manipulation was performed only on 1 of the C1 or C2, from 1 direction (left or right) for each participant according to palpation findings. With the participant placed in the supine position and the clinician positioned at the intervention side (right or left) of the table, the Activator V instrument was placed horizontally on the transverse process of the C1 vertebra, and a thrust force was applied in the lateral to medial direction. With the patient in the prone position and the clinician at the intervention side of the table, the Activator V instrument was placed on the pedicle-lamina junction of the C2 vertebra and a thrust force was applied in the posterior to anterior, inferior to superior, and slightly lateral to medial direction of the facet joint. [26]
Immediately after manipulation, participants were repositioned for Doppler USG evaluations. Blood flow parameters of the relevant arteries monitored with Doppler USG were recorded as numerical data by the same physician.
Sample Size
A minimum study population of 14 individuals per group was determined according to a previous study. [24] Considering Doppler variation, VF rate as a function of average velocity and area, and ultrasound machine variability, their study used a significance level of 5%; a power of 80%; a standard deviation in blood flow velocity of 18 cm/s, and a mean percentage change in blood flow velocity of 26%.
Randomization
The 30 participants with NNP were randomized into equal MSM (n = 15) and ISM (n = 15) groups. Randomization occurred directly after baseline measurements and screening for inclusion in the study. An online randomization website (Research Randomizer, version 4.0) was used to generate the allocation of treatment via an investigator with no clinical involvement in the study. Thirty random basis sequences without blocks were generated by numbered index cards (1-30), folded, and placed in sealed and opaque envelopes. Then, another investigator (SY) opened each envelope and assigned the participants to the groups according to the selected index card.
Blinding
As groups received different treatment applications, participants and study implementers were not blinded. The assessor who applied the Doppler USG was blinded.
Statistical Methods
Statistical Package for Social Science version 22.0 for Windows software (IBM) was used for statistical analyses. The Kolmogorov-Smirnov test was performed to assess the distribution of the data. Demographics and clinical baseline variables (age, sex, smoking, NPS, NDI, systolic and diastolic blood pressures, intimal thickness, and cervical range of motion) were assessed using the independent samples t test and ?2 test. Intragroup analyses were performed using the paired samples t test and the Wilcoxon test for preintervention and postintervention values, while intergroup analyses were performed using the independent samples t test and the Mann-Whitney U test for preintervention and postintervention differences. The significance level was set at P < .05.
Results
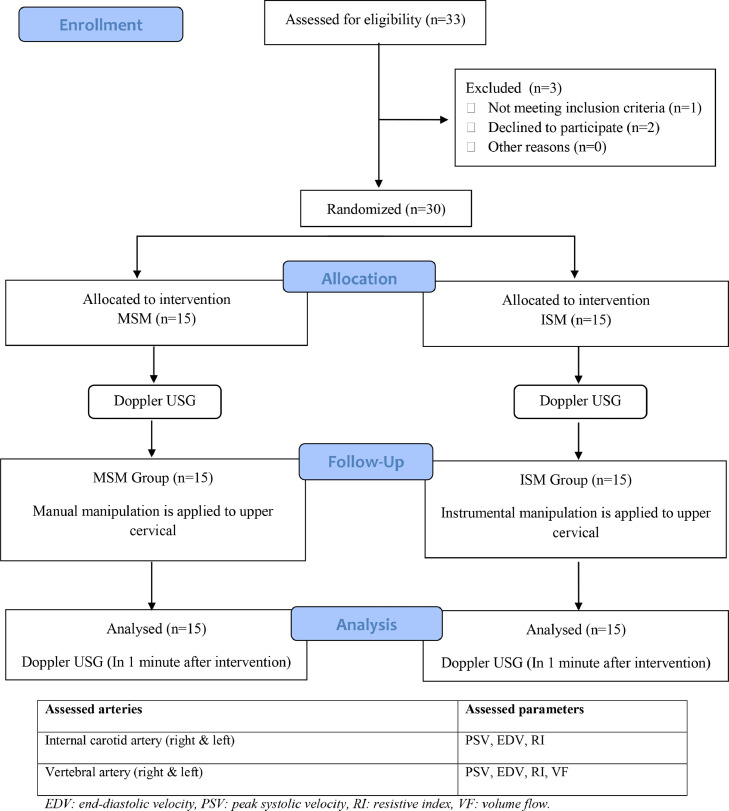
Figure 1

Table 1

Table 2

Table 3 The flow diagram for participants is given in Figure 1. Two participants were unable to participate, and 1 participant had arterial wall abnormality in Doppler USG investigation before intervention, so they were excluded from the study.
Assessment of Descriptive Data
Both the MSM and ISM groups consisted of 11 (73.3%) women and 4 (26.7%) men. There were no statistically significant differences between study groups in terms of baseline physical and characteristics of the participants (age, smoking, NPS and NDI values, mean systolic and diastolic blood pressures, vessel intimal thicknesses, active and passive normal joint movements of the neck) (P > .05 for all) (Table 1).
Intragroup and Intergroup Comparisons of Internal Carotid Artery Hemodynamic Changes
Changes in 6 parameters (ipsilateral and contralateral PSV, EDV, and RI) in ICAs for the groups are shown in Table 2. There was no statistically significant difference in the intragroup analysis (P > .05). In the intergroup analysis, ipsilateral ICA PSV showed a statistically significant difference (P = .031). We believe that this difference is due to the increase in PSV after the application in the ISM group, while it decreased in the MSM group. No significant change was observed in the other parameters (P > .05).
Intragroup and Intergroup Comparisons of Vertebral Artery Hemodynamic Changes
The changes in 8 parameters (ipsilateral and contralateral PSV, EDV, RI, and VF) in VAs in the ipsilateral and contralateral of ISM and MSM applications are shown in Table 3.
Intragroup and intergroup comparisons did not show any statistically significant change in any of the parameters, although changes were observed in all parameters (P > .05) (Table 3).
No adverse effects were reported by any participant after MSM or ISM.
Discussion
To our knowledge, this is the first study comparing hemodynamic measurements before and after manual and instrumental spinal manipulations on VAs and ICAs in individuals with chronic NNP. Single-session MSM and ISM applications to the upper cervical region did not appear to result in any significant hemodynamic effects on ipsilateral and contralateral VAs and ICAs.
There are numerous studies involving blood flow changes in the VA due to cervical rotation in the literature.12,13,24 It has been hypothesized that the rotational movement involved in cervical manipulation by means of creating tension in the VA is a possible occlusion in the VA.27 A meta-analysis10 reported that cervical spine rotation prevailed in the decrease of contralateral VA flow in individuals with and without vertebrobasilar insufficiency, particularly in patients with VA insufficiency. In contrast, other studies in the literature have reported that the blood flow of the VA on the ipsilateral side of cervical rotation is greater.28 In the current study, neither MSM nor ISM caused a significant change.
In our study, PSV was used to determine stenosing of ICA and VA. Peak systolic velocity was the most reliable parameter in determining stenosis of the carotid and VAs. Peak systolic velocity in the area of stenosis was consistent with the severity of the lumen narrowing. In stenosis of 50% or greater, PSV rises above its normal limits. End-diastolic velocity does not change in stenosis of the ICA below 50%; however, as stenosis increases, an increase in EDV may be observed. Except for severe stenosis cases, the hemodynamics in the stenosed cervical artery increase.29
A study by Trattnig et al found that normal, neutral values of VA PSV stood between 19 and 98 cm/s (average 56 cm/s).30 In the current study, we found mean values ??for PSV in the ISM group for ipsilateral and contralateral VAs of 48.5 cm/s and 43.3 cm/s, respectively. In the MSM group, PSV was 48.2 cm/s for the ipsilateral VA and 46.9 cm/s for the contralateral VA. It has been reported that the PSV of the ICA in the normal population is <125 cm/s, and the EDV is <40 cm/s.31 In our study, since premanipulation and postmanipulation values ??fell within the normal range for both groups, postmanipulation stenosing was not suggested. The significant difference in intergroup comparison of the ipsilateral ICA PSV is thought to be due to the decrease of the PSV in the MSM group after the manipulation and the increase of PSV in the ISM group. Premanipulation and postmanipulation intragroup assessments were not significant. Hence, it was concluded that both manipulations did not cause a significant decrease in the hemodynamic parameters of ICAs.
While there is information in the literature that the Activator device is safe,32 to date, there has been no study that evaluates the effect of the Activator treatment on the cervical arteries. Since the Activator treatment applies a force that can move the vertebra, physiological changes in the area of ??application via the Activator instrument are within the realm of possibility.33 The applied force during Activator application has been reported to be 1 mm of translation and 0.5° of rotation in 19 ms in animal models.34 However, it is unclear whether this force affects cervical artery hemodynamics. Reported information and the inadequate literature about the subject led us to investigate the effect of the Activator instrument on the cervical arteries.
No standard vertebral segment has been determined for imaging the VA using Doppler USG.28 According to Bernoulli's principle, due to the compression or stretching during cervical rotation, the blood flow velocity at or just beyond the narrowing point of the vessel increases.35 This may result in spurting and turbulent flow immediately downstream from the region of distortion that may evoke a local thrombogenic response, leading to vertebral artery stroke.12 Considering this principle, we performed Doppler USG measurements at the V3 segment of the VA, which is the level at which manipulations were applied.
Doppler USG is a non-invasive, reliable, and repeatable method to evaluate the extracranial portions of the carotid and VAs and to determine possible vascular pathologies.36,37 In the current study, Doppler USG was applied for measurement purposes prior to manipulation. Patients with a possible vascular pathology (vascular hypoplasia, genetic weakness in the vascular wall) were excluded from the study. Cervical arterial dysfunction is an umbrella term covering a wide spectrum of pathologies, from atherosclerosis to mechanical trauma of the vessels. Patients with neck pain or headache may present for manipulative treatment that could possibly be caused by cervical artery dysfunction.38
Limitations
Participants were only evaluated immediately after manipulation; thus, we only measured short-term effects. Therefore, possible changes to the arteries after the evaluation are unknown.
Manual manipulation of the C1 vertebra was performed using rotational forces, while manipulation with the Activator was performed in the lateral direction. Thus, forces were not similar between these 2 manipulations and may have had different effects on cervical artery blood flow.
In our study, we had a small sample size. A small sample size reduces the power of the study and increases the margin of error. Since individuals with vertebrobasilar insufficiency were not included in the study, the hemodynamic effects following manual or instrumental manipulation on pathological vessels were not measured and are therefore unknown.
Conclusion
The present study found that manual and instrumental spinal manipulations applied to the upper cervical region in people with chronic NNP did not result in significant changes in the ICA and VA blood flow parameters.
Practical Applications
This study compared the hemodynamic effects of manual vs instrumented spinal manipulation in participants with chronic nonspecific neck pain.
Intragroup analysis exhibited no statistically significant difference between the 2 groups.
This study found that manual and instrumental spinal manipulations applied to the upper cervical spine in participants with chronic nonspecific neck pain did not appear to alter blood flow parameters of the vertebral arteries and internal carotid arteries.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
References
McLean SM, May S, Klaber-Moffett J, Sharp DM, Gardiner E.
Risk factors for the onset of non-specific neck pain: a systematic review.
J Epidemiol Community Health. 2010;64(7):565572.Vincent K, Maigne JY, Fischhoff C, Lanlo O, Dagenais S.
Systematic review of manual therapies for nonspecific neck pain.
Joint Bone Spine. 2013;80(5):508515.Hidalgo B, Hall T, Bossert J, Dugeny A, Cagnie B, Pitance L.
The efficacy of manual therapy and exercise for treating
non-specific neck pain: a systematic review.
J Back Musculoskelet Rehabil. 2017;30(6):11491169.Coulter ID, Crawford C, Hurwitz EL, Vernon H, Khorsan R, Booth MS and Herman PM.
Manipulation and Mobilization for Treating Chronic Nonspecific Neck Pain:
A Systematic Review and Meta-Analysis for an Appropriateness Panel
Pain Physician 2019 (Mar); 22 (2): E55E70R. Bryans, P. Decina, M. Descarreaux, et al.,
Evidence-Based Guidelines for the Chiropractic Treatment of Adults With Neck Pain
J Manipulative Physiol Ther 2014 (Jan); 37 (1): 4263Gemmel H, Miller P.
Relative effectiveness and adverse effects of cervical manipulation,
mobilization and the activator instruments with sub-acute
non-specific neck pain: results from a stopped randomised trial.
Chiropr Osteopat. 2010;18(20):20.Bergmann TF, Peterson DH.
3rd ed. Mosby; St. Louis, MO: 2011.
Chiropractic Technique, Principles and Procedures.Pickar JG.
Neurophysiological Effects of Spinal Manipulation
Spine J (N American Spine Society) 2002 (Sep); 2 (5): 357371Colloca CJ, Keller TS, Black P, Normand MC, Harrison DE, Harrison DD.
Comparison of mechanical force of manually assisted chiropractic adjusting instruments.
J Manipulative Physiol Ther. 2015;28(6):414422.Mitchell J.
Vertebral artery blood flow velocity changes associated with cervical
spine rotation: a meta-analysis of the evidence with
implications for professional practice.
J Man Manip Ther. 2009;17(1):4657.Kranenburg HAR, Tyer R, Schmitt M, et al.
Effects of head and neck positions on blood flow in the vertebral,
internal carotid, and intracranial arteries: a systematic review.
J Orthop Sports Phys Ther. 2019;49(10):688697.Quesnele JJ, Triano JJ, Noseworthy MD, Wells GD.
Changes in Vertebral Artery Blood Flow Following Various
Head Positions and Cervical Spine Manipulation
J Manipulative Physiol Ther. 2014 (Jan); 37 (1): 2231Erhardt JW, Windsor BA, Kerry R, et al.
The immediate effect of atlanto-axial high velocity thrust techniques
on blood flow in the vertebral artery: a randomized controlled trial.
Man Ther. 2015;20(4):614622.Parwar BL, Fawzi AA, Arnold AC, Schwartz SD.
Horner's syndrome and dissection of the internal carotid artery
after chiropractic manipulation of the neck.
Am J Ophthalmol. 2001;131(4):523524.Chung CL, Cτtι P, Stern P, L'Espιrance G.
The association between cervical spine manipulation and carotid
artery dissection: a systematic review of the literature.
J Manipulative Physiol Ther. 2015;38(9):672676.Herzog W, Tang C, Leonard T.
Internal Carotid Artery Strains During High-Speed,
Low-Amplitude Spinal Manipulations of the Neck
J Manipulative Physiol Ther. 2015 (Nov); 38 (9): 664671Wuest, S, Symons, B, Leonard, T, and Herzog, W.
Preliminary Report: Biomechanics of Vertebral Artery
Segments C1-C6 During Cervical Spinal Manipulation
J Manipulative Physiol Ther. 2010 (May); 33 (4): 273278Symons, B.,Leonard, T.R.,Herzog, W.,2002.
Internal Forces Sustained by the Vertebral Artery
During Spinal Manipulative Therapy
J Manipulative Physiol Ther 2002 (Oct); 25 (8): 504510Huggins T, Boras AL, Gleberzon BJ, Popescu M, Bahry LA.
Clinical effectiveness of the activator adjusting instrument in the management
of musculoskeletal disorders: a systematic review of the literature.
J Can Chiropr Assoc. 2012;56(1):4957.Magee DJ. 6th ed.
Saunders Elsevier; St. Louis, MO: 2014.
Orthopedic Physical Assessment.Telci EA, Karaduman A, Yakut Y, Aras B, Simsek IE, Yagli N.
The cultural adaptation, reliability, and validity of neck disability index
in patients with neck pain: a Turkish version study.
Spine (Phila Pa 1976) 2009;34(16):17321735.Young IA, Dunning J, Butts R, Mourad F, Cleland JA.
Reliability, construct validity, and responsiveness of the neck disability index
and numeric pain rating scale in patients with mechanical
neck pain without upper extremity symptoms.
Physiother Theory Pract. 2019;35(12):13281335.Norkin CC. Norkin CC, White DJ.
Measurement of Joint Motion: A Guide to Goniometry. 5th ed.
F.A. Davis Company; Philadelphia: 2016.
The cervical spine; pp. 411467.Bowler N, Shamley D, Davies R.
The effect of a simulated manipulation position on internal carotid
and vertebral artery blood flow in healthy individuals.
Man Ther. 2011;16:8793.Rivett DA, Sharples KJ, Milburn PD.
Effect of Premanipulative Tests on Vertebral Artery and
Internal Carotid Artery Blood Flow: A Pilot Study
J Manipulative Physiol Ther 1999 (Jul); 22 (6): 368375Fuhr AW.
2nd ed. Mosby; St. Louis, Missouri: 2009.
The Activator Method.Rothwell DM, Bondy SJ, Williams JI.
Chiropractic Manipulation and Stroke:
A Population-based Case-control Study
Stroke 2001 (May); 32 (5): 1054-1060Mitchell J, Keene D, Dyson C, Harvey L, Pruvey C, Phillips R.
Is cervical spine rotation, as used in the standard vertebrobasilar
insufficiency test, associated with a measurable change in
intracranial vertebral artery blood flow?
Man Ther. 2004;9:220227.Yurdakul S, Aytekin S.
Doppler ultrasound imaging of the carotid and vertebral arteries.
Turk Kardiyol Dern Ars. 2011;39(6):508517.Trattnig S, Hubsch P, Schuster H, Polzleitner D.
Color-coded Doppler imaging of normal vertebral arteries.
Stroke. 1990;21(8):12221225.Seηil M.
2nd ed. Akademisyen Kitabevi; Bas?m, Ankara: 2013.
Temel Ultrasonografi ve Doppler; pp. 479498.Taylor SH, Arnold ND, Biggs L, et al.
A Review of the Literature Pertaining to the Efficacy, Safety,
Educational Requirements, Uses and Usage of Mechanical
Adjusting Devices: Part 2 of 2
J Canadian Chiropractic Assoc 2004 (Mar); 48 (12): 152161Cohen FL.
Cerebral hemorrhage following chiropractic activator
treatment-case report and review of literature.
J Neurol Surg. 2016;77(4):162167.Terret AGJ.
NCMIC Group Inc; West Des Moines, IA: 2001.
Current Concepts in Vertebrobasilar Complications Following Spinal Manipulation
An 8-page Executive SummaryGanong WF.
22nd ed. McGraw-Hill; New York, NY: 2005.
Review of Medical Physiology.Hallerstam S, Rosfors S.
Blood flow and flow resistance in vertebral arteries of patients
with and without carotid atherosclerosis.
Clin Physiol Funct Imaging. 2004;24:96102.Yazici B, Erdogmus B, Tugay A.
Cerebral blood flow measurements of the extracranial carotid
and vertebral doppler ultrasonography in healthy adults.
Diagn Interv Radiol. 2005;11(4):195198.Cassidy JD, Boyle E, Cote P, et al.
Risk of Vertebrobasilar Stroke and Chiropractic Care:
Results of a Population-based Case-control
and Case-crossover Study
Spine (Phila Pa 1976) 2008 (Feb 15); 33 (4 Suppl): S176183
Return to STROKE AND CHIROPRACTIC
Since 12-23-2022


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |