The Treatment of Neck Pain-Associated Disorders
and Whiplash-Associated Disorders:
A Clinical Practice GuidelineThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Manipulative Physiol Ther. 2016 (Oct); 39 (8): 523–564 ~ FULL TEXT
OPEN ACCESS André E. Bussières, DC, PhD, Gregory Stewart, BPE, DC, Fadi Al-Zoubi, PT, MSc,
Philip Decina, DC, Martin Descarreaux, DC, PhD, Jill Hayden, DC, PhD,
Brenda Hendrickson, BN, MN, Cesar Hincapié, DC, PhD, Isabelle Pagé, DC, MSc,
Steven Passmore, DC, PhD, John Srbely, DC, PhD, Maja Stupar, BSc, DC, PhD,
Joel Weisberg, BSc, DC, Joseph Ornelas, DC, PhD
School of Physical and Occupational Therapy,
Faculty of Medicine, McGill University,
Montreal, QC, Canada;
Département Chiropratique,
Université du Québec à Trois-Rivières,
Trois-Rivières, QC, Canada.
Andre.bussieres@mcgill.ca
Thanks to JMPT for permission to reproduce this Open Access article! OBJECTIVE: The objective was to develop a clinical practice guideline on the management of neck pain-associated disorders (NADs) and whiplash-associated disorders (WADs). This guideline replaces 2 prior chiropractic guidelines on NADs and WADs.
METHODS: Pertinent systematic reviews on 6 topic areas (education, multimodal care, exercise, work disability, manual therapy, passive modalities) were assessed using A Measurement Tool to Assess Systematic Reviews (AMSTAR) and data extracted from admissible randomized controlled trials. We incorporated risk of bias scores in the Grading of Recommendations Assessment, Development, and Evaluation. Evidence profiles were used to summarize judgments of the evidence quality, detail relative and absolute effects, and link recommendations to the supporting evidence. The guideline panel considered the balance of desirable and undesirable consequences. Consensus was achieved using a modified Delphi. The guideline was peer reviewed by a 10-member multidisciplinary (medical and chiropractic) external committee.
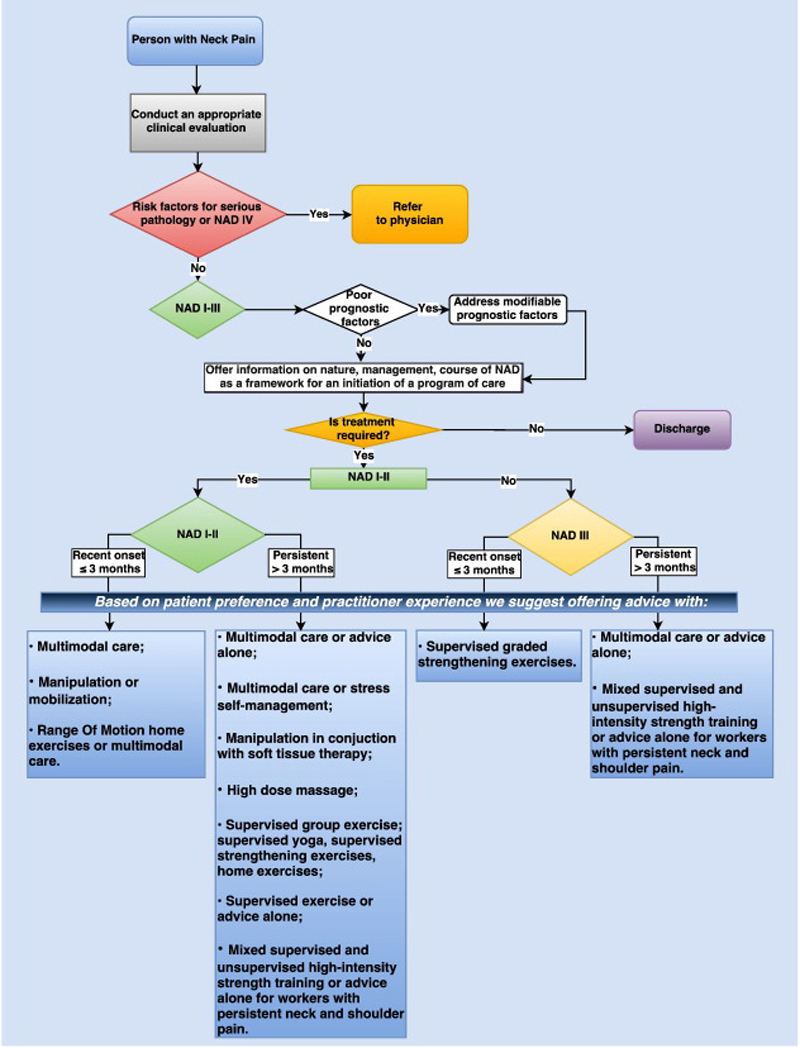
RESULTS: For recent-onset (0-3 months) neck pain, we suggest offering multimodal care; manipulation or mobilization; range-of-motion home exercise, or multimodal manual therapy (for grades I-II NAD); supervised graded strengthening exercise (grade III NAD); and multimodal care (grade III WAD).
For persistent (>3 months) neck pain, we suggest offering multimodal care or stress self-management; manipulation with soft tissue therapy; high-dose massage; supervised group exercise; supervised yoga; supervised strengthening exercises or home exercises (grades I-II NAD); multimodal care or practitioner's advice (grades I-III NAD); and supervised exercise with advice or advice alone (grades I-II WAD).
For workers with persistent neck and shoulder pain, evidence supports mixed supervised and unsupervised high-intensity strength training or advice alone (grades I-III NAD).
CONCLUSIONS: A multimodal approach including manual therapy, self-management advice, and exercise is an effective treatment strategy for both recent-onset and persistent neck pain.
KEYWORDS: Chiropractic; Disease Management; Musculoskeletal Disorders; Neck Pain; Practice Guideline; Therapeutic Intervention; Whiplash Injuries
From the FULL TEXT Article:
Introduction
Neck pain and its associated disorders (NAD), including headache and radiating pain into the arm and upper back, are common and result in significant social, psychological, and economic burden. [1–4] Neck pain, whether attributed to work, injury, or other activities, [5] is a prevalent source of disability and a common reason for consulting primary health care providers, including chiropractors, physical therapists, and primary care physicians. [6] The estimated annual incidence of neck pain measured in 4 studies ranged between 10.4% and 21.3%, with a higher incidence noted in office and computer workers. [7] Although some studies report that between 33% and 65% of people have recovered from an episode of neck pain at 1 year, most cases follow an episodic course over a person’s lifetime, and thus, relapses are common. [7] Neck pain is a leading cause of morbidity and chronic disability worldwide. [5, 8] In 2008 the Bone and Joint Decade Task Force on Neck Pain and Its Associated Disorders reported that 50% to 75% of individuals with neck pain also report pain 1 to 5 years later. [4] Several modifiable and nonmodifiable environmental and personal factors influence the course of neck pain, including age, previous neck injury, high pain intensity, self-perceived poor general health, and fear avoidance. [7]
Neck pain related to whiplash-associated disorders (WADs) most commonly results from motor vehicle accidents. [9, 10] Whiplash-associated disorders disrupt the daily lives of adults around the world and are associated with considerable pain, suffering, disability, and costs. [3, 11] Whiplash-associated disorders are defined as an injury to the neck that occurs with sudden acceleration or deceleration of the head and neck relative to other parts of the body, typically occurring during motor vehicle collisions. [10, 12] The majority of adults with traffic injuries report pain in the neck and upper limb pain. Other common symptoms of WADs include headache, stiffness, shoulder and back pain, numbness, dizziness, sleeping difficulties, fatigue, and cognitive deficits. [9, 10] The global yearly incidence rate of emergency department visits as a result of acute whiplash injuries after road traffic crashes is between 235 and 300 per 100,000. [3, 13, 14] In 2010, there were 3.9 million nonfatal traffic injuries in the United States. [11] The economic costs of motor vehicle crashes that year totaled USD $242 billion, including $23.4 billion in medical costs and $77.4 billion in lost productivity (both market and household). [11] In Ontario, traffic collisions are a leading cause of disability and health care use and expenditures, resulting in the automobile insurance system paying nearly CND $4.5 billion in accident benefits in 2010. [15]
More than 85% of patients experience neck pain after a motor vehicle accident, often associated with sprains and strains to the back and extremities, headache, psychological symptomatology, and mild traumatic brain injury. [10] Whiplash injuries have an effect on general health, with recovery in the short term reported by 29% to 40% of individuals with WAD in Western countries that have compensation schemes for whiplash injuries. [16, 17] The median time to first reported recovery is estimated at 101 days (95% confidence interval: 99–104) and about 23% are still not recovered after 1 year. [13]
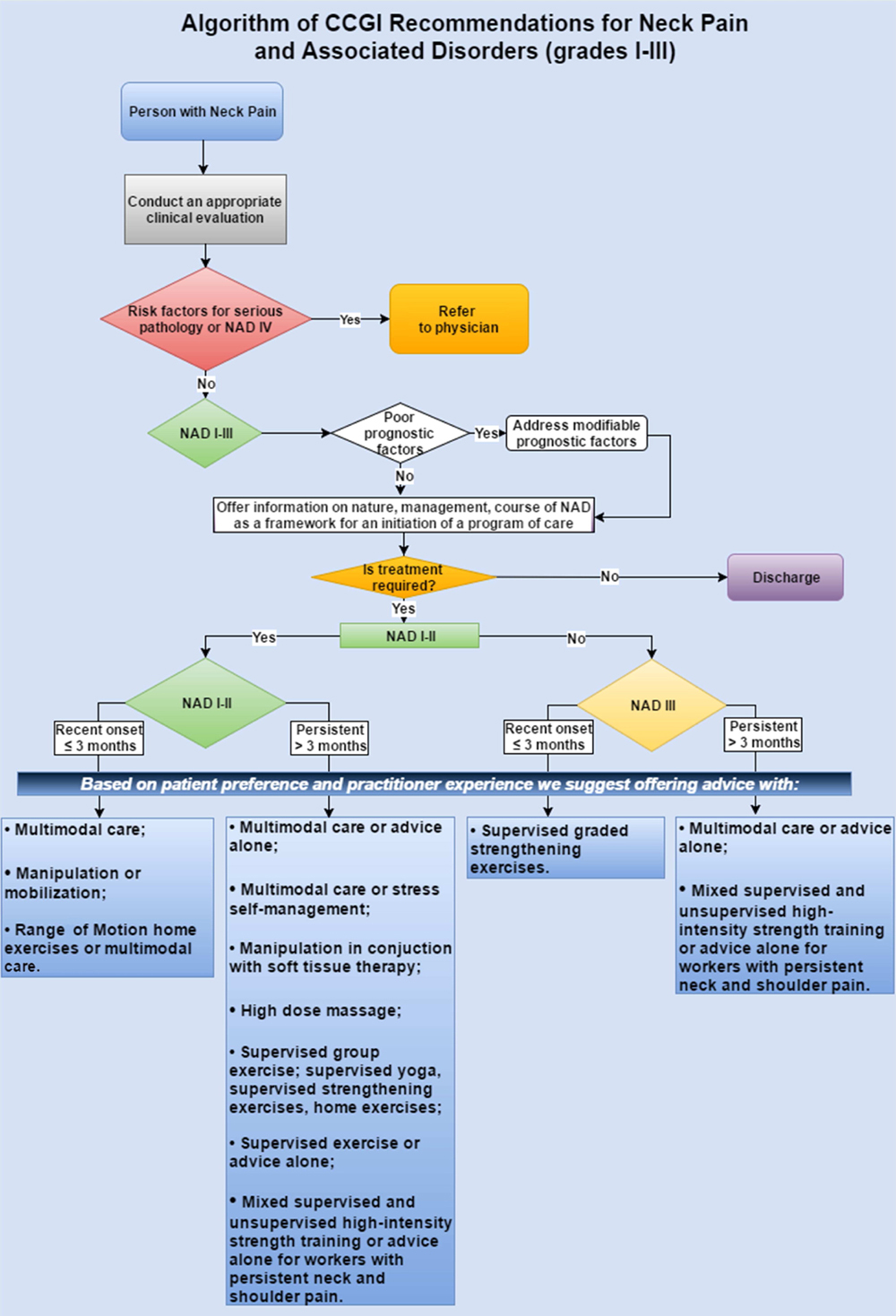
The 2000–2010 Bone and Joint Decade Task Force on Neck Pain and its Associated Disorders recommended that all types of neck pain, including WADs, [18] be included under the classification of NAD. [19] NAD can be classified into 4 grades, distinguished by the severity of symptoms, signs, and impact on activities of daily life (Table 1).
Table 1. Classification of Neck Pain–Associated Disorders (NAD)
and Whiplash-Associated Disorders (WAD)
FROM: The 2000–2010 Bone and Joint Decade Task Force on Neck Pain
and Its Associated Disorders Classification of NAD [18]
I No signs or symptoms suggestive of major structural pathology
and no or minor interference with activities of daily living
II No signs or symptoms of major structural pathology, but major
interference with activities of daily living
III No signs or symptoms of major structural pathology, but presence
of neurologic signs such as decreased deep tendon reflexes,
weakness or sensory deficits
IV Signs or symptoms of major structural pathologyThe clinical management of musculoskeletal disorders, and neck pain in particular, can be complex and often involves combining multiple interventions (multimodal care) to address its symptoms and consequences. [19] In this guideline, multimodal care refers to treatment involving at least 2 distinct therapeutic methods, provided by 1 or more health care disciplines. [20] Manual therapy (including spinal manipulation), medication, and home exercise with advice are commonly used multimodal treatments for recent-onset and persistent neck pain. [21, 22] Thus, there is a need to determine which treatments or combinations of treatments are more effective for managing NAD and WAD.
Rationale for Developing This Guideline
The Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration [20] recently updated the systematic reviews from the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders (Neck Pain Task Force). [23] Consequently, it was deemed timely to update the recommendations of 2 chiropractic guidelines on NAD (2014) [24] and WAD (2010) [25] produced by the Canadian Chiropractic Association and the Canadian Federation of Chiropractic Regulatory and Educational Accrediting Boards (the “Federation”) into a single guideline.
Scope and Purpose
The aim of this clinical practice guideline (CPG) was to synthesize and disseminate the best available evidence on the management of adults and elderly patients with recent onset (0-3 months) and persistent (>3 months) neck pain and its associated disorders, with the goal of improving clinical decision making and the delivery of care for patients with NAD and WAD grades I to III.
Guidelines are “Statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options.” [26]
The target users of this guideline are chiropractors and other primary care health care providers delivering conservative care to patients with NADs and WADs, as well as policymakers. We define conservative care as treatment designed to avoid invasive medical therapeutic measures or operative procedures.
OPTIMa published a closely related guideline in the European Spine Journal. [27] Although we reached similar results, OPTIMa developed recommendations using the modified Ontario Health Technology Advisory Committee (OHTAC) framework. [28] In contrast, our guideline used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. GRADE provides a common, sensible, and transparent approach to grading quality (or certainty) of evidence and strength of recommendations (http://www.gradeworkinggroup.org). GRADE was the highest scoring instrument among 60 evidence grading systems [29] and has been determined to be reproducible among trained raters. [30] GRADE is now considered a standard in guideline development and has been adopted by many international guideline organizations and journals. [31] The Canadian Chiropractic Guideline Initiative (CCGI) guideline panel considered available high-quality systematic reviews, updated the search of the peer-reviewed published reports up to December 2015, and then used the GRADE approach to formulate recommendations for the management of neck pain and associated disorders.
Framework
To inform its work, the CCGI considered recent advances in methods to conduct knowledge synthesis, [32] derive evidence-based recommendations, [31, 33] adapt high-quality guidelines, [34] and develop [35] and increase the uptake of CPGs. [36, 37] An overview of CCGI structure and methods is provided in Appendix 1.
Methods
Ethics
Because no novel human participant intervention was required and secondary analyses were considered, the research presented in this guideline is exempt from institutional ethics review board approval.
Selection of Guideline Development Panelists
The CCGI project lead (A.B.) appointed 2 co-chairs (J.O. and G.S.) for the guideline development group and nominated the project executive committee and the remaining guideline panelists. J.O. served as the lead methodologist on the guideline panel. G.S. helped ensure geographic representation of the panel and advised on specific duties of panel members, time commitment, and decision-making process for reaching consensus (development of key questions and of recommendations). To ensure a broad representation, the guideline panel included clinicians (P.D., J.W.), clinician researchers (F.A., M.D., C.H., S.P., I.P., J.S.) methodologists (J.O., A.B., M.S., J.H.), a professional leader/decision maker (G.S.), and 1 patient advocate (B.H.) to ensure that patient values and preferences were considered. One observer (J.R.) monitored the 3 face-to-face meetings of the guideline panel held in Toronto (June and September 2015 and April 2016).
All CCGI members, including guideline panelists and peer reviewers, were required to disclose any potential conflict of interest by topic before participation and during the guideline development process. There was no self-declaration of conflicts of interest among the panel or the reviewers.
Key Question Development
Six topic areas (exercise, multimodal care, education, work disability, manual therapy, passive modalities) on the conservative management of NAD and WAD grades I to III were covered in 5 recent systematic reviews by the OPTIMa Collaboration, [38–42] among a total of 40 reviews on the management of musculoskeletal disorders. [20] The panel met over 2 days in June 2015 to brainstorm about potential key questions.
Search Update and Study Selection
The panel assessed the quality of eligible systematic reviews using the AMSTAR tool [43] and its 11 criteria
(http://amstar.ca/Amstar_Checklist.php).
Because the last search dates of included systematic reviews were 2012, [40, 41] 2013, [38, 39, 42] and 2014, [42] the panel updated the literature searches in Medline and Cochrane Central databases on December 24, 2015 using the published search strategies. We used a 2-phase screening process to select additional eligible studies. In phase 1, 2 independent reviewers screened titles and abstracts to determine the relevance and eligibility of studies. In phase 2, the same pairs of independent reviewers screened full-text articles to make a final determination of eligibility. Reviewers met to resolve disagreements and reach consensus on the eligibility of studies in both phases, with arbitration by a third reviewer if needed. Studies were included if they (1) met the PICO (population, intervention, comparator, outcome) criteria and (2) were randomized controlled trials (RCTs) with an inception cohort of at least 30 participants per treatment arm with the specified condition, because this sample size is considered the minimum needed for non-normal distributions to approximate the normal distribution. [44]
Data Abstraction and Quality Assessment
Data were extracted from the included studies identified in each systematic review, including study design, participants, intervention, control, outcomes, and funding. The internal validity of included studies was assessed by the OPTIMa collaboration using the Scottish Intercollegiate Guidelines Network (SIGN) criteria. [45] For articles retrieved from the updated search, pairs of independent reviewers critically appraised the internal validity of eligible studies using the SIGN criteria, [46] similar to the OPTIMa collaboration reviews. Reviewers reached consensus through discussion. A third reviewer was used to resolve disagreements if consensus could not be reached. A quantitative score or a cutoff point to determine the internal validity of studies was not used. Instead, the SIGN criteria were used to assist reviewers in making an informed overall judgment on the risk of bias of included studies. [47]
Synthesis of Results
J.O. extracted data from scientifically admissible studies into evidence tables. A second reviewer (A.B.) independently checked the extracted data. We performed a qualitative synthesis of findings and stratified results based on the type and duration of the disorder (ie, recent [symptoms lasting <3 months] vs persistent [symptoms lasting >3 months]).
Recommendation Development
We used the Guideline Development Tool
(http://www.guidelinedevelopment.org),
and assessed the quality of the body of evidence for our outcomes of interest by applying the GRADE approach. [48] We used the evidence profiles to summarize the evidence. [49] The quality of evidence rating (high, moderate, low, or very low) reflects our confidence in the estimate of the effect to support a recommendation and considers the strengths and limitations of the body of evidence stemming from risk of bias, imprecision, inconsistency, indirectness of results, and publication bias. [50] Assessment of quality of evidence was carried out in the context of its relevance to the primary care setting.
Using the Evidence to Decisions (EtD) Framework
(http://www.decide-collaboration.eu/etd-evidence-decision-framework),
the panel formally met in September 2015 and April 2016 to consider the balance of desirable and undesirable consequences to determine the strength of each recommendation, using informed judgment on the quality of evidence and effect sizes, resource use, equity, acceptability, and feasibility. To make a recommendation, the panel needed to express an average judgment that was beyond neutral with respect to the balance between desirable and undesirable consequences of an intervention, as outlined in the EtD. We defined the strength rating of a recommendation (strong or weak) as the extent to which the desirable consequences of an intervention outweigh its undesirable consequences. A strong recommendation can be made when the desirable consequences clearly outweigh the undesirable consequences. In contrast, a weak recommendation is made when, on the balance of probabilities, the desirable consequences likely outweigh the undesirable consequences. [49, 51]
The panel provided recommendations based on the evidence if statistically and clinically significant differences were found. The panel followed a 2-step process in making a recommendation. We first agreed that there should be evidence of clinically meaningful changes occurring over time in the study population and that a single consensus threshold of clinical effectiveness should be applied consistently. We reached a consensus decision that a 20% change in the outcome of interest within any study group was required to make a recommendation. The decision to use a 20% threshold was informed by current published reports and relevant available minimal clinically important differences (MCIDs). [52–55] However, MCIDs can vary across populations, settings, and conditions and depending on whether within-group or between-group differences are being assessed. Therefore, the panel considered MCID values for the most relevant outcomes (ie, 10% for visual analog scale [VAS] or Neck Disability Index [NDI; 5/50 on the NDI], 20% for numerical rating scale [NRS]) and chose the more conservative of these values as the threshold when evaluating between group differences. [52, 54]
Second, the results from relevant studies were used to formulate a recommendation where appropriate. A treatment determined to be effective (with statistically significant differences between baseline and follow-up scores and clinical significance based on the MCID applied in the study) was recommended by our panel. If a study found 2 or more treatments to be equally effective based on our threshold, then the panel recommended all equivalently effective treatments.
The EtD Frameworks were completed and recommendations were drafted over a series of conference calls with panel members after making judgments about 4 decision domains:quality of evidence (confidence in estimates of effect);
balance of desirable (eg, reduced pain and disability) and undesirable outcomes (eg, adverse reactions);
confidence about the values and preferences for the target population; and
resource implications (costs). [56, 57]A synthesis of our judgments about the domains determined the direction (ie, for or against a management approach) and the strength of recommendations (the extent to which one can be confident that the desirable consequences of an intervention outweigh the undesirable consequences). A specific format was followed to formulate recommendations using patient description and the treatment comparator. [56] Remarks were added for clarification if needed. If the desirable and undesirable consequences were judged to be evenly balanced and the evidence was not compelling, the panel decided not to write any recommendation.
A modified Delphi technique was used at an in-person meeting to achieve consensus on each recommendation. [58] Using an online tool (www.polleverywhere.com), panelists voted their level of agreement with each recommendation (including quality of evidence and strength of recommendation) based on a 3-point scale (yes, no, neutral). Before voting, panelists were encouraged to discuss and provide feedback on each recommendation in terms of suggested wording edits or general remarks. To achieve consensus and be included in the final manuscript, each recommendation had to have at least 80% agreement with a response rate of at least 75% of eligible panel members. All recommendations achieved consensus in the first round.
Peer Review
A 10-member external committee composed of stakeholders, end-users, and researchers from Canada, the United States, and Lebanon (Appendix 2) independently reviewed the draft manuscript, recommendations, and supporting evidence. The AGREE II instrument was used to assess the methodological quality of the guideline. [35] Feedback received was collected and considered in a revised draft for a second round of review. Chairs of the guideline panel provided a detailed response to reviewers’ comments. For a glossary of terms, please see Appendix 3.
Results
Key Question Development
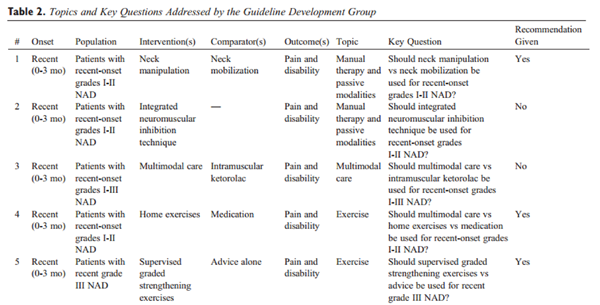
Thirty-two standardized key questions were developed in line with the PICO (population, intervention, comparator, outcome) format. The panel recognized overlap in content and relevance among some key questions. After combining 3 questions, we ultimately addressed a total of 29 key questions (Table 2).
Study Selection and Quality Assessment: OPTIMa Reviews
OPTIMa searches yield 26,335 articles screened. [38–42] After removal of duplicates and screening, 26,273 articles did not meet selection criteria, leaving 109 articles eligible for critical appraisal. Fifty-nine studies (62 articles) published from 2007 to 2013 were deemed scientifically admissible and included in the synthesis (Appendix 4). Each review used was rated as either moderate or high quality (AMSTAR score 8–11). [59]
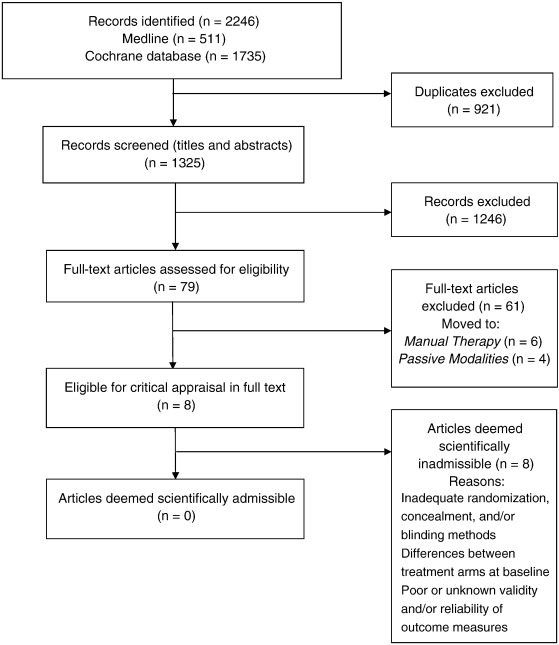
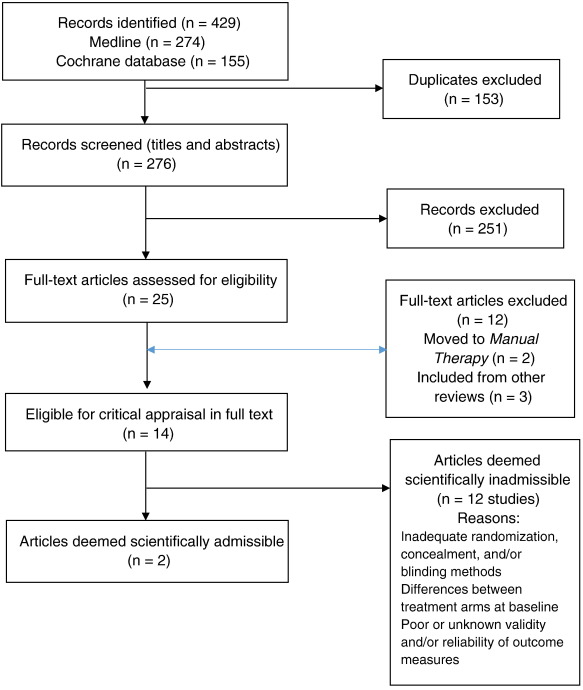
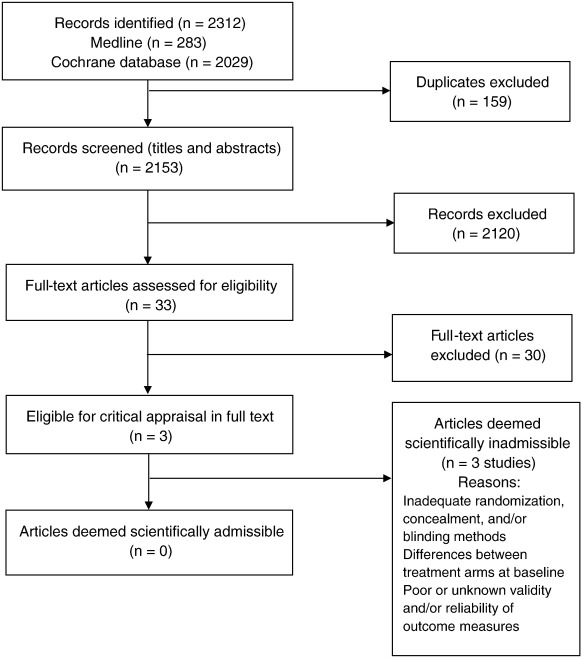
Search Update and Study Selection
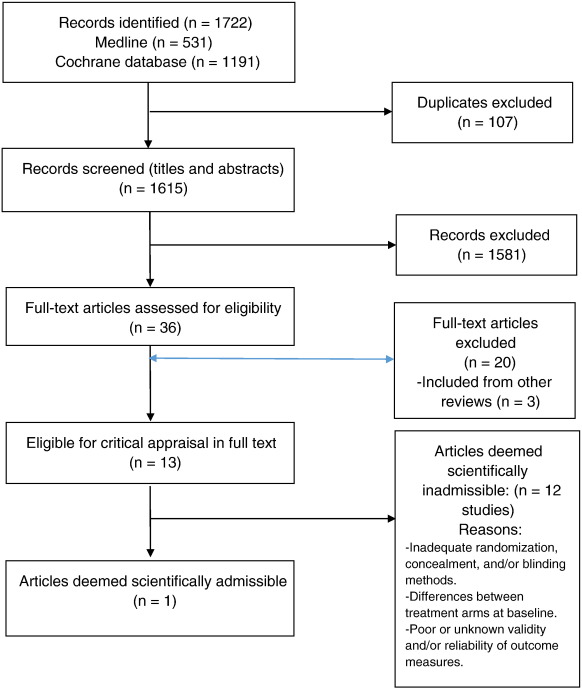
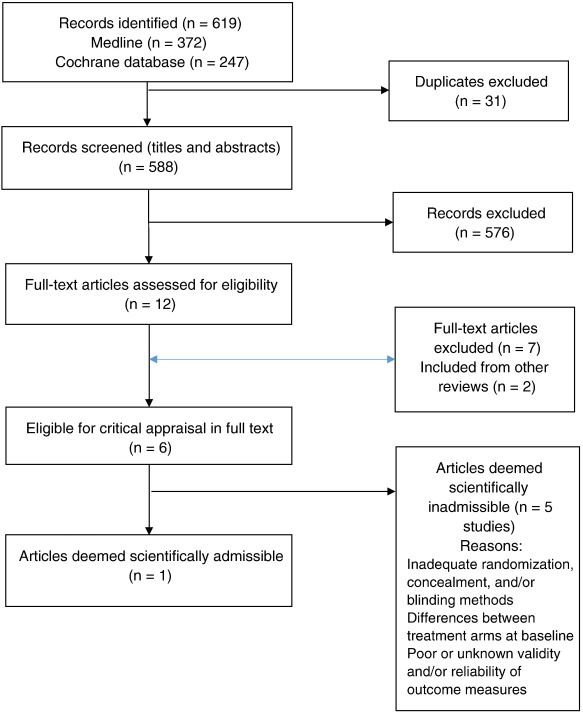
Our updated search yielded 7,784 articles. We removed 1,411 duplicates and screened 6,373 articles for eligibility (Figures 1–5). After screening, 6,321 articles did not meet our selection criteria (phase 1), leaving 52 articles for full-text review (phase 2) and critical appraisal (studies on the topic of multimodal care (n = 12), structured patient education (n = 3), exercise (n = 8), work disability interventions (n = 13), manual therapy (n = 4), soft tissues (n = 2), and passive modalities (n = 6). Of the 52 RCTs, 4 scientifically admissible studies were included in our synthesis. The remaining articles failed to address the key question (n = 1); selected population (n = 2), outcomes (n = 13), or intervention (n = 11); had no between estimates (n = 19); or were duplicates (n = 1) or a secondary analysis of an included study (n = 1) (Appendix 5).
Quality Assessment and Synthesis of Results
The GRADE evidence profile and risk of bias within included studies are presented in Tables 3–15
(See pages 534–543) and Appendix 6, respectively.
Recommendations
We present recommendations as follows:
Recent-onset (0-3 months) grades I to III NAD
Recent-onset (0-3 months) grades I to III WAD
Persistent (>3 months) grades I to III NAD
Persistent (>3 months) grades I to III WAD
Recommendations for Recent-Onset (0-3 Months) Grades I to III NAD
Manual Therapy
Key Question 1: Should neck manipulation vs neck mobilization be used for recent-onset (0-3 months) grades I to II NAD?
Summary of Evidence
One RCT by Leaver et al. [60] evaluated the effectiveness of neck manipulation or neck mobilization delivered by physiotherapists, chiropractors, or osteopaths for recent-onset grades I to II neck pain (≥2 NRS). All patients received advice, reassurance, or a continued exercise program as indicated for 4 treatments over 2 weeks unless recovery was achieved or a serious adverse event occurred. There was no statistically significant difference in Kaplan-Meier recovery curves between groups for recovery from neck pain and recovery of normal activity, and no statistically significant differences between groups for pain, disability, or other outcomes (function, global perceived effect, or health-related quality of life) at any follow-up point (Table 3).
One other RCT by Dunning et al. [61] evaluated the effectiveness of a single high-velocity, low-amplitude (thrust) manipulation (n = 56) directed to the upper cervical spine (C1–C2) and upper thoracic spine (T1–T2) compared with a (nonthrust) mobilization (n = 51) directed to the same anatomical regions for 30 seconds for patients with neck pain. Findings indicated a greater reduction in pain (NPRS) and disability (NDI) in the thrust manipulation group compared with the mobilization at 48 hours. No serious adverse events were reported. Minor adverse events were not collected. This study did not inform our recommendation because (1) patient complaints were not recent onset (mean duration >337 days in both groups), and (2) outcomes were measured at 48 hours only. The Guideline Development Group (GDG) considered this an important study limitation because one cannot assume these benefits would have carried on for a longer period. The panel acknowledged, however, that some patients may value obtaining fast pain relief even if temporary.
The panel determined that the overall certainty in the evidence was low, with large desirable relative to undesirable effects. The relative small cost of providing the option would make it more acceptable to stakeholders and feasible to implement. Although the panel decided the desirable and undesirable consequences were closely balanced, the following statement was provided:
Recommendation: For patients with recent (0-3 months) grades I to II NAD, we suggest manipulation or mobilization based on patient preference. (Weak recommendation, low-quality evidence)
Exercise
Key Question 2: Should integrated neuromuscular inhibition technique be used for recent-onset (0-3 months) grades I to II NAD?
Summary of Evidence
Nagrale et al. [62] reported non–clinically significant differences for neck pain and disability outcomes at 4 weeks. This study suggested that a soft tissue therapy intervention to the upper trapezius, combining ischemic compression, strain-counterstrain, and muscle energy technique, provides similar clinical benefit compared with muscle energy technique alone. Participants were required to have neck pain of less than 3 months’ duration.
The panel determined moderate certainty in the evidence, with small desirable and undesirable effects and no serious adverse events. Low costs are required for the intervention and no specific equipment is needed, with the exception of training to provide the technique. Because the intervention is widely practiced and taught, it is acceptable and feasible to implement. However, its effects on health equities cannot be determined. Overall, the panel decided the balance between the desirable and undesirable consequences was uncertain, and more evidence is needed before a recommendation can be made.
Multimodal Care
Key Question 3: Should multimodal care vs intramuscular ketorolac be used for recent (0-3 months) grades I to III NAD?
Summary of Evidence
McReynolds et al. [63] presented short-term outcomes of pain intensity and concluded that sessions of multimodal care (manipulation, soft tissue techniques) provided equivalent outcomes to an intramuscular injection of ketorolac. However, the follow-up time of 1 hour is generally atypical and the dosing was determined to be incomplete for multimodal care as reported. Furthermore, the study was limited to an emergency setting only.
The panel determined low certainty in the clinical evidence, with small desirable and undesirable effects. There is relatively low risk for multimodal care, considering the reported outcomes were equal. From a clinician standpoint, resources required are small assuming no additional staff are needed. However, one practitioner gave most multimodal therapies. Expenses may vary depending on the definition of multimodal care. This option should not create health inequities, except for those who cannot access clinicians or choose to pay out of pocket, and would be feasible to implement. Professional associations would generally support the option, yet extended multimodal therapies can incur additional costs, which can be unfavorable to both payors and patients. Overall, the balance between the desirable and undesirable consequences is uncertain and more research is needed in this area before any recommendation can be made.
Exercise
Key Question 4: Should multimodal care vs home exercises vs medication be used for recent-onset (0-3 months) grades I to II NAD?
Summary of Evidence
One RCT by Bronfort et al. [22] evaluated the efficacy of multimodal care over 12 weeks compared with a 12-week home exercise and advice program or medication on neck pain (11-box NRS) and disability (NDI) in 181 adult patients with acute and subacute neck pain (2-12 weeks’ duration and a score of ≥3 on a 10-point scale). Multimodal care by a chiropractor (mean of 15.3 visits, range 2-23) included manipulation and mobilization, soft tissue massage, assisted stretching, hot and cold packs, and advice to stay active or modify activity as needed. Daily home exercise was to be done up to 6 to 8 times per day (individualized program including self-mobilization exercise of the neck and shoulder joints) with advice by a physical therapist (two 1-hour sessions, 1-2 weeks apart on posture and activity of daily living). Medication prescribed by a physician included nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, opioid analgesic, or muscle relaxants (dosage was not reported). The results displayed in Table 4 indicated that multimodal care and home exercises and advice were as effective as medication in reducing pain and disability at short term (26 weeks). However, medication was associated with a higher risk for adverse events (mostly gastrointestinal symptoms and drowsiness in 60% of participants) than home exercises. The choice of medications was based on the participant’s history and response to treatment. Clinicians and patients should be aware that current evidence is insufficient to determine the effectiveness of long-term opioid therapy for improving chronic pain and function. Importantly, evidence supports a dose-dependent risk for serious harms, including increased risk for overdose, dependence, and myocardial infarction. [64]
Recommendation: For patients with recent (0-3 months) neck pain grades I to II, we suggest either range-of-motion home exercises, medication, or multimodal manual therapy for reduction in pain and disability. (Weak recommendation, moderate-quality evidence)
Remark: Home exercises included education self-care advice, exercises, and instruction on activities of daily living. Medication included NSAIDs, acetaminophen, muscle relaxant, or a combination of these. Multimodal manual therapy included manipulation and mobilization with limited light soft tissue massage, assisted stretching, hot and cold packs, and advice to stay active or modify activity as needed.
Key Question 5: Should supervised graded strengthening exercises vs advice be used for recent-onset (0-3 months) grade III NAD?
Summary of Evidence
One RCT by Kuijper et al. [65] evaluated the effectiveness of supervised strengthening exercises compared with advice to stay active for recent-onset grade III neck pain. This RCT reported that strengthening exercises (n = 70) were more effective than advice to stay active (n = 66). [65] Trial participants were followed at 3 weeks, 6 weeks, and 6 months. Based on panel consensus, outcomes determined to be important in the assessment of effectiveness in this RCT included neck and arm pain (VAS) and disability (NDI). These outcomes were both statistically and clinically significant (Table 5).
In this RCT, the strengthening exercise program was delivered by physiotherapists 2 times per week for 6 weeks. [65] It included supervised graded strengthening exercises for the shoulder and daily home exercises to strengthen the superficial and deep neck muscles (mobility, stability, and muscle strengthening). Participants in the comparison group were advised to continue daily activities. Both groups were allowed to use painkillers. See Key Question 6 for a recommendation on cervical collar.
Recommendation: For patients with recent (0-3 months) grade III neck and arm pain, we suggest supervised graded strengthening exercises* rather than advice alone.† (Weak recommendation, moderate-quality evidence)
Remark: *Supervised graded strengthening exercises consisted of strengthening and stability exercises twice a week for 6 weeks with daily home exercises (which included mobility, stability, and muscle strengthening).
†Advice alone consisted of maintaining activity of daily living without specific treatment.
Passive Physical Modalities
Key Question 6: Should cervical collar vs graded strengthening exercise program be used for recent-onset (0-3 months) grade III NAD?
Summary of Evidence
One RCT by Kuijper et al. [65] randomly assigned 205 patients with recent-onset neck cervical radiculopathy (NAD grade III) to 1 of 3 groups (1): Rest and semi-hard cervical collar for 3 weeks, then weaned off during weeks 3–6 (2); physiotherapy (mobilizing and stabilizing the cervical spine, standardized graded neck strengthening exercises twice per week for 6 weeks, and education to do daily home exercises); or (3) a control group (wait and see with advice to continue daily activities). All patients received written and oral reassurance about the usually benign course of the symptoms and were allowed painkillers.
Wearing a semi-hard cervical collar or receiving standardized graded strengthening exercise program and home exercises for 6 weeks provided similar improvements in arm pain (VAS), neck pain (VAS), or disability (NDI) compared with a wait-and-see policy at 6 weeks. There were no between-group differences at 6 months.
Because of uncertainty about potential for iatrogenic disability associated with the prolonged use of cervical collar, [27, 42] one recommendation made in the current guideline favoring strengthening exercise programs over advice, and the lack of consensus among the guideline panel, the GDG decided not to make a recommendation against the use of cervical collar (first vote on the proposed recommendation with direct results from the study [11% agree, 11% neutral, 78% disagree, 1 abstained]). A second vote favored also removing the remark from the recommendation (27% agree, 9% neutral, 64% disagree, 1 did not vote). Choice should be based on patient’s preference and management changed if recovery is slow. [66]
Key Question 7: Should low-level laser therapy be used for recent-onset (0-3 months) grade III NAD?
Summary of Evidence
One RCT by Konstantinovic et al. [67] evaluated the effectiveness of low-level laser therapy (LLLT) delivered 5 times per week for 3 weeks compared with placebo (inactive laser treatment) for recent-onset grade III neck pain. LLLT leads to statistically but not clinically significant improvements in neck pain and disability at 3 weeks compared with placebo. Transitional worsening in pain (20%) and persistent nausea (3.33%) were observed in the LLLT group, whereas no adverse events were reported in the placebo group.
The panel determined the overall certainty of the evidence was moderate, with small desirable effects and minor adverse events. LLLT can be expensive. If practitioners choose not to purchase, it may negatively affect health equities. However, the option is acceptable to stakeholders and is relatively easy to implement. The panel was uncertain about the balance between desirable and undesirable consequences and voted against making a recommendation because of a lack of clear evidence (LLLT was no better than placebo but both groups demonstrated within-group change over time).
Work Disability Prevention Interventions
Key Questions 8 and 9: Should work disability prevention interventions vs fitness and strengthening exercise program be used for recent-onset nonspecific work-related upper limb disorders?
Should work disability prevention interventions be used for recent-onset work-related neck and upper limb complaints?
In reviewing the evidence on work disability prevention interventions, [41] the GDG concluded that the balance between desirable and undesirable consequences was “closely balanced or uncertain.” As a result, the guideline panel was unable to formulate recommendations for these key questions, yet future research is very likely to either positively or negatively support the various types of work disability prevention interventions.
Although some benefits were reported favoring computer-prompted and instructed exercise interventions, [68] the incremental self-reported improvement was insufficient to formulate a recommendation considering (1) a follow-up period of 8 weeks in reviewed studies is too short to estimate long-term sustained benefits; and (2) the potential costs related to programming and worker instruction may be significant.
Overall, it appears that adding computer-prompted exercises (with workplace breaks), or workplace breaks alone, to a program of ergonomic modification and education improves self-perceived recovery and symptomatic benefits in computer workers with neck and upper back complaints. [41] However, it is unclear whether the addition of computer-prompted exercises to the various established workplace interventions alters perceived or objective health outcomes. Future research may identify added benefits in order for stakeholders to consider the extra cost as being surmountable.
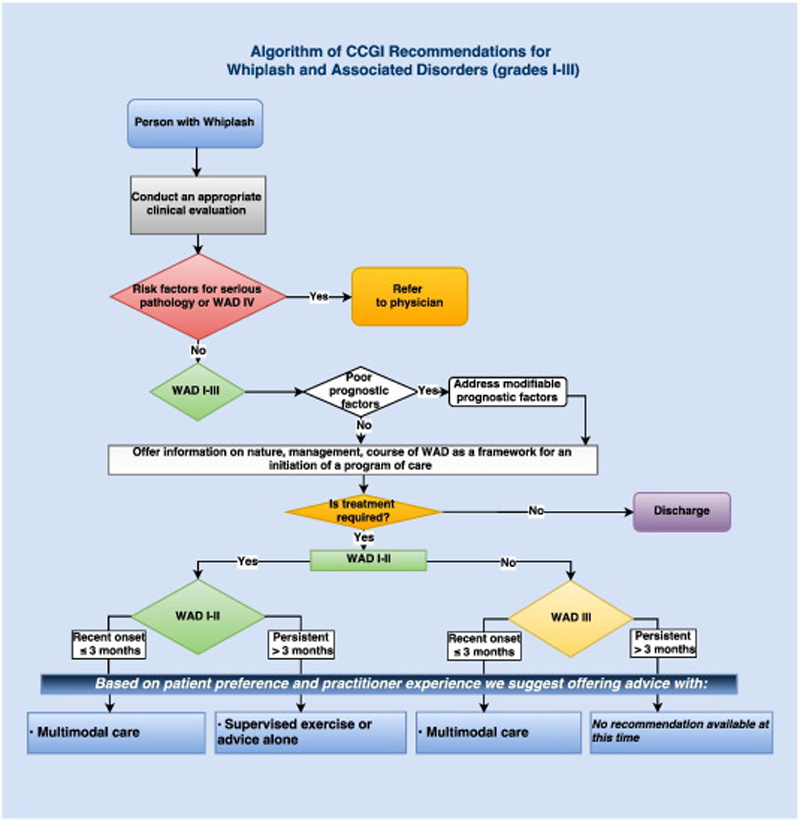
Recommendations for Recent-Onset (0-3 Months) Grades I to III WAD
Multimodal Care
Key Question 10: Should multimodal care vs education be used for recent (0-3 months) grades I to III WAD?
Summary of Evidence
A 2-part RCT by Lamb et al. [69] evaluated the effectiveness of oral advice compared with written material for improving pain (self-rated neck pain) and disability (NDI) in patients with recent-onset grades I to III WAD. Lamb et al. [69] included a total of 3,851 participants with a history of WAD grades I to III of less than 6 weeks’ duration who sought treatment at an emergency department. A total of 2,253 participants received active management advice in the emergency department incorporating oral advice and the Whiplash Book, which included reassurance, exercises, encouragement to return to normal activities, and advice against using a collar; 1,598 participants received usual care advice, including verbal and written advice along with anti-inflammatory medication, physiotherapy, and analgesics. No between-group difference was observed in self-rated neck pain and disability at 12-month follow-up and no difference in workdays lost was observed at 4-month follow-up (Table 6).
Lamb et al. [69] included 599 participants with WAD grades I to III that persisted for 3 weeks after attending emergency departments. Three hundred participants were treated by a physiotherapist (maximum 6 sessions over 8 weeks) includingpsychological strategies (goal setting or pacing, coping, reassurance, relaxation, pain and recovery),
self-management advice (posture and positioning),
exercises (shoulder complex mobilization and range of motion [ROM]; cervical and scapular stability and proprioception), and cervical and thoracic spine Maitland mobilization and manipulation; a total of 299 received single-session reinforcement advice from a physiotherapist during their previous visit to emergency department.No difference in self-rated disability was identified at 4-month follow-up; however, greater reductions in workdays lost after 8-month follow-up were determined with self-management advice over single-session reinforcement. Similar findings were found in an earlier study. [70]
Recommendation: For adult patients with recent (0-3 months) WAD grades I to III, we suggest multimodal care over education alone. (Weak recommendation, moderate-quality evidence)
Remark: Multimodal care may consist of manual therapy (joint mobilization, other soft tissue techniques), education, and exercises.
Structured Education
Key Question 11: Should structured patient education vs education reinforcement be used for recent-onset (0-3 months) WAD?
Summary of Evidence
Lamb et al. [69] reported outcomes at 4 months for self-rated disability, identifying no clinically significant differences between groups. The study suggested that oral advice and an educational pamphlet provide similar benefits.
The panel determined moderate quality in the clinical evidence, yet uncertain desirable effects with small, minor, and transient adverse events. Relatively few resources would be required for the intervention, and wide dissemination of educational materials through electronic tools can help reduce inequities. The option is acceptable to stakeholders and feasible to implement. Overall, the desirable consequences probably outweigh the undesirable consequences. The panel determined this topic and its evidence has substantial overlap with Key Question 10. Therefore, one recommendation was made, addressing both topics.
Recommendations for Persistent (>3 Months) Grades I to III NAD
Exercise
Key Question 12: Should supervised exercise (ie, qigong exercise) vs no treatment (wait listing) be used for persistent (>3 months) grades I to II NAD?
Summary of Evidence
Two RCTs (Table 7) evaluated the effectiveness of supervised qigong compared with supervised exercise therapy and no treatment on neck pain (101-point VAS), disability (NDI), and Neck Pain and Disability Scale in a total of 240 patients with chronic neck pain (>6 months). [71, 72] Rendant et al. [82] reported that, in adults with chronic neck pain, supervised qigong is more effective than no treatment and as effective as exercise therapy in reducing neck pain and disability at 3 and 6 months. Conclusions regarding the effectiveness of these 2 interventions compared with no treatment in patients aged older than 55 years cannot be drawn from the included studies.
In their study of these interventions for neck pain in elderly patients, von Trott et al. [71] observed a reduction in pain and disability in both intervention groups at 3 and 6 months (although not statistically significant). The quality of the evidence was downgraded to low based on the SIGN criteria (concealment method not reported). In the von Trott et al. study, the interventions consisted of two 45-minute sessions per week for 3 months (a total of 24 sessions), [71] whereas in the Rendant et al. study, interventions consisted of 12 treatments in the first 3 months and 6 treatments in the following 3 months (total of 18 sessions). [72] Exercise therapy in both studies included repeated active cervical rotations and strengthening and flexibility exercises in the form of Dantian qigong [71] or Neiyanggong qigong. [72] Similar minor transient side effects were reported in both the intervention and comparison groups.
Recommendation: For adult patients with persistent (>6 months) neck pain grades I to II, we suggest supervised group exercises* to reduce neck pain and disability. (Weak recommendation, moderate-quality evidence)
Remark: Patients received 18 to 24 group sessions during a period of 4 to 6 months. Patients considered had a rating of 40/100 on a pain scale (VAS). The intervention group reached suggested MCID level of 10% difference for pain and functional outcomes.
*Exercises included qigong or ROM, flexibility, and strengthening exercises. No evidence of significant effect in the elderly population.
Key Question 13: Should supervised yoga vs education be used for persistent (>3 months) grades I to II NAD?
Summary of Evidence
Yoga is an ancient Indian practice involving postural exercises, breathing control, and meditation. [20] One RCT by Michalsen et al. [73] evaluated the effectiveness of Iyengar yoga compared with a self-care/exercise program on neck pain (VAS) and disability (NDI) in 76 patients with chronic neck pain (pain for at least 3 months and a score of more than 40 mm on a 100-mm VAS). Yoga consisted of a weekly 90-minute session for 9 weeks of a wide range of postures aimed to enhance flexibility, alignment, stability, and mobility. The self-care/exercise group had to practice for 10 to 15 minutes at least 3 times a week a series of 12 exercises focusing on muscle stretching and strengthening and joint mobility. The results indicated that yoga is more effective for reducing neck pain and disability at short term (4 and 10 weeks) than self-care/exercise (Table 8). No serious adverse events were reported in either group. In this study, the quality of evidence was downgraded to low because blinding was “poorly addressed.” [45]
One RCT by Jeitler et al. [74] evaluated the effectiveness of Jyoti meditation compared with exercise on neck pain (VAS). The results showed that Jyoti meditation (sitting motionless, repeating a mantra, and visual concentration while keeping the eyes closed) is more effective than exercise (established and previously used self-care manual for specific exercise and education for chronic neck pain). [74] Because Jyoti meditation only includes 1 of the 3 components of yoga (ie, meditation), Jeitler et al. [74] was not considered in developing the following recommendation.
Recommendation: For patients with persistent (>3 months) grades I to II neck pain and disability, we suggest supervised yoga over education and home exercises for short-term improvement in neck pain and disability. (Weak recommendation, low-quality evidence)
Remark: Baseline intensity of pain was more than 40/100 and duration was at least 3 months. Yoga was specific to the Iyengar type, with a maximum of 9 sessions over 9 weeks.
Key Question 14: Should supervised strengthening exercises vs home ROM or stretching exercises be used for persistent (>3 months) grades I to II NAD?
Summary of Evidence
Three RCTs evaluated the effectiveness of supervised strengthening exercises compared with home exercises for grades I to II neck pain and disability. [38] Two RCTs (Hakkinen et al. [75] and Salo et al. [76]) reported no significant between group differences at 1 year for primary or secondary outcomes. One RCT (N = 170) reported that supervised strengthening exercises were more effective than home ROM exercises. [77] Two smaller RCTs (N = 107) found that both treatments are equally effective. [75, 76] All 3 trials had a follow-up of 1 year. Based on our panel’s consensus, outcomes determined to be important in the assessment of effectiveness for these RCTs included pain (NRS) and disability (NDI).
In the RCT by Evans et al. [77] the strengthening exercise program (delivered by exercise therapists) was determined to be more effective than home exercises. The program included 20 supervised sessions over a period of 12 weeks and consisted of neck and upper body dynamic resistance strengthening program with and without spinal manipulative therapy. [77] Conversely, the home exercises included an individualized program of neck and shoulder self-mobilization with initial advice regarding posture and daily activities (Table 9). In the 2 RCTs demonstrating equivalence, the strengthening program included 10 supervised sessions over 6 weeks of isometric exercises for the neck flexors and extensors, dynamic shoulder and upper extremity exercises, abdominal and back exercises, and squats. [43, 44]
A fourth RCT by Maiers et al. [78] assessed the effectiveness of supervised rehabilitative exercises in combination with and compared with home exercises alone for persistent neck pain in individuals aged 65 years or older. All participants in the study received 12 weeks of care. One group received 20 supervised 1-hour exercise sessions in addition to home exercises. Home exercises consisted of four 45- to 60-minute sessions to improve flexibility, balance, and coordination and enhance trunk strength and endurance. Participants also received instructions on pain management, practical demonstrations of body mechanics (lifting, pushing, pulling, and rising from a lying position), and massaging to stay active. Results favored supervised rehabilitative exercises combined with home exercises over home exercise for pain (NRS) and disability (NDI) at 12 weeks. However, between-group differences did not reach statistical significance.
Recommendation: For patients with persistent (>3 months) grades I to II neck pain, we suggest supervised strengthening exercises or home exercises. (Weak recommendation, low-quality evidence)
Remark: For reduction in pain, supervised strengthening exercises, provided along with ROM exercises and advice, were evaluated at 12 weeks within 20 sessions. Home exercises include stretching or self-mobilization.
Key Question 15: Should strengthening exercises vs general strengthening exercises be used for persistent (>3 months) grades I to II NAD?
Summary of Evidence
Griffiths et al. [79] presented non–clinically significant outcomes for neck pain and disability among patients with persistent neck pain and concluded there is no added benefit of incorporating specific isometric exercise to a general exercise program. Dosages were up to 4 sessions per 6-week period, with advice for 5 to 10 times at home. The general exercise program consisted of postural exercise, active ROM, 5 to 10 times daily with reinforcement.
The panel determined there is low certainty in the clinical evidence and uncertainty in the desirable effects of the intervention. Isometric exercises have little anticipated adverse effects, require minimal resources, and are generally acceptable to stakeholders and feasible to implement. Yet uncertainty remains regarding their effects on health equity and the overall balance between desirable and undesirable consequences. More research is needed in this area before a recommendation can be made.
Key Question 16: Should combined supervised strengthening, ROM, and flexibility exercises vs no treatment (wait listing) be used for persistent (>3 months) grades I to II NAD?
Summary of Evidence
von Trott et al. [71] and Rendant et al. [72] presented significant outcomes for reduction in neck pain and disability that favor combined strengthening, ROM, and flexibility exercises. Both studies address different populations and lead to similar outcomes (von Trott et al. [71] addressed elderly populations).
The panel determined there was moderate certainty in the clinical evidence, with large desirable and small undesirable anticipated effects. Yet there may be differences in adverse events for strengthening vs ROM and flexibility exercises, along with the challenges of such adverse events being self-reported. For example, strengthening exercises likely coincide with short-term pain after the intervention. Further, significant space may be required for exercises, which may incur large costs that need to be considered up front. As a result, there is uncertainty about the feasibility to implement and whether this could widely affect health inequalities. However, the option would be acceptable to stakeholders. Overall, the desirable consequences would probably outweigh the undesirable consequences. The panel determined this topic and its evidence has substantial overlap with Key Question 12 (qigong was considered exercise). Therefore, 1 recommendation was made, addressing both topics.
Manual Therapy
Key Question 17: Should multimodal care vs self-management be used for persistent (>3 months) grades I-II NAD?
Summary of Evidence
One RCT by Gustavsson et al. [80] evaluated the effectiveness of self-management of persistent musculoskeletal tension type neck pain for grades I to II neck pain. They compared treatment effects of a multicomponent pain and stress self-management group intervention (n = 77) to individually administered multimodal physical therapy (n = 79). Measures of pain (NRS) and disability (NDI) were collected at baseline and at 10 and 20 weeks. Both groups had within-group differences for decreased pain intensity and disability. At the 20-week follow-up after an average of 7 sessions, based on the measures used, the multicomponent pain and stress self-management group intervention had a greater treatment effect on coping with pain and patients’ self-reported pain control and disability than the multimodal care group. The initial treatment effects were largely maintained over a 2-year follow-up period (Table 10). [81]
Recommendation: For patients with persistent (>3 months) neck pain and associated disorders grades I to II, we suggest multimodal care* or stress self-management† based on patient preference, prior response to care, and resources available. (Weak recommendation, low-quality evidence)
Remark: *Individualized multimodal care may include manual therapy (manipulation, mobilization, massage, traction), acupuncture, heat, transcutaneous electrical nerve stimulation, exercise, and/or ultrasound.
†Stress self-management may include relaxation, balance and body awareness exercises, pain and stress self-management lectures, and discussion.
The multimodal care group received an average of 7 (range 4–8) sessions, compared with 11 (range 1–52) sessions for the stress self-management group over 20 weeks.
Education
Key Question 18: Should structured patient education vs massage therapy be used for persistent (>3 months) NAD?
Summary of Evidence
Sherman et al. [82] reported non–clinically significant outcomes at 4 weeks for disability. This study suggests a mailed self-care book and a course in massage therapy provide similar clinical benefits for patients with persistent neck pain.
The panel determined the overall certainty of the evidence was low, with relatively large anticipated effects and no serious adverse events noted from intervention (some headaches possibly). There is uncertainty in the costs required, including necessary staff, equipment, and materials. Yet this option is feasible to implement in most settings and has strong implications for reducing health inequities. As a preventive strategy, the intervention is acceptable to stakeholders, including the chiropractic practitioners, patients, and policymakers. The panel was uncertain about the balance between the desirable and undesirable consequences. Additional high-quality studies are needed in this area before any recommendation can be made.
Manual Therapy
Key Question 19: Should manipulation be used for persistent grades I to II NAD?
Summary of Evidence
Evans et al. [77] compared spinal manipulation in addition to 20 weeks of supervised exercise therapy (20 sessions) to supervised exercise therapy alone in adults with persistent grades I to II neck pain, whereas Maiers et al. [78] compared spinal manipulation in addition to home exercises (20 sessions maximum) to home exercise alone in seniors with persistent grades I to II neck pain. Pain and disability outcomes at 12 and 52 weeks did not reach statistical significance in between-group differences, except for pain level at 12 weeks in the Maiers study. [78] A third RCT by Lin et al. [83] allocated 63 persistent neck pain patients (NAD I–II) to the experimental group (n = 33) treated with cervical spine manipulation and traditional Chinese massage (TCM) compared with TCM alone (n = 30) over 3 weeks. Results favored cervical manipulation with TCM over TCM alone for pain (NPS) and disability (Northwick Park Neck Disability Questionnaire) at 3 months (Table 11).
The panel concluded low certainty in the evidence, with small desirable and undesirable effects of the intervention. Few resources are required for the intervention, and it is probably acceptable to stakeholders and feasible to implement. Although the panel decided the desirable and undesirable consequences were closely balanced, the following statement was provided.
Recommendation: For patients with persistent grades I to II NAD, we suggest manipulation in conjunction with soft tissue therapy. (Weak recommendation, low-quality evidence)
Remark: Evaluated after eight 20-minute sessions (over a 3-week period). Does not include manipulation as a standalone treatment.
Manual Therapy
Key Question 20: Should massage vs no treatment (wait listing) be used for persistent (>3 months) grades I to II NAD?
Summary of Evidence
Sherman et al. [82] and Lauche et al. [84] reported non–clinically significant differences in outcomes for disability at 4 and 12 weeks, respectively. Sherman et al. [82] suggested Swedish and/or clinical massage with verbal self-care advice provides similar clinical benefit to a self-care book for disability outcomes. Lauche et al. [84] suggested cupping massage and progressive muscle relaxation lead to similar changes in disability. Sherman et al. [85] reported outcomes for neck pain and disability at 4 weeks and suggested that higher doses of massage provide superior clinical benefit (Table 12).
The panel determined low certainty in the evidence, with small desirable and undesirable effects. Additional costs may be needed to get clinical benefit. Sherman et al. [85] suggested a minimum of 14 hours of staff time needed. Because of the costs associated with high-dose massage, it may not be entirely acceptable to patients or payors. However, this option is feasible and relatively easy to implement in educated and affluent populations similar to subjects primarily studied. [85] Overall, the panel decided the desired consequences probably outweigh the undesirable consequences and suggest offering this option.
Recommendation: For patients with persistent (>3 months) grades I to II NAD, we suggest high-dose massage over no treatment (wait listing) based on patient preferences and resources available. (Weak recommendation, low-quality evidence)
Remark: Interventions were given 3 times for 60 minutes a week for 4 weeks. Lower dosages and duration did not have therapeutic benefit, and we cannot suggest offering as an option.
Passive Physical Modalities
Key Question 21: Should LLLT be used for persistent (>3 months) grades I to II NAD?
Summary of Evidence
After full-text screening and review, no studies addressing between-group differences among outcomes of pain or disability were included to inform this key question. The lack of evidence and uncertainty in the overall balance between desirable and undesirable consequences led the panel to decide not to write a recommendation for this topic at this time. More high-quality studies are needed in this area before certainty in judgments or recommendations can be made.
Key Question 22: Should transcutaneous electrical nerve stimulation vs multimodal soft tissue therapy program be used for persistent (>3 months) grades I to II NAD?
Summary of Evidence
After full-text screening and review, no studies addressing between-group differences among outcomes of pain or disability were included to inform this key question. The lack of evidence and uncertainty in the overall balance between desirable and undesirable consequences led the panel to decide not to write a recommendation for this topic at this time. More high quality studies are needed in this area before certainty in judgments or recommendations can be made.
Key Question 23: Should cervical traction be used for grade III NAD (variable duration)?
Summary of Evidence
After full-text screening and review, no studies addressing between-group differences among outcomes of pain or disability were included to inform this key question. The lack of evidence and uncertainty in the overall balance between desirable and undesirable consequences led the panel to decide not to write a recommendation for this topic at this time. More high-quality studies are needed in this area before certainty in judgments or recommendations can be made.
Multimodal Care
Key Question 24: Should multimodal care vs continued practitioner care be used for persistent grades I to III NAD?
Summary of Evidence
One RCT by Walker et al. [86] evaluated the effectiveness of multimodal care for neck pain with or without unilateral upper extremity symptoms (grades I–III). They compared treatment effects of combined multimodal care and home exercises (n = 47) to multimodal minimal intervention (n = 47). Both intervention groups received on average of 2 sessions per week for 3 weeks. No interventions were rendered after 6 weeks. Baseline self-reported questionnaires included neck and arm pain (VAS) and disability (NDI). All measures were repeated at 3, 6, and 52 weeks. Patients in the multimodal care and home exercise group had significantly greater reduction in short-term neck pain and in short-term and long-term disability compared with the multimodal minimal intervention group (Table 13).
A secondary analysis of the Walker et al. study [87] determined that patients receiving both cervical thrust and nonthrust manipulations did no better than the group receiving cervical nonthrust manipulations only. This underpowered secondary analysis prohibits any definitive statement regarding the presence or absence of a treatment advantage of one approach over the other. The reduction in pain reported by Walker’s multimodal care and exercise group compared favorably to the change scores reported by other studies, including Hoving et al. [88, 89]
In an RCT, Monticone et al. [90] evaluated the effectiveness of multimodal care for persistent neck pain. They compared treatment effect of multimodal care alone (n = 40) to multimodal care in conjunction with cognitive behavioral treatment (n = 40). Both groups had a reduction in pain (NRS) and disability (NPDS), but there were no clinically significant differences between the groups at 52 weeks. The addition of a cognitive behavioral treatment did not provide greater outcomes than multimodal care alone.
Recommendation: For patients presenting with persistent neck pain grades I to III, we suggest clinicians offer multimodal care* and/or practitioner advice† based on patient preference. (Weak recommendation, low-quality evidence)
Remark: *Multimodal care and exercises may consist of thrust/nonthrust joint manipulation, muscle energy, stretching, and home exercises (cervical retraction, deep neck flexor strengthening, cervical rotation ROM).
†Multimodal minimal intervention may consist of postural advice, encouragement to maintain neck motion and daily activities, cervical rotation ROM exercise, instructions to continue prescribed medication, and therapeutic pulsed (10%) ultrasound at 0.1 W/cm2 for 10 minutes applied to the neck and cervical ROM exercises.
Exercise
Key Question 25: Should group exercises vs education or advice be used for workers with persistent neck and shoulder pain?
Summary of Evidence
We have combined the key questions for “Should structured patient education vs exercise programs be used for persistent neck pain and associated disorders in workers?” and “Should workplace-based exercises vs advice be used for neck pain in workers?”
One large cluster RCT (n = 537) by Zebis et al. [91] evaluated the effectiveness of strength training in the workplace compared with receiving advice to stay physically active on nonspecific neck and shoulder pain intensity. The findings indicated a similar reduction in neck and shoulder pain intensity at 20 weeks for the exercise program compared with advice (Table 14). The intervention consisted of 3 sessions per week, each lasting 20 minutes, for up to 20 weeks (total of 60 sessions).
The workplace exercise program consisted of high-intensity strength training relying on principles of progressive overload and involved local neck and shoulder muscles strengthening with 4 different dumbbell exercises and 1 exercise for the wrist extensor muscles. More than 15% of workers assigned to the workplace exercise group reported minor and transient complaints. The comparison group reported no adverse events.
A subgroup analysis [92] of the primary Zebis et al. study [91] included 131 women with a baseline neck pain rating of at least 30 mm VAS from the 537 male and female participants. Results favored specific resistance training over advice to stay active for pain (VAS) at 4 weeks. This study was not included because findings were already considered in the primary study.
Recommendation: For workers with persistent neck and shoulder pain, we suggest mixed supervised and unsupervised high-intensity strength training or advice alone. (Weak recommendation, moderate-quality evidence)
Remark: For reduction in pain intensity, 3 sessions per week, each lasting 20 minutes, over a 20-week period. Exercise includes strengthening. Extra resources are likely required for complete exercise intervention implementation.
Structured Patient Education
Key Question 26: Should structured patient education vs exercise programs be used for persistent (>3 months) NAD in workers?
Summary of Evidence
Andersen et al. [93] reported non–clinically significant outcomes at 10 weeks for neck and shoulder pain, suggesting weekly e-mailed information on general health behaviors and shoulder abduction exercise programs provide similar clinical benefit. Yet implementation of high-intensity strength training exercises in industrial workplaces (implementation of exercise into day-to-day life and to increase active leisure time) is generally supported. [94, 95] In another RCT, pain reduction was significantly greater than in the group receiving advice alone. [91] Findings from Zebis et al. [91] are also included in the exercise intervention section of this guideline.
The panel determined moderate certainty in the clinical evidence, with small desirable and undesirable effects of the intervention. The resources required are relatively small, assuming the practitioner presents the education to the patient. Health inequities would be positively affected, and the intervention would be acceptable to stakeholders and feasible to implement. The panel decided not to repeat these findings in the current section. The panel felt that the benefits of increasing the frequency and intensity of exercise regimes was not restricted to those working in an industrial environment or to any specific population subgroup with the exception of older adults.
Work Disability Prevention Interventions
Key Questions 27–29: Should work-based hardening vs clinic-based hardening be used for persistent (>3 months) work-related rotator cuff tendinitis?
Should work disability prevention interventions be used for persistent neck and shoulder pain?
Should work disability prevention interventions be used for persistent (>3 months) upper extremity symptoms?
Summary of Evidence
In reviewing the evidence on work disability prevention interventions, [41] the GDG concluded that the balance between desirable and undesirable consequences was “closely balanced or uncertain” for Key Questions 27–29. As a result, the guideline panel was unable to formulate recommendations for these key questions, yet future research is very likely to either positively or negatively support the various types of work disability prevention interventions.
Recommendations for Persistent (>3 Months) Grades I to III WAD
Exercise
Key Question 30: Should supervised general exercise and advice vs advice alone be used for persistent (>3 months) grades I to II WAD?
Summary of Evidence
In an RCT, Stewart et al. (2007) [96] evaluated the effectiveness of 3 advice sessions alone compared with 3 advice sessions combined with 12 exercise sessions over 6 weeks on neck pain (NRS) and disability (NDI) among 134 patients with persistent grades I to II WAD. The results, presented in Table 15, indicated that supervised exercises with advice are as effective as advice alone at long term (12 months). Advice included standardized education, reassurance, and encouragement to resume light activity and consisted of 1 consultation and 2 follow-up phone contacts. However, the quality of the evidence was downgraded to low based on SIGN criteria (randomization and outcome measurement were “poorly addressed”) and the low number of participants and events. [45]
A pragmatic trial assigned 172 patients with persistent WAD grades I to II to receive a comprehensive 12-week exercise program (20 sessions including manual therapy technique the first week [no manipulation] and cognitive behavioral therapy delivered by physiotherapists) or advice (1 session and telephone support). [97] The comprehensive exercise program was not more effective than advice alone for pain reduction or disability, although findings favored a comprehensive physiotherapy exercise program over advice.
The panel determined low certainty in the evidence, with small desirable and undesirable effects and no serious adverse events (5 patients who received the comprehensive exercise program and 4 who received advice had minor transient adverse events). Overall, the panel decided the balance between the desirable and undesirable consequences such as costs was uncertain, and more evidence is needed before a recommendation can be made.
In a 20-week cluster RCT, Gram et al. (2014) [98] randomly assigned 351 office workers to 2 training groups receiving the same total amount of planned exercises 3 times per week, with 1 group supervised throughout the intervention period and the other receiving minimal supervision only initially, and a reference group (without exercise). Although results indicated that supervised training at the workplace reduced neck pain, results were not clinically significant and both training groups improved independently of the extent of supervision. The panel decided not to consider this study in formulating a recommendation because exercise was not directly compared with advice and an important loss to follow-up occurred across groups. Although supervised exercise appears to be beneficial, costs can be high. This could possibly be mitigated, however, by offering group treatment, which may increase compliance and accountability with a supervised group.
Recommendation: For patients with persistent (>3 months) grades I to II WAD, we suggest supervised exercises with advice or advice alone based on patient preference and resources available. (Weak recommendation, low-quality evidence)
Remark: Extra resources may be required for supervised exercises.
Multimodal Care
Key Question 31: Should multimodal care vs self-management program be used for persistent (>3 months) grade II WAD?
Summary of Evidence
Jull et al. [99] reported no clinically or statistically significant outcomes for pain and disability at 10 weeks. They suggested that multimodal care (exercises, mobilization, education, and ergonomic advice) provided similar outcomes to a self-management program based on an educational booklet (mechanism of whiplash, reassurance of recovery, stay active, ergonomic advice, exercise). Care did not include high-velocity manipulation. Although this study is specific to physiotherapists, it is well within the scope of chiropractors (manual therapists).
One other RCT by Jull et al. [100] evaluated the effectiveness of multidisciplinary individualized treatments for patients with acute whiplash (<4 weeks postinjury). Patients randomly assigned to pragmatic intervention (n = 49) could receive medication including opioid analgesia, multimodal physiotherapy, and psychology for post-traumatic stress over 10 weeks. No significant differences in frequency of recovery (NDI ≤ 8%) between pragmatic and usual care groups was found at 6 or 12 months. There was no improvement in current nonrecovery rates at 6 months (63.6%, pragmatic care; 48.8%, usual care), indicating no advantage of the early multiprofessional intervention.
The panel determined low certainty in the clinical evidence, with small desirable and undesirable effects reported. Yet there were relatively small costs and resources required to implement the intervention. Electronic dissemination of the educational component of multimodal care may reduce health inequities. The option may be acceptable to clinicians (assuming collaborative care approaches), policymakers, and patients and is likely feasible to implement in usual care settings. Overall, the balance between the desirable and undesirable consequences is uncertain, and no recommendation is given at this time. Further studies need to be conducted in this area and should involve multimodal care including high-velocity procedures or manipulation.
Education
Key Question 32: Should structured patient education vs advice be used for persistent (>3 months) WAD?
Summary of Evidence
Stewart et al. (2007) [96] reported non–clinically significant between differences for pain and disability outcomes at 6 weeks. This study suggested that adding a physiotherapy-based graded exercise program to a structured advice intervention provided similar clinical benefit as structured education alone.
The panel determined low certainty of the evidence, with low desirable and undesirable anticipated effects. The main complaints were muscle pain, knee pain, and spinal pain with mild headaches. [96] The small resources required for the intervention may reduce health inequities, and the option is acceptable to stakeholders and feasible to implement in most settings.
The panel determined that this key question had substantial overlap with Key Question 5 and decided to make 1 recommendation addressing both topics.
Discussion
This evidence-based guideline establishes the best practice for the management of NAD and WAD resulting from or aggravated by a motor vehicle collision and updates 2 previous guidelines on similar topics. [24, 25] This guideline covers recent-onset (0-3 months) and persistent (>3 months) NADs and WADs grades I to III. It does not cover the management of musculoskeletal thoracic spine or chest wall pain.
The primary outcomes reported in the selected studies were neck pain intensity and disability. Although all recommendations included in this guideline are based on low risk of bias RCTs, the overall quality of evidence is generally low considering other factors considered by GRADE such as imprecision, and thus the strength of recommendations is weak at this time.
Weak recommendations mean that clinicians need to devote more time to the process of shared decision making and ensure that the informed choice reflects patient values and preferences. [56] Interventions not described in this guideline cannot be recommended for the management of patients with NAD or WAD because of a lack of evidence about their effectiveness and safety
(Table 16 see page 550).
A recent systematic review and meta-analysis by Wiangkham (2015) [101] on the effectiveness of conservative management for acute WAD grade II included 15 RCTs, all assessed as high risk of bias (n = 1,676 participants), across 9 countries. Authors concluded that conservative interventions (noninvasive treatment), including active mobilization exercises, manual techniques, physical agents, multimodal therapy, behavioral approaches, and education, are generally effective for recent-onset WAD grade II to reduce pain in the medium and long term and to improve cervical ROM in the short term compared with standard or control intervention. [101] Although findings from the Wiangkham review are generally in line with those from the systematic reviews we included in this guideline, [24, 25] the pooling of high risk of bias and of clinically heterogeneous trials seriously challenges the validity of this more recent review.
Similarities and Differences With Recommendations by the OPTIMa Collaboration
First, the recommendations for the management of minor injuries of the neck were recently released by the Ministry of Finance of Ontario in collaboration with the OPTIMa Collaboration [20] and published as a separate guideline. [27] They considered the risks of bias of included RCTs using the SIGN criteria [45] and the guideline recommendations developed using the modified OHTAC framework, [28] based on 3 decision determinants (1): overall clinical benefit (evidence of effectiveness and safety) (2); value for money (evidence of cost-effectiveness where available); and (3) consistency with expected societal and ethical values. In the current guideline, we used the GRADE approach, which, in addition to considering risk of bias of included RCTs, takes into account 4 other factors (imprecision, inconsistency, indirectness, publication bias) to rate the confidence in effect estimates (quality of evidence) for each outcome. [102] As a result of imprecision of estimates in several RCTs, the overall quality of admissible studies was deemed low. GRADE considers similar decision determinants as the modified OHTAC to develop recommendations when subsequently making an overall rating of confidence in effect estimates across all outcomes based on those outcomes considered critical to a particular recommendation. [56] Accordingly, the guideline panel was asked to consider this low quality of evidence when judging the “desirable” consequences. When the benefits of important outcomes slightly outweighed undesirable effects of the intervention, a weak recommendation was made (ie, suggestions for care). This is likely to involve ensuring patients understand the implications of the choices they are making, possibly using a formal decision aid. [56] However, if the judgment was “closely balanced or uncertain,” no recommendation could be made.
Second, OPTIMa [20] recommended that interventions should only be provided in accordance with published evidence for effectiveness, including parameters of dosage, duration, and frequency, and within the most appropriate phase. The emphasis during the early phase (0–3 months) should be on education, advice, reassurance, activity, and encouragement. Health care professionals should be encouraged to consider watchful waiting and clinical monitoring as evidence-based therapeutic options during the acute phase. For injured persons requiring therapy, time-limited and evidence-based interventions should be implemented on a shared decision-making basis, an approach that equally applies to patients in the persistent phase (4–6 months). Despite using slightly different methods to derive recommendations, the 2 processes generally led to similar guidance.
Third, OPTIMa [20] reported that the following interventions are not recommended for recent-onset NAD:structured patient education alone (either verbal or written);
strain-counterstrain or relaxation massage;
cervical collar;
electroacupuncture (electrical stimulation of acupuncture points with acupuncture needles or electrotherapy applied to the skin), a topic not covered in our guideline; electric muscle stimulation;
heat (clinic based).Similarly for persistent NAD, programs solely of clinic-based supervised high-dose strengthening exercises, strain-counterstrain or relaxation massage, relaxation therapy for pain or disability outcomes, transcutaneous electrical nerve stimulation (TENS), electric muscle stimulation, pulsed shortwave diathermy, heat (clinic based), electroacupuncture, and botulinum toxin injections are not recommended.
In contrast, based on the RCT by Zebis et al. [91] the current guideline suggests offering multimodal care and/or patient education for industrial workers presenting with neck pain grades I to III. Although structured patient education used alone cannot be expected to yield large benefits for patients with neck pain, this strategy may be of benefit during the recovery of patients with persistent WAD when used as an adjunct therapy. [40] For persistent neck pain (grades I-II), Gustavsson et al. [80] reported that multimodal care combining manual therapy (spinal manipulation, mobilization, massage, traction) and passive modalities (heat, TENS, exercise, and/or ultrasound) reduced neck disability. It should be noted, however, that past reviews were unable to make any definitive conclusions about the effectiveness of TENS as an isolated treatment for acute pain [103] or chronic pain [104] in adults, nor about the effectiveness of heat therapy. [105, 106]
A comparison of the recommendations with 2 previous chiropractic guidelines [24, 25] reveals that a multimodal approach including manual therapy, advice, and exercise remains the overall recommended strategy of choice for the treatment of neck pain. However, treatment modalities included in recommended multimodal care differed according to the quality of the evidence available at the time.
The 2010 guideline on the management of WAD developed treatment recommendations based on low-quality evidence from 8 available RCTs and 3 cohort studies. [25] Overall, recommendations for recent and persistent WAD are similar (multimodal care, and supervised exercise and multidisciplinary care, respectively). The 2014 guideline on neck pain [24] developed 11 treatment recommendations from 41 RCTs. The current guideline developed 13 recommendations from 26 low risk of bias RCTs. In line with the 2014 guideline [24] for recent-onset neck pain, the current recommendations suggest offering multimodal care including mobilization, advice, and exercises. The current guideline recommendations also suggest offering supervised graded strengthening and stability exercises. Similar to the 2014 guideline for persistent neck pain (grades I-II), [24] the current recommendations suggest offering multimodal care consisting of manual therapy (spinal manipulation therapy or mobilization) and exercises. Details on specific exercise modalities are now provided, including suggestions for supervised and unsupervised exercises, strength training, and supervised group exercises such as workplace exercise programs and supervised yoga.
Adverse Events
This guideline did not specifically review the evidence on adverse events from treatments. However, in the review by Wong et al. [42] on manual therapy and passive modalities, 22 of the low risk of bias RCTs addressed the risk of harm from conservative care. Most adverse events were mild to moderate and transient (mostly increased stiffness and pain at the site of treatment, with a mean rate of about 30%). No serious neurovascular adverse events were reported. Another review of published RCTs and prospective cohort studies confirmed that around half of people treated with manual therapy can expect minor to moderate adverse events after treatment, but that the risk of major adverse events is small. [107] The pooling of data from RCTs of manual therapy on the incidence of adverse events indicated that the relative risk of minor or moderate adverse events was similar for manual therapy and exercise treatments, and for sham/passive/control interventions.
A patient-centered holistic and collaborative view of the needs of the patient with pain and disability is encouraged. [108, 109] Although chiropractors are not responsible for pharmacologic management, they should have sufficient knowledge about pharmacologic agents and their adverse events. One eligible RCT [22] found home exercises and advice to be as effective as medication (acetaminophen, NSAIDs, muscle relaxant, and opioid analgesic) in reducing pain and disability at short term for patients with acute or subacute neck pain grades I to II. However, medication was associated with a higher risk for adverse events. Of interest, recent evidence suggests that acetaminophen is not effective for managing low back pain, [110, 111] and the effectiveness of long-term opioid therapy for improving chronic pain and function is uncertain. [64] However, a dose-dependent risk for serious harms is associated with long-term use of opioid (increased risk for overdose, opioid abuse and dependence, fractures, myocardial infarction, and use of medications to treat sexual dysfunction). [64] Risk of unintentional opioid overdose injury appears to be particularly important in the first 2 weeks after initiation of long-acting agents. [112, 113]
Recommendations
I. Stakeholders
Choosing a Care Provider
A range of health care providers (chiropractors, general medical practitioners, physiotherapists, registered massage therapists, and osteopaths) deliver care for NADs and WADs. [108, 114] Considering the level of skills required to deliver manual therapy, including spinal manipulative therapy and other forms of therapies (eg, prescription of specific exercise) and based on individual patient preference, cervical spine manipulation as part of multimodal care should be delivered by properly trained licensed professionals. [115]
II. Practitioners
Best Practice Recommendations-Initial Assessment and Monitoring
This guideline specifically addresses the treatment of NAD and WAD grades I to III. Importantly, our panel supports the following 5 best practice recommendations on patients care outlined in the OPTIMa guideline [27]:Clinicians should
(1) rule out major structural or other pathologic conditions as the cause of neck pain–associated disorders before classifying as grade I, II, or III
(2); assess prognostic factors for delayed recovery
(3); educate and reassure patients about the benign and self-limited nature of the typical course of NAD grades I to III and the importance of maintaining activity and movement (4); refer patients with worsening symptoms and those who develop new physical or psychological symptoms for further evaluation at any time during their care; and
(5) reassess the patient at every visit to determine whether additional care is necessary, the condition is worsening, or the patient has recovered.Patients reporting significant recovery should be discharged. Similar recommendations were formulated by the Neck Pain Task Force [116] and in prior practitioner guides on the management of WAD and NAD by chiropractors. [24, 25]
Benefits of Physical Activity and Self-management
Educating patients about the benefits of being physically active and participating in their care has become the standard of care internationally. Despite the benefits of therapeutic exercise for managing chronic neck pain and the strong evidence favoring regular physical activity to reduce related comorbidities, care providers fail to routinely prescribe these to patients. [117–120] When prescribed, the amount of supervision and types of exercises do not follow practice guidelines and are not linked to the degree of patient impairment. [118, 121] On the patient side, adherence to prescribed exercise programs is often low. [122]
The promotion of physical activity, including exercise, is a first-line treatment considered important in the prevention and treatment of musculoskeletal pain and its related comorbidities (eg, coronary heart disease, type 2 diabetes, and depression). [123–126] For a minority of patients with chronic spine pain, clinician-delivered interventions and pharmacologic treatments are appropriate; and in fewer cases, multidisciplinary pain management or surgery may be indicated. [118]
People with musculoskeletal pain will often adopt an inactive lifestyle. Unfortunately, physical inactivity is associated with important adverse health effects, including increased risks of coronary heart disease, type 2 diabetes, and breast and colon cancers, and shorter life expectancy in general. [127] The World Health Organization [128] provided clear guidance on physical activity for health for children, adults, and elders. In addition, recent research suggests that WAD patients with high levels of passive coping strategies have slower pain and disability recovery. [129] Self-management support (SMS) strategies aimed at increasing physical activity and active coping strategies are key to effectively managing spinal pain and related comorbidities. [124, 125, 130–134] The CCGI developed a theory-based knowledge translation (KT) intervention targeting identified barriers to professional behavior change to increase the uptake of SMS strategies among Canadian chiropractors. [135] Interviews of clinicians identified 9 theoretical domains as likely relevant (ie, factors perceived to influence the use of multimodal care to manage nonspecific neck pain). [135] The intervention, comprising a webinar and a learning module on Brief Action Planning, is a highly structured SMS strategy that allows patient-centered goals [136] and is being pilot-tested among Canadian chiropractors (ongoing pilot trial). [137] Care providers are encouraged to perform periodic clinical revaluations and to monitor patient progression of self-management strategies while discouraging dependence on passive treatment.
III. Research
Overall, the quality of the research on conservative management of NADs and WADs remains low, partly explaining that only weak recommendations could be formulated for clinical practice. Further, the reporting of RCTs remains suboptimal. [138] Past recommendations for improving the quality of the research still apply. [24, 25] Future research should aim to clarify the role of spinal manipulation therapy alone or as part of multimodal care for the management of recent neck pain and have adequate frequency and length of follow-up. For instance, a large number of patient visits to the emergency departments each year are for acute neck and arm pain resulting from WADs. [14, 139] A small RCT suggested that cervical spine manipulation is a reasonable alternative to intramuscular NSAID for immediate pain relief in these patients. [63] However, the small sample size, comparison of a single session of spinal manipulation to an NSAID injection, and a 1-day follow-up was not representative of clinical practice.
Few recent adequately controlled high-quality research studies of chiropractic care for NADs have been published. In addition, studies included in the reviews did not estimate the maximum therapeutic benefits (ie, best dosage for treatment under evaluation). Well-designed clinical trials with sufficient numbers of participants, longer-term treatments, and follow-up periods are needed to increase the confidence in the recommendations and to advance our understanding of effective and cost-effective conservative care, and spinal manipulation, for the management of patients with NADs and WADs.
Dissemination and Implementation Plan
Evidence-based practice aims to improve clinical decision making and patient care. [140, 141] When followed, CPGs have the potential to improve health outcomes and the efficiency of the health care system. [142–144] However, low adherence to CPGs has been noted across health care sectors [145] and in the management of musculoskeletal conditions, including NADs and WADs. [77, 101, 102] Such gaps contribute to wide geographic variations in the use and quality of health care services. [146]
Efforts to bridge the “research-practice gap” have led to a growing interest in KT. [145, 147] Knowledge translation is defined as the exchange, synthesis, and ethically sound application of knowledge to improve health and provide more effective health services. [148] Knowledge translation aims to bridge the research-practice gap and improve patient outcomes by promoting the integration and exchange of research and evidence-based knowledge into clinical practice.
To prepare for guideline implementation, we considered the Guideline Implementation Planning Checklist [149] and available strategies and supporting evidence [141, 150] to increase guideline uptake. Although effects of KT interventions tend to be modest, they are likely important at a population health level. [37]
To raise awareness, chiropractic professional organizations are encouraged to inform their members of new CCGI guidelines and tools easily accessible on our website (www.chiroguidelines.org). The guideline implementation tools framework was used to clarify the objectives of the tools; identify end users and the context and setting where tools will be used; provide instructions for use; and describe methods to develop the tools and related evidence and to evaluate the tools. [151]
Implementation tools designed to increase guideline uptake includepractitioner and patients’ handouts (Figure 8, Appendix 7)
algorithms (Figures 6 and 7)
webinars, videos, and learning modules (http://www.cmcc.ca/CE)
point-of-care checklists; and health status reminders. [152–154]The CCGI has established a network of opinion leaders across Canada (www.chiroguidelines.org). Based on successful efforts to implement a WAD guideline in Australia using opinion leaders among regulated physiotherapists, chiropractors, and osteopaths, [155] the CCGI is planning a series of implementation studies among Canadian chiropractors. [137] We will also pilot within chiropractic practice-based research networks. [156] Monitoring guideline use in chiropractic is challenging because the use of electronic health records to routinely collect clinical practice information is not common in Canada and those using electronic health records often collect different indicators. [157] Nonetheless, the frequency of downloads (posting of the open access guideline on the CCGI website) and number of registering participants and completion of educational online material (webinar, video, and learning module) will be monitored monthly as proxy measures of guideline uptake.
Figure 8. Canadian Chiropractic Guideline Initiative
(CCGI) patient information sheet.

Guideline Update
The methods for updating the guideline will be as follows:1) Monitoring changes in evidence, available interventions, importance and value of outcomes,
resources available or relevance of the recommendations to clinicians (limited systematic
literature searches each year for 3–5 years and survey to experts in the field annually)
2) assessing the need to update (relevance of the new evidence or other changes, type and scope
of the update), and
3) communicating the process, resources, and timeline to the Guideline Advisory Committee of the
CCGI, who will submit a recommendation to the Guideline Steering Committee to make a
decision to update and schedule the process. [158–163]
Strengths and Limitations
Shortcomings for this guideline include the low quantity and quality of supporting evidence found during the searches. Most of the downgrading of evidence supporting the outcomes occurred because of imprecision. In addition, our updated search of the published reports included 2 databases (Medline and Cochrane Central Register of Controlled Trials) but was limited to the English published reports, which possibly excluded some relevant studies. This, however, is an unlikely source of bias.164, 165 Qualitative studies that explored the lived experience of patients were not included. Thus, this review cannot comment on how patients valued and experienced their exposure to manual therapies or passive physical modalities. Although the composition of the guideline panel was diverse, with experienced methodologists, expert clinicians, and stakeholder and patient representatives, only 1 member was from another health discipline (physiotherapist). The scope of this guideline focused on selected outcomes such as pain and disability, although included studies assessed several additional outcomes.
Conclusion
This CPG supersedes the original (2005) and revised (2014) neck pain guideline as well as the 2010 whiplash-associated guidelines produced by the Canadian Chiropractic Association (CCA); Canadian Federation of Chiropractic Regulatory and Educational Accrediting Boards (CFCREAB).
People should receive care based on evidence-based therapeutic options. Based on patient preference and resources available, a mixed multimodal approach including manual therapy and advice about self-management and exercise (supervised/unsupervised or at home) may be an effective treatment strategy for recent-onset and persistent NAD and WAD grades I to III. Progress should be regularly monitored for evidence of benefit, in particular on the basis of pain alleviation and reduction of disability.
Practical ApplicationsA multimodal approach including manual therapy, self-management advice,
and exercise can be an effective treatment strategy for recent-onset and
persistent neck pain– and whiplash-associated disorders.
Appendix 1. CCGI Executive Summary: Structure and Methods
The Clinical Practice Guideline Initiative was launched by the Canadian Chiropractic Association (CCA) and the Canadian Federation of Chiropractic Regulatory and Education Accrediting Boards (CFCREAB or Federation) over a decade ago to develop clinical practice guidelines (CPGs) to improve chiropractic care delivery in Canada.
This is now known as the Canadian Chiropractic Guideline Initiative (CCGI), a 6-year project (2012–2018) funded by chiropractic stakeholders in Canada (www.chiroguidelines.org).
CPGs aim to describe appropriate care based on the best available scientific evidence and broad consensus while promoting efficient use of resources. The Institute of Medicine revised the definition of CPGs as: ‘Statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options.’ [1]
The scope of the Canadian Chiropractic Guideline Initiative (CCGI) is limited to non-specific musculoskeletal disorders. CPGs produced by the CCGI are developed using the best available evidence and involve stakeholders in a transparent and collaborative manner. Stakeholders include professional organizations, health care professionals, consumers, and organizations that fund or carry out research.
CPGs should address multiple dimensions of decision making, including: effectiveness; harm events; quality of life; health-service delivery issues (i.e., dissemination and implementation); provider and patient compliance; and resources use and costs. While guidelines can encourage practitioners to conform to best practices and lead to improvements in care, reviews have demonstrated that dissemination of guidelines alone is rarely sufficient to optimise care. To date, very little knowledge translation research has addressed evidence-practice gaps in chiropractic.
Recent advances on methods to conduct knowledge synthesis, [2] derive evidence-based recommendations, [3] adapt high quality guidelines, [4] and increase the uptake of Clinical Practice Guidelines (CPG) [5, 6] have prompted an update of the development, dissemination, implementation, evaluation, and revision (DIER-Plan) published in the Journal of the Canadian Chiropractic Association in 2004. [7] The initial report described the CCA/CFCRB-CPG and its origins and mapped the plan for the DIER-Plan of each CPG. The report updates the structure, methods and procedures to develop, update or adapt guidelines, and proposes strategies to help disseminate and implement CPGs. Original research in knowledge translation (KT) is undertaken to reduce the evidence-practice gap.
Structure
To accomplish its complex tasks, the CCGI is made of a Guideline Steering Committee and a Guideline Advisory Committee, a Guideline Development Group, a Guideline Implementation Group, graduate students, and an External Review Group. Committee members originate from several countries and represent a range of clinical and scientific disciplines or specialties (Appendix 1 Figure 1).
Methods and procedures
1. Developing, updating or adapting guidelines
The analytic framework of a guideline is a key element in guideline development. [8] In this critical stage, the group defines which questions must be answered to arrive at a recommendation, which types of evidence and information are relevant to the analysis, and by what criteria that evidence will be evaluated. The analytic work encompasses the examination of scientific evidence, expert opinion, clinical experience, and other relevant information and the use of decision rules to translate that information into recommendations. It obliges the group to make explicit, a priori decisions about the outcomes of interests to arrive at a recommendation. The end product of the process is captured in the analytic logic of the guideline, the rationale for the recommendations.
Steps from stakeholders’ consultation to the release of the guidelines include:
The Steering Committee commissioned to develop the guideline.
The Guideline Advisory Committee (GAC) determines the feasibility of the requested guideline.
The Steering Committee refers clinical guideline topics to the Editor of the CCGI.
Provincial and national organisations representing practitioners register as stakeholders. They will be consulted throughout the guideline development process.
Committee and Group members register interest.
The scope, approach, and output of the process are developed. This process sets out what the guideline will – and will not – cover.
Guideline Development Group is established during the scoping phase.
Based on recent high-quality studies, a multidisciplinary expert panel considers the evidence of effectiveness, safety, cost-effectiveness, societal and ethical values, and patient experiences when formulating recommendations.
Consultations take place on the draft guideline (external review phase).
The final guideline is produced and submitted for publication.
Paper-based and web-based practitioner guides are produced.
2. Disseminating and implementing guidelines
Despite available evidence for optimal management of back and neck pain, poor adherence to guidelines and wide variations in services have been noted in the area of primary care such as care for back pain. [9] By themselves, CPGs cannot overcome the multitude of barriers to clinician adherence. [10] Several factors, involving the clinicians, patients, and the health care environment, influence decision making or clinical behaviours. [11] Competing beliefs and other factors such as environmental issues can prevent practitioners from using best evidence. Ongoing effort is needed to identify these modifiable determinants of clinicians’ guideline adherence to encourage evidence-based practice. Given the very slow pace of converting evidence into routine practice (between 1 to 2 decades), [12] Knowledge Translation (KT) has emerged as an important consideration to promote evidence-based practice. [13] KT research is the scientific study of the determinants, processes and outcomes of dissemination and implementation. [13]
Putting knowledge into action (practice) is a dynamic, iterative, and complex process. Success requires an integrated approach where all involved parties work together to select, tailor, and implement KT interventions.
KT research
Distribution of printed educational material such as posting of guidelines on the web and Continuing Education conference and workshops are widely used strategies to improve knowledge, awareness, attitudes, skills, professional practice and patient outcomes. While these are effective, they generally have small to modest impacts on practice. [14, 15] Specifically, research is needed:1) to address the complex process of bridging research and practice in a variety of real-world settings, and
2) to conduct research that balances rigor with relevance and employs study designs and methods appropriate for KT research.The KT Committee will undertake primary research projects to help understand barriers and enablers to guideline uptake, develop implementation tools alongside of CPGs, and develop and evaluate intervention to improve process of care and patient care.
Dissemination of the work undertaken by the Guideline Initiative
Guidelines produced, results and a set of preliminary conclusions of the research undertaken by the CCGI will be presented at various professional and scientific meetings, as well as in a series of articles to be published in publications that are considered to have the greatest impact and readership in the musculoskeletal area for our target audience. In addition, Clinical Practice Guidelines and tools to help disseminate and implement the guideline recommendations will be posted on the CCGI website (www.chiroguidelines.org), and links to the CCGI will be provided from stakeholder websites.
Appendix 1 Figure 1. Structure of the Canadian Chiropractic Guideline Initiative (CCGI) 2012–2018.
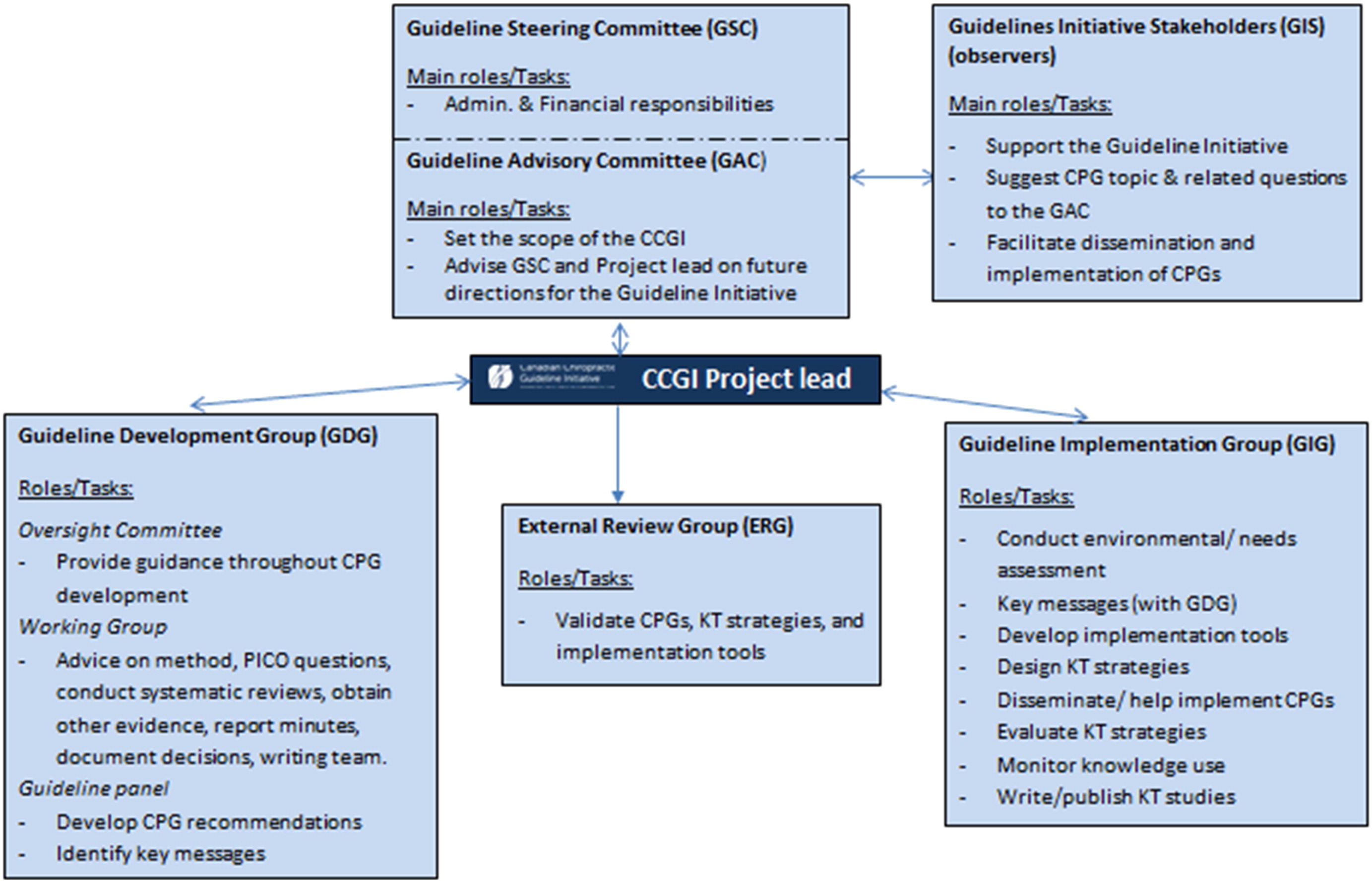
References:
Graham G, Mancher M., Miller Wolman, D., Greenfield, S., Steinberg, E. Editors.
Clinical Practice Guidelines We Can Trust.
Institute of Medicine, Shaping the Future for Health.
Washington, DC: The National Academies Press. 2011.Tricco A, Tetzlaff J, Moher D.
The art and science of knowledge synthesis.
J Clin Epidemiol. 2011;64(1):11-20.GRADE Working Group.
Grading the quality of evidence and the strength of recommendations (GRADE). Available at:
http://www.gradeworkinggroup.org/ Accessed October 4, 2016.ADAPTE Manual and Resource Toolkit V2.
Guideline International Network (G-I-N) Adaptation Working Group.
Available from:
http://www.g-i-n.net/working-groups/adaptationDECIDE Collaboration.
Developing and Evaluating Communication Strategies to Support Informed Decisions and Practice Based on Evidence (DECIDE).
Available at: http://www.decide-collaboration.eu/Grimshaw J, Eccles M, Lavis J, Hill S, Squires J.
Knowledge translation of research findings.
Implementation Sci. 2012;7(1):50.The Canadian Chiropractic Association and the Canadian Federation of Chiropractic RegulatoryBoards Clinical Practice Guidelines Development Initiative (The CCA/CFCRB-CPG) development, dissemination, implementation, evaluation, and revision (DevDIER) plan.
J Can Chiropr Assoc. 2004;48(1):56-72.Shekelle, P, Woolf, S, Grimshaw, JM, Schunemann, HJ, and Eccles, MP.
Developing Clinical Practice Guidelines: Reviewing, Reporting, and Publishing Guidelines;
Updating Guidelines; and the Emerging Issues of Enhancing Guideline Implementability
and Accounting for Comorbid Conditions in Guideline Development
Implementation Science 2012 (Jul 4); 7: 62Chenot J, Scherer M, Becker A, et al.
Acceptance and perceived barriers of implementing a guideline for managing low back in general practice.
Implementation Sci. 2008;3:7.Cabana M, Rand C, Powe N, Wu AW, Wilson MH, Abboud PA, Rubin,HR.
Why don't physicians follow clinical practice guidelines? A framework for improvement.
JAMA. 1999;282:1458-1465.Matchar DB, Westermann-Clark EV, McCrory DC, Patwardhan M, Samsa G, Kulasingam S, Myers E, Sarria-Santamera A, Lee A, Gray R, Liu K;
Agency for Healthcare Research and Quality. Dissemination of Evidence-based Practice Center Reports.
Ann Intern Med. 2005;142(2):1120-1125.Sussman S, Valente T, Rohrbach L, Skara S, Pentz M.
Translation in the health professions: Converting science into action.
Eval Health Prof. 2006;29:7-32.Curran JA, Grimshaw JM, Hayden JA, Campbell B.
Knowledge translation research: The science of moving research into policy and practice.
J Continuing Edu Health Pro. 2011;31(3):174-180.Forsetlund L, Bjørndal A, Rashidian A, Jamtvedt, O'Brien MA, Wolf F, Davis D, Odgaard-Jensen J, Oxman AD.
Continuing education meetings and workshops: effects on professional practice and health care outcomes.
Cochrane Database Syst Rev. 2009;(2):CD003030.Giguère A, Légaré F, Grimshaw J, Turcotte S, Fiander M, Grudniewicz A, Makosso-Kallyth S, Wolf FM, Farmer AP, Gagnon MP.
Printed educational materials: effects on professional practice and health care outcomes.
Cochrane Database of Systematic Reviews 2012(10):CD004398.
Appendix 2. Composition of the External Guideline Review Committee

Appendix 3. Glossary of Terms (Adapted from Côté et al. [20]).
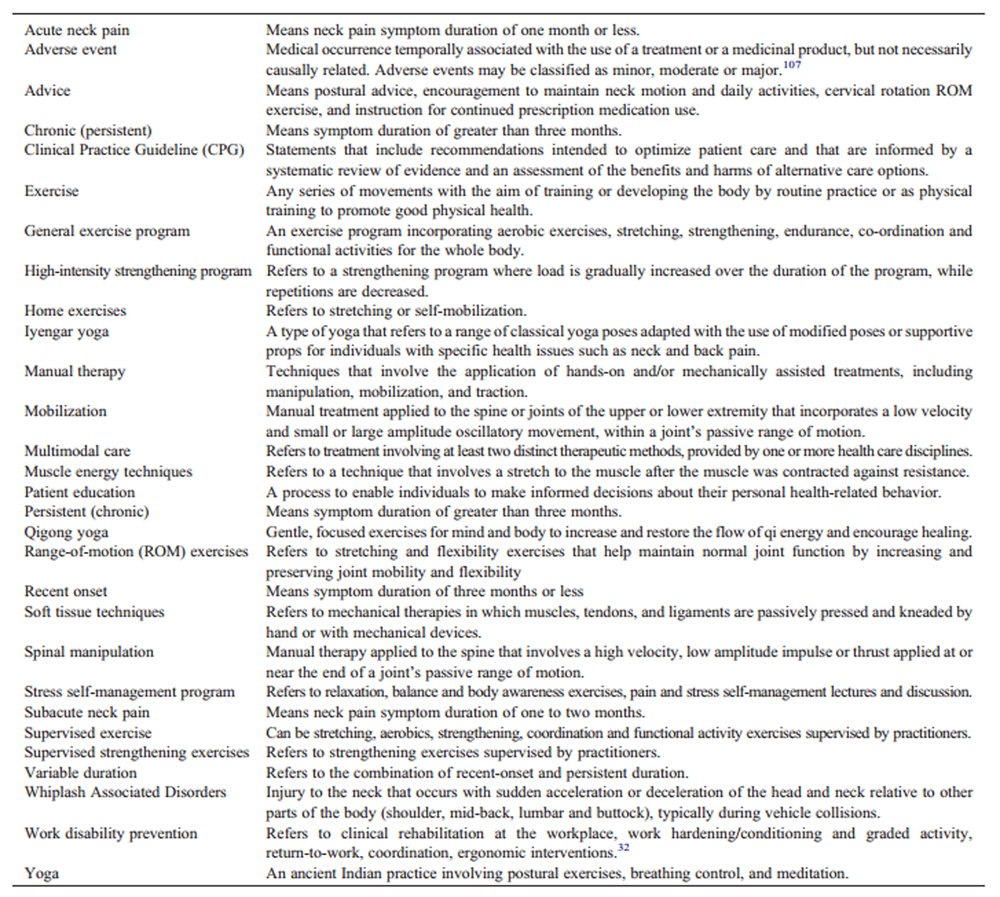
Appendix 4. OPTIMa Flowcharts
Figure 4.1 OPTIMa flowchart – Southerst et al. [38]
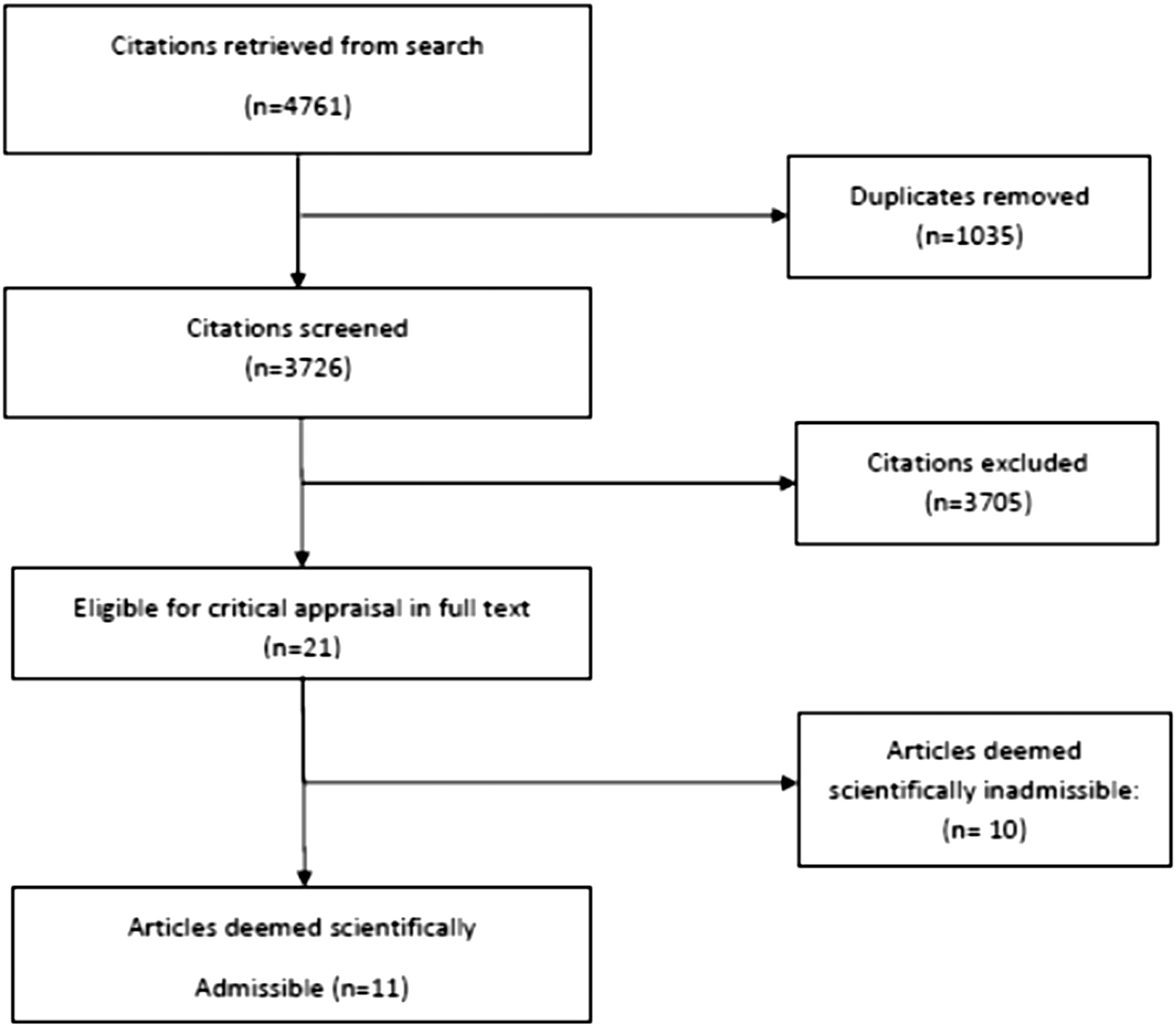
Figure 4.2 OPTIMa flowchart – Sutton et al. (2014) [39]

Figure 4.3 OPTIMa flowchart – Yu et al. (2014) [40]
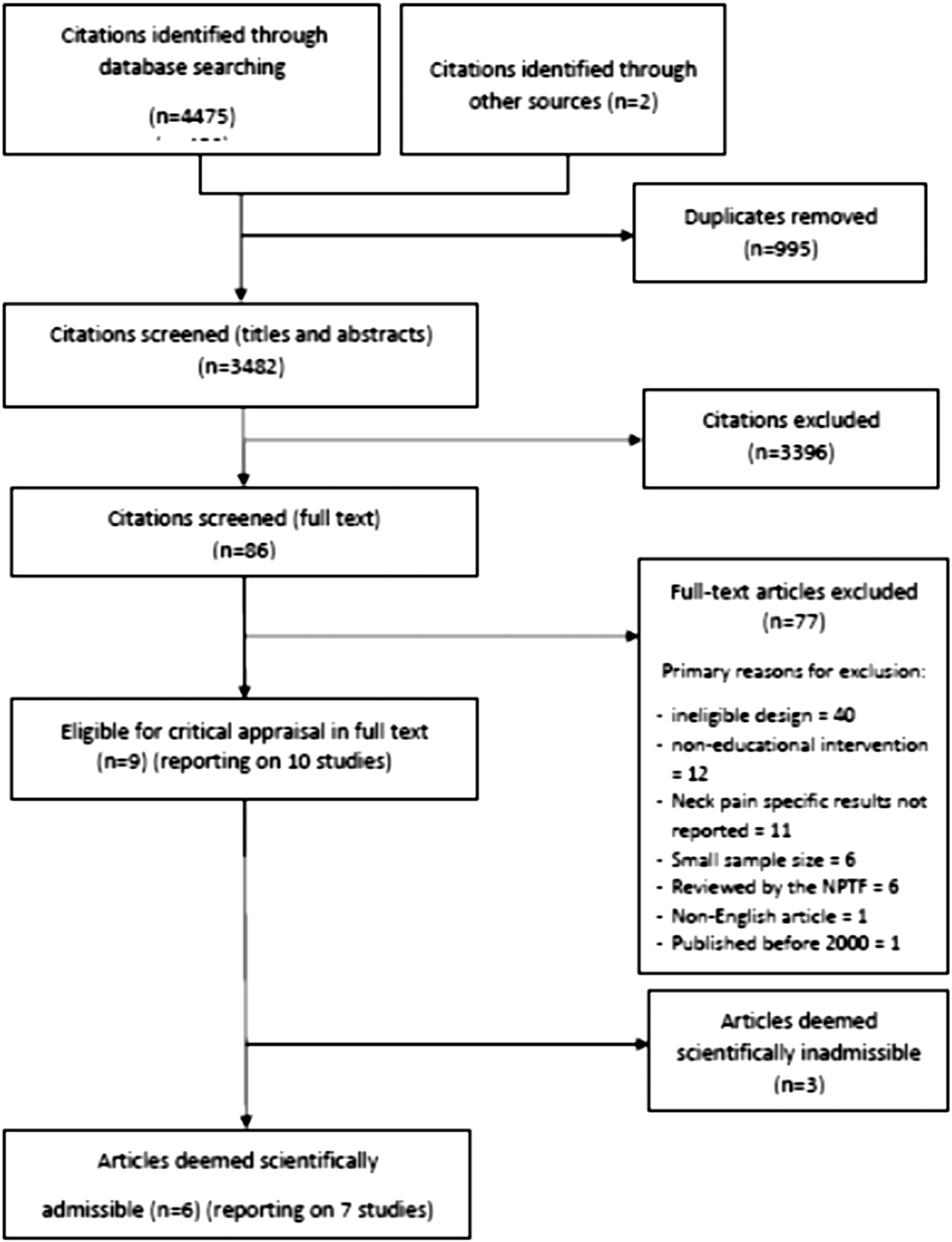
Figure 4.4 OPTIMa flowchart – Varatarajan et al. (2014) [41]
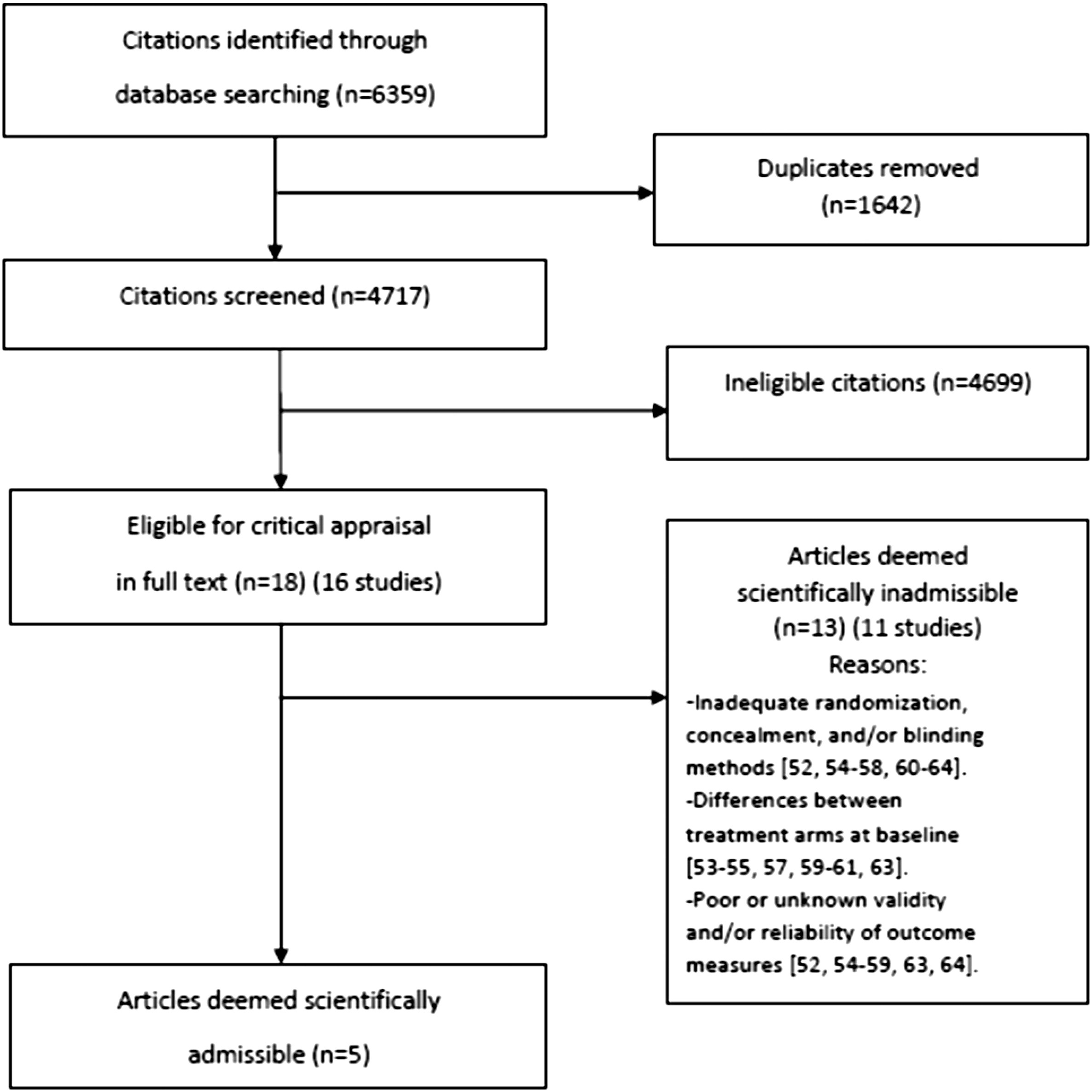
Appendix 5. Included and Excluded Articles After Full Text Review (n=53)
from Updated Search in Medline and Cochrane Central Register
of Controlled Trials for five OPTIMa Reviews
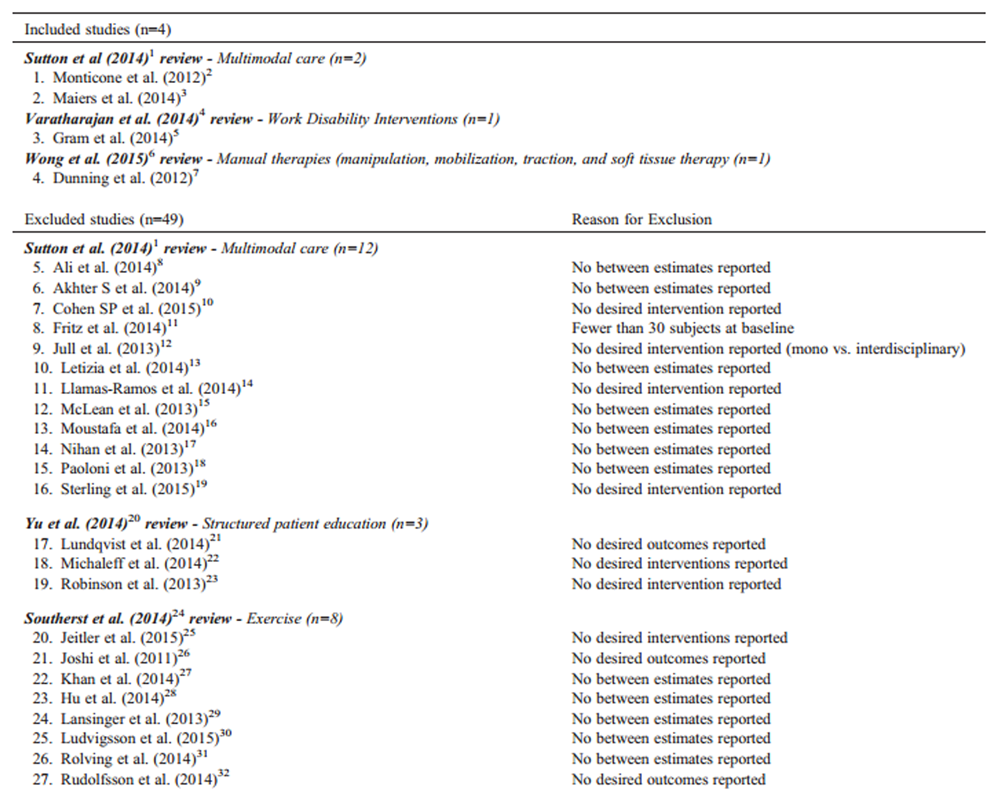
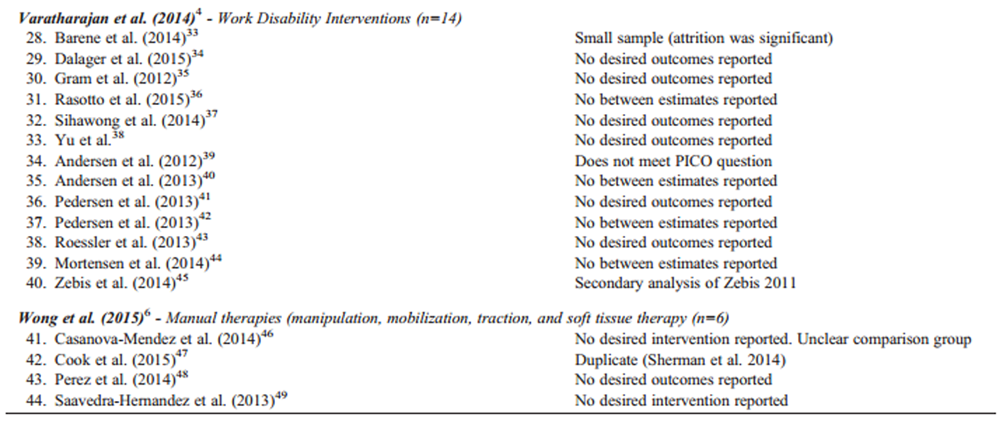
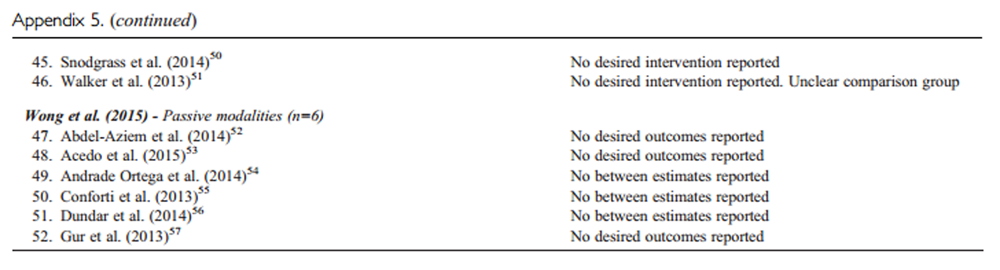
References:
Sutton D, Cote P, Wong J, et al.
Is multimodal care effective for the management of patients with whiplash-associated disorders or neck pain and associated disorders? A systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration.
Spine J. 2014;S1529-9430(14):00650-0.Monticone M, Baiardi P, Vanti C, et al.
Chronic neck pain and treatment of cognitive and behavioural factors: results of a randomised controlled clinical trial.
Euro Spine J. 2012;21(8):1558-1566.Maiers M, Bronfort G, Evans R, Hartvigsen J, Svendsen K, Bracha Y, et al.
Spinal Manipulative Therapy and Exercise For Seniors with Chronic Neck Pain
Spine J. 2014 (Sep 1); 14 (9): 1879–1889Varatharajan S, Côté P, Shearer H, et al.
Are Work Disability Prevention Interventions Effective for the Management of
Neck Pain or Upper Extremity Disorders? A Systematic Review by the
Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration.
J Occup Rehabil. 2014;24(4):692-708.Gram B, Andersen C, Zebis MK, et al.
Effect of Training Supervision on Effectiveness of Strength Training for Reducing Neck/Shoulder Pain and Headache in Office Workers: Cluster Randomized Controlled Trial.
BioMed Ress Int. 2014;2014-2019.Wong JJ , Shearer HM , Mior S , et al .
Are Manual Therapies, Passive Physical Modalities, or Acupuncture
Effective for the Management of Patients with Whiplash-associated
Disorders or Neck Pain and Associated Disorders? An Update
of the Bone and Joint Decade Task Force on Neck Pain and
Its Associated Disorders by the OPTIMa Collaboration
Spine J. 2016 (Dec); 16 (12): 1598-1630Dunning J, Cleland J, Waldrop M, et al.
Upper cervical and upper thoracic thrust manipulation versus nonthrust mobilization in patients with mechanical neck pain: a multicenter randomized clinical trial.
J Orthop Sports Phys Ther. 2012;42(1):5-18.Ali A, Shakil-ur-Rehman S, Sibtain FT.
The efficacy of sustained natural apophyseal glides with and without isometric exercise training in non-specific neck pain.
Pak J Med Sci. 2014;30(4):872-4.Akhter S, Khan M, SS. A, Soomro R.
Role of manual therapy with exercise regime versus exercise regime alone in the management of non-specific chronic neck pain.
Pak J Pharma Sci. 2014;27(6 Suppl):2125-2128.Cohen S, Hayek S, Semenov Y, et al.
Epidural Steroid Injections, Conservative Treatment, or Combination Treatment for Cervical Radicular PainA Multicenter, Randomized, Comparative-effectiveness Study.
Anesthesiology. 2014;121(5):1045-1055.Fritz J, Thackeray A, Brennan G, et al.
Exercise Only, Exercise With Mechanical Traction, or Exercise With Over-Door Traction for Patients With Cervical Radiculopathy, With or Without Consideration of Status on a Previously Described Subgrouping Rule: A Randomized Clinical Trial.
JOSPT. 2014;44(2):45-57.Jull G, Kenardy J, Hendrikz J, et al.
Management of acute whiplash: a randomized controlled trial of multidisciplinary stratified treatments.
Pain. 2013;154(9):1798-1806.Letizia M, Cataldo P, Barbera G, et al.
Alpha-Lipoic Acid and Superoxide Dismutase in the Management of Chronic Neck Pain: A Prospective Randomized Study.
Drugs R D. 2014;14(1):1-7.Llamas-Ramos R, Pecos-Martín D, Gallego-Izquierdo T, et al.
Comparison of the Short-Term Outcomes Between Trigger Point Dry Needling and Trigger Point Manual Therapy for the Management of Chronic Mechanical Neck Pain: A Randomized Clinical Trial.
J Orthop Sports Phys Ther. 2014;44(11):852-861.McLean S, Klaber Moffett J, Sharp D, et al.
A randomised controlled trial comparing graded exercise treatment and usual physiotherapy for patients with non-specific neck pain (the GET UP neck pain trial).
Man Ther. 2013;18(3):199-205.Moustafa I, Diab A.
Multimodal treatment program comparing 2 different traction approaches for patients with discogenic cervical radiculopathy: a randomized controlled trial.
J Chiropr Med 2014;13(3):157-167.Nihan C, Sebnem Koldas D, Deniz E, Saime A.
The effectiveness of portable audio biofeedback device in myofascial pain syndrome in neck and upper trapezius muscles.
J Musculoskelet Pain. 2013;21(3):217-223. Available at:
http://www-tandfonline com.proxy3.library.mcgill.ca/doi/full/10.3109/10582452.2013.828148Paoloni M, Tavernese E, Cacchio A, et al.
Patient-oriented rehabilitation in the management of chronic mechanical neck pain: a randomized controlled trial. European journal of physical & rehabilitation medicine.
Euro J Phys Rehabil Med. 2013;49(3):273-281.Sterling M, Vicenzino B, Souvlis T, et al.
Dry-needling and exercise for chronic whiplash-associated disorders: a randomized single-blind placebo-controlled trial.
Pain. 2015;156(4):635-643.Yu H, Côté P, Southerst D, Wong J, et al.
Does structured patient education improve the recovery and clinical outcomes of patients with neck pain? A systematic review from the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration.
Spine J. 2014;pii: S1529-9430(14).Lundqvist L, Zetterlund C, Richter H.
Effects of Feldenkrais method on chronic neck/scapular pain in people with visual impairment: a randomized controlled trial with one-year follow-up.
Arch Phys Med Rehabil. 2014;95(9):1656-1661.Michaleff Z, Maher C, Lin C-WC, et al.
Comprehensive physiotherapy exercise programme or advice for chronic whiplash (PROMISE): a pragmatic randomised controlled trial.
Lancet. 2014;384(9938):133-141.Robinson J, Theodore B, Dansie E, et al.
The role of fear of movement in subacute whiplash-associated disorders grades I and II.
Pain. 2013;154(3):393-401.Southerst D, Nordin M, Côté P, et al.
Is Exercise Effective for the Management of Neck Pain and
Associated Disorders or Whiplash-associated Disorders?
A Systematic Review by the Ontario Protocol for Traffic
Injury Management (OPTIMa) Collaboration
Spine J 2016 (Dec); 16 (12): 1503–1523Jeitler M, Brunnhuber S, Meier L, et al.
Effectiveness of jyoti meditation for patients with chronic neck pain and psychological distress-a randomized controlled clinical trial.
J Pain. 2015;16(1):77-86.Joshi V, Bellad A.
Effect of yogic exercises on symptoms of musculoskeletal disorders of upper limbs among computer users: a randomised controlled trial
Indian J. Med. Sci. 2011;65(11):424-428.Khan M, Soomro R, Ali S.
The effectiveness of isometric exercises as compared to general exercises in the management of chronic non-specific neck pain.
Pakistan J Pharml Sci. 2014;27(5 Suppl):1719-1722.Hu Z, Tang Z, Wang S, et al.
A 12-words-for-life-nurturing exercise program as an alternative therapy for cervical spondylosis: A randomized controlled trial.
Evid Based Complement Alternat Med. 2014;2014:1-7.Lansinger B, Carlesson J, Kreuter M, et al.
Health-related quality of life in persons with long-term neck pain after treatment with qigong and exercise therapy respectively.
Euro J Physiother. 2013;15(3):111-117.Ludvigsson M, Peterson G, O'Leary S, et al.
The effect of neck-specific exercise with, or without a behavioral approach, on pain, disability, and self-efficacy in chronic whiplash-associated disorders: a randomized clinical trial.
Clin J Pain. 2015;31(4):294-303.Rolving N, Christiansen D, Andersen L, et al.
Effect of strength training in addition to general exercise in the rehabilitation of patients with non-specific neck pain. A randomized clinical trial.
Euro J Phys Rehabil Med. 2014;50(6):617-626.Rudolfsson T, Djupsjobacka M, Hager C, et al.
Effects of neck coordination exercise on sensorimotor function in chronic neck pain: a randomized controlled trial.
J Rehabil Med. 2014;46(9):908-914.Barene S, Krustrup P, Holtermann A.
Effects of the workplace health promotion activities soccer and zumba on muscle pain, work ability and perceived physical exertion among female hospital employees.
PloS one 2014;9(12).Dalager T, Bredahl T, Pedersen M, et al.
Does training frequency and supervision affect compliance, performance and muscular health? A cluster randomized controlled trial.
Man ther. 2015;20(5):657-665.Gram B, Holtermann A, Bultmann U, et al.
Does an exercise intervention improving aerobic capacity among construction workers also improve musculoskeletal pain, work ability, productivity, perceived physical exertion, and sick leave?: A randomized controlled trial.
J Occup Environ Med. 2012;54(12):1520-1526.Rasotto C, Bergamin M, et al.
A tailored workplace exercise program for women at risk for neck and upper limb musculoskeletal disorders: a randomized controlled trial.
J Occup Environ Med. 2015;57(2):178-183.Sihawong R, Janwantanakul P, Jiamjarasrangsi W.
Effects of an exercise programme on preventing neck pain among office workers: a 12-month cluster-randomised controlled trial.
Occu Environ Med. 2014;71(1):63-70.Yu W, Yu IT, Wang X, et al.
Effectiveness of participatory training for prevention of musculoskeletal disorders: a randomized controlled trial.
Int Arch Occup Environ Health. 2013;86(4):431-440.Andersen C, Andersen. L, Gram B, et al.
Influence of frequency and duration of strength training for effective management of neck and shoulder pain: a randomised controlled trial. British Journal of Sports Medicine.
Br J Sports Med. 2012;46(14):1004-1010.Andersen C, Andersen L, Pedersen M, et al.
Dose-response of strengthening exercise for treatment of severe neck pain in women.
J Strength Cond Res. 2013;27(12):3322-3328.Pedersen M, Zebis M, Langberg H, et al.
Influence of self-efficacy on compliance to workplace exercise.
Int J Behav Med. 2013;20(3):365-370.Pedersen M, Andersen C, Zebis M, et al.
Implementation of specific strength training among industrial laboratory technicians: long-term effects on back, neck and upper extremity pain.
BMC Musculoskelet Disord. 2013;14(1):1-11.Roessler K, Rugulies R, ilberg R, et al.
Does work-site physical activity improve self-reported psychosocial workplace factors and job satisfaction? A randomized controlled intervention study.
Int Arch Environ Occup Health. 2013;86(8):861-864.Mortensen P, Larsen A, Zebis M, et al.
Lasting effects of workplace strength training for neck/shoulder/arm pain among laboratory technicians: natural experiment with 3-year follow-up.
Biomed Res Int. 2014; 2014: 845851.Zebis MK, Andersen CH, Sundstrup E, et al.
Time-Wise Change in Neck Pain in Response to Rehabilitation with Specific Resistance Training: Implications for Exercise Prescription.
PLoS ONE. 2014;9(4):e93867.Casanova-Méndez A, Oliva-Pascual-Vaca Á, Rodriguez-Blanco C, et al.
Comparative short-term effects of two thoracic spinal manipulation techniques in subjects with chronic mechanical neck pain: A randomized controlled trial.
Man Ther. 2014;19(4):331-337.Cook A, Wellman R, Cherkin D, et al.
Randomized clinical trial assessing whether additional massage treatments for chronic neck pain improve 12- and 26-week outcomes.
Spine J. 2015;15(10):2206-2215.Izquierdo Pérez H, Alonso Perez J, Gil Martinez A, et al.
Is one better than another?: A randomized clinical trial of manual therapy for patients with chronic neck pain.
Man Ther. 2014;19(3):215-221.Saavedra-Hernández M, Arroyo-Morales M, Cantarero-Villanueva I, et al.
Short-term effects of spinal thrust joint manipulation in patients with chronic neck pain: a randomized clinical trial.
Clin. Rehabil. 2013;27(6):504-512.Snodgrass S, Rivett D, Sterling M, et al.
Dose optimization for spinal Treatment effectiveness: A randomized controlled trial investigating the effects of high and low mobilization forces in patients with neck pain.
J Orthop Sports Phys Ther. 2014;44(3):141-152.Walker BF, Hebert JJ, Stomski NJ, Losco B, French SD.
Short-term Usual Chiropractic Care for Spinal Pain: A Randomized Controlled Trial
Spine (Phila Pa 1976). 2013 (Nov 15); 38 (24): 2071–2078Abdel-Aziem A, Draz A, Battecha K, et al.
Effect of ultrasound combined with conventional therapy on neck pain, function, and disability in patients with cervical spondylosis: A randomized placebo-controlled trial.
J Musculoskelet pain. 2014;22(2):199-205.Acedo A, Antunes A, Dos Santos A, et al.
Upper trapezius relaxation induced by tens and interferential current in computer users with chronic nonspecific neck discomfort: An electromyographic analysis.
J Back Musculoskelet rehabil. 2015;28(1):19-24.Andrade Ortega JA, Cerón Fernández E, García Llorent R et al.
Microwave diathermy for treating nonspecific chronic neck pain: A randomized controlled trial.
Spine J. 2014;14(8):1712-1721.Conforti M, Fachinetti G.
High power laser therapy treatment compared to simple segmental physical rehabilitation in whiplash injuries (1° and 2° grade of the Quebec Task Force classification) involving muscles and ligaments.
Muscles Ligaments Tendons J. 2013;3(2):106-111.Dundar U, Turkmen U, Toktas H, et al.
Effect of high-intensity laser therapy in the management of myofascial pain syndrome of the trapezius: a double-blind, placebo-controlled study.
Lasers Med Sci. 2014;30(1):325-332.Gur A, Koca I, Karagullu H, et al.
Comparison of the efficacy of ultrasound and extracorporeal shock wave therapies in patients with myofascial pain syndrome: A randomized controlled study.
J Musculoskelet Pain. 2013;31(3):210-216.
[186]
Appendix 6. Tables of Risk of Bias for Accepted RCTs on Neck Pain based on
Scottish Intercollegiate Guidelines Network (SIGN) Criteria
Table 6.1 (Southerst et al.[38]):
Risk of Bias for Scientifically Admissible RCTs Based on the SIGN Criteria [166]
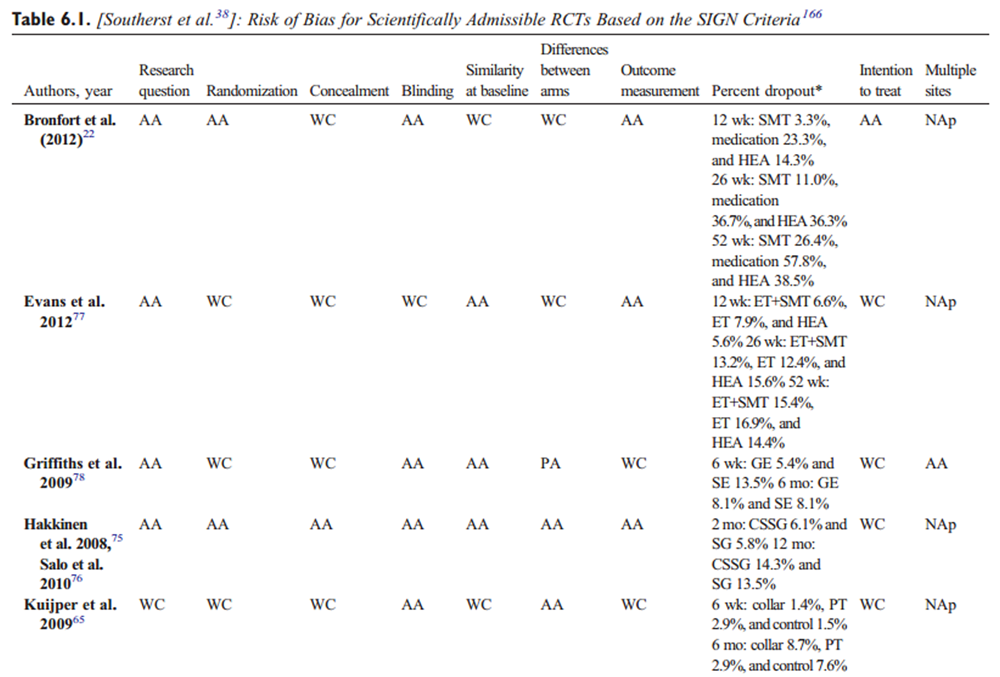
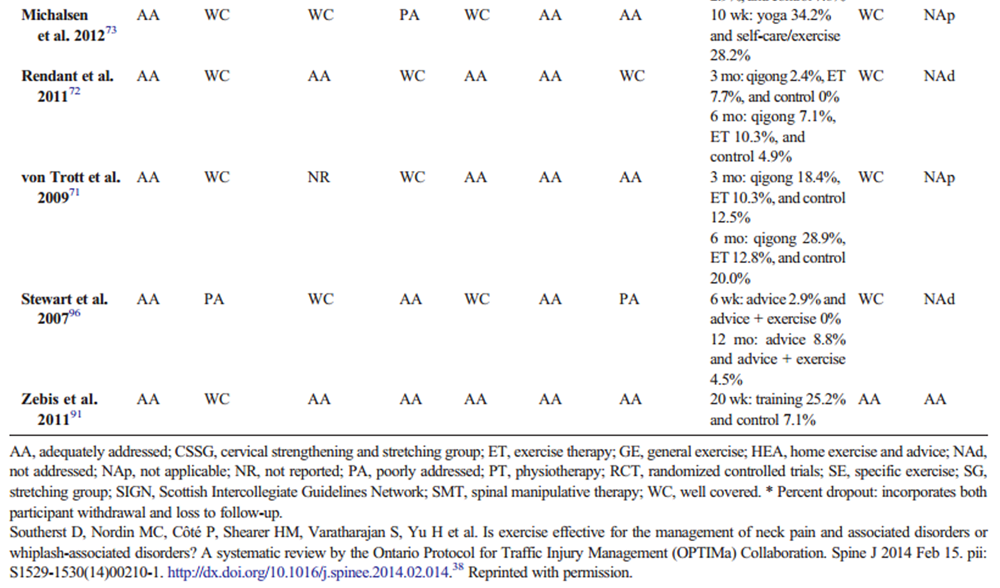
Table 6.2 (Sutton et al.[39]):
Risk of Bias for Scientifically Admissible RCTs Based on the SIGN Criteria [186]
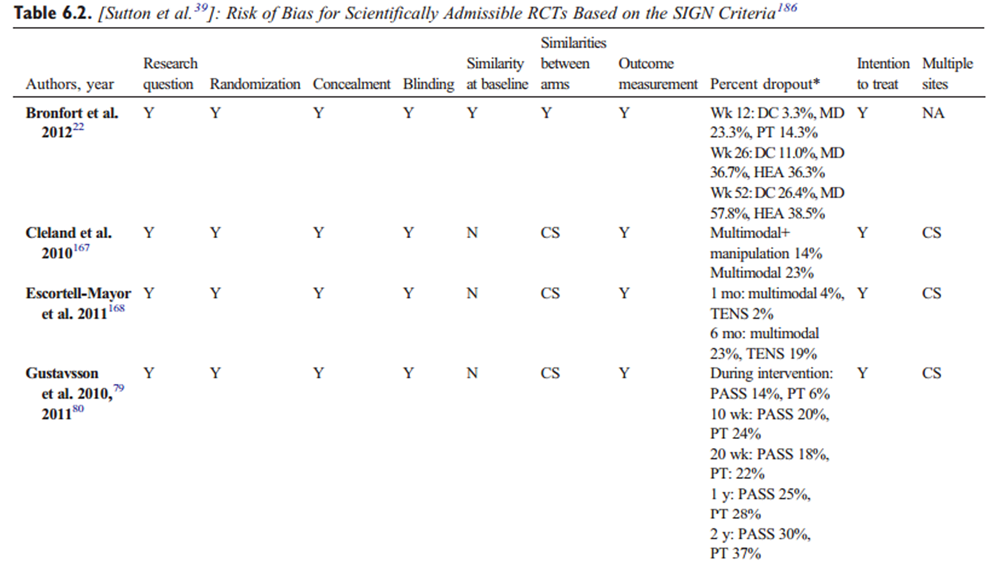
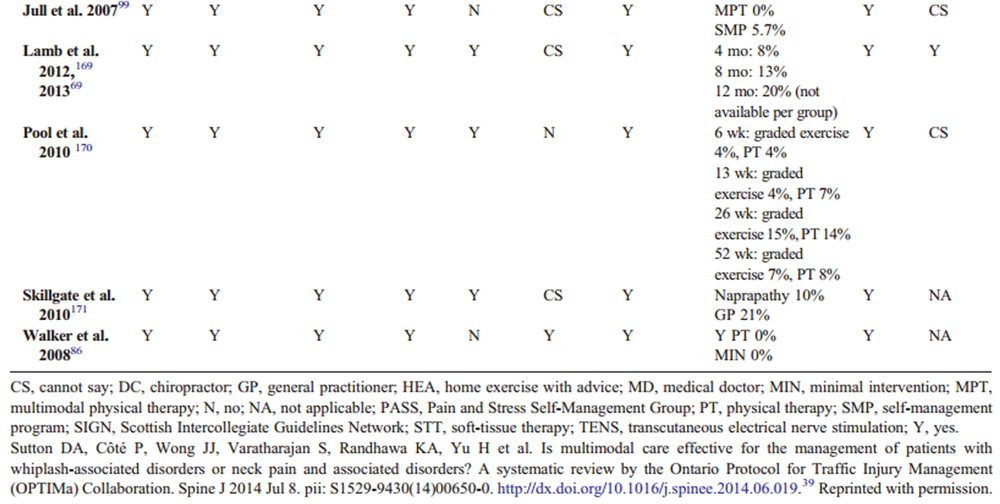
Table 6.3 (Yu et al.[40]):
Risk of Bias for Accepted RCTs Based on SIGN Criteria [186]
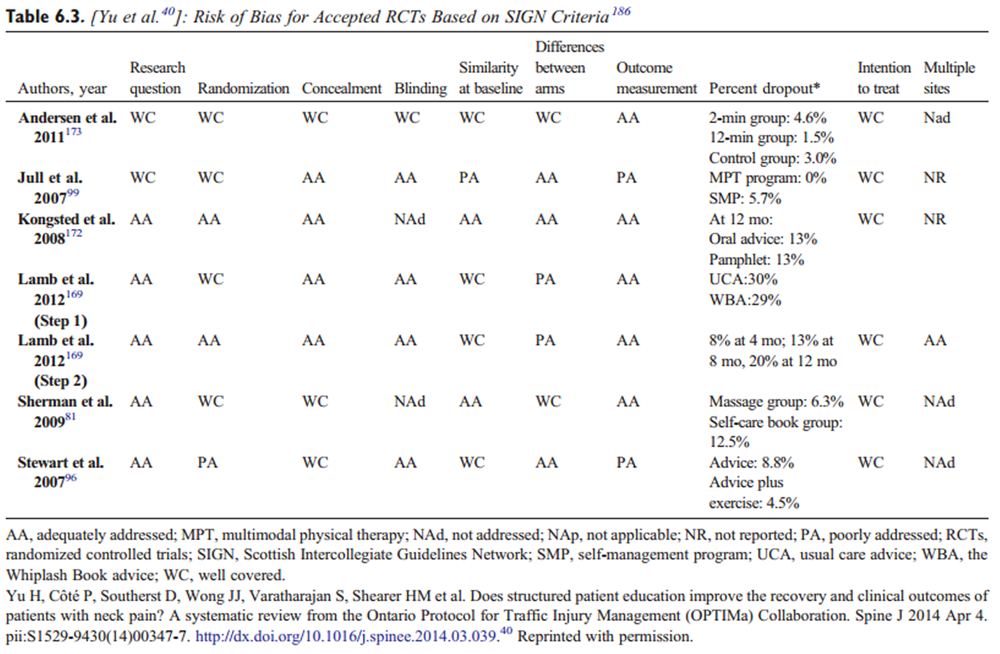
Table 6.4 (Varatharajan et al.[41]):
Risk of Bias for Accepted RCTs Based on SIGN Criteria [186]
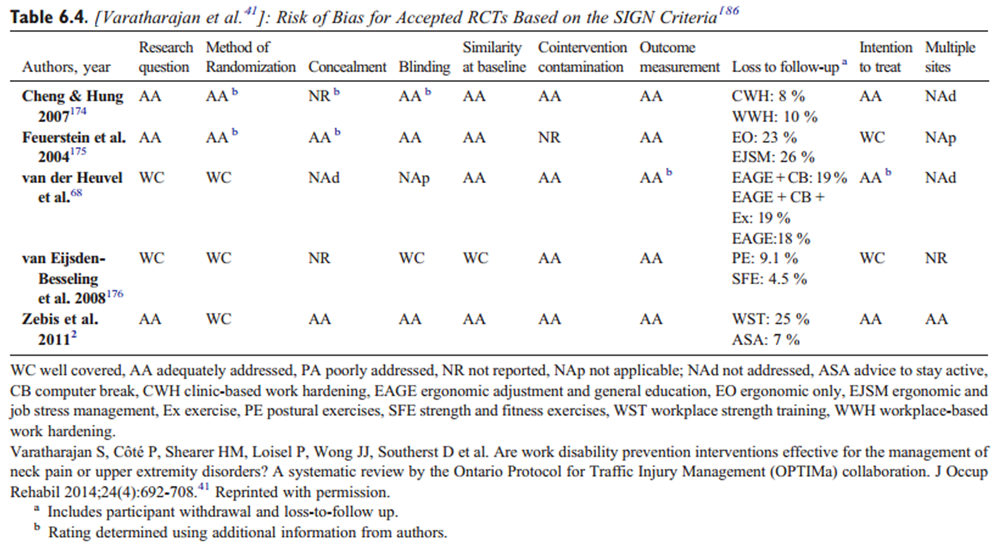
Table 6.5 (Wong et al.[42]):
Risk of Bias for Accepted RCTs on Neck Pain based on SIGN Criteria [186]
Refer to pages 564–565
Appendix 7. Educational Executive Summary of Recommendations on
Neck Pain and Associated Disorders for Practitioners
You may also enjoy this Executive Summary handout
prepared by the Canadian Chiropractic Guidline Initiative
NOTE: Just to avoid confusion: The citation numbers in this Appendix refer to the Reference section contained within this Appendix, and do NOT refer to the 189 references listed at the bottom of this long article.
Neck pain and its associated disorders (NAD) are common and result in significant social, psychological, and economic burden. [1] In light of recent research evidence, [2–5] an update to the recommendations of the management of Neck Pain Associated Disorders and Whiplash-Associated Disorders (WAD) was timely. The Guideline Development Group of the Canadian Chiropractic Guideline Initiative (CCGI) considered recently published systematic reviews on NAD and WAD from the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. [6]
This educational executive summary provides an overview of recommendations for clinical practice issued by CCGI in a new clinical practice guideline on the management of NAD and WAD. The full guideline and accompanying documents are available from the CCGI website at www.chiroguidelines.org.
Summary of recommendations: A multimodal approach including manual therapy, self-management advice and exercise is an effective treatment strategy for both recent onset and persistent neck pain and whiplash associated disorders.
Table 7.1 Classification of Neck Pain Associated Disorders (NAD) [7]
and Whiplash Associated Disorders (WAD) [8]

SUMMARY OF RECOMMENDATIONS FOR
GRADES I–II NECK PAIN AND ASSOCIATED DISORDERS (NAD)
For recent-onset (0-3 months) neck pain grades I-II, based on patient preference and practitioner experience we suggest offering advice with:
- multimodal care;
- manipulation or mobilization;
- Range of motion home exercises or multimodal manual therapy.
For recent-onset (0-3 months) neck pain grade III, based on patient preference and practitioner experience we suggest offering advice with:
- supervised graded strengthening exercises.
For persistent (> 3 months) neck pain grades I-II, based on patient preference and practitioner experience we suggest offering advice with:
- multimodal care or stress self-management;
- multimodal care or advice alone;
- manipulation in conjunction with soft tissue therapy;
- supervised yoga; supervised group exercise; supervised strengthening exercises or home exercises;
- mixed supervised and unsupervised high-intensity strength training or advice alone for workers with persistent neck and shoulder pain;
- high dose massage.
For persistent (> 3 months) neck pain grade III, based on patient preference and practitioner experience we suggest offering advice with:
- multimodal care or advice alone;
- mixed supervised and unsupervised high-intensity strength training or advice alone for workers with persistent neck and shoulder pain.
SUMMARY OF RECOMMENDATIONS FOR
GRADE I–III WHIPLASH AND ASSOCIATED DISORDERS (WAD)
For recent onset (0-3 months) whiplash grades I-III, based on patient preference and practitioner experience we suggest offering advice with:
- multimodal care.
For persistent (> 3 months) whiplash grades I-II, based on patient preference and practitioner experience we suggest offering advice with:
- supervised exercise or advice alone.
Detailed Recommendations
Recommendations for Recent-Onset Neck Pain Associated Disorders (NAD)
For patients with recent (0-3 months) neck pain and associated disorders grades I-II, we suggest manipulation or mobilization based on patient preference.
For patients with recent (0-3 months) neck pain grades I-II, we suggest ROM home exercises or multimodal manual therapy for reduction in pain and disability.Remark: Home exercises include education self-care advice, exercises and instruction of activity of daily living. Medication including non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen and muscle relaxant is an alternative. Multimodal manual therapy includes manipulation and mobilization with limited light soft-tissue massage, assisted stretching, hot and cold packs, and advice to stay active or modify activity as needed.
For patients with recent (0-3 months) grade III neck and arm pain, we suggest supervised graded strengthening exercises* rather than advice alone**.Remark: *Supervised graded strengthening exercises consisted of strengthening and stability exercises twice a week for 6 weeks with daily home exercises (which included mobility, stability and muscle strengthening). **Advice consisted of maintaining activity of normal life without specific treatment.
Recommendations for Recent-Onset Whiplash Associated Disorders (WAD)
For adult patients with recent (0-3 months) WAD grades I-III, we suggest multimodal care over education alone.Remark: Multimodal care may consist of manual therapy (joint mobilization), other soft tissue techniques, education and exercises.
Recommendations for Persistent Neck Pain Associated Disorders (NAD)
For adult patients with persistent (over 6 months duration) neck pain grades I-II, we suggest supervised group exercise* to reduce neck pain and disability.Remark: Patients received 18-24 group sessions during a period of 4 to 6 months. Patients considered had a rating of 40/100 on a pain scale (VAS). The intervention group reached suggested MCID level of 10% difference for pain and functional outcomes. *Exercises included qigong or ROM, flexibility and strengthening exercises. No evidence of significant effect in the elderly population.
For patients with persistent (over 3 months) grades I-II neck pain and disability, we suggest supervised yoga over education and home exercises for short-term improvement in neck pain and disability.Remark: Baseline intensity of pain was more than 40/100 and at least 3 months duration. Yoga was specific to Iyengar type, with a maximum of 9 sessions over 9 weeks.
For patients with persistent (over 3 months) grades I-II neck pain, we suggest supervised strengthening exercises or home exercises.Remark: For reduction in pain, supervised strengthening exercises, provided along with ROM exercises and advice, interventions were evaluated at 12 weeks within 20 sessions. Home exercises include stretching or self-mobilization.
For patients with persistent (over 3 months) neck pain and associated disorders grades I-II, we suggest multimodal care* or stress self-management** based on patient preference, prior response to care and resources available.Remark: *Individualized multimodal care may include manual therapy (manipulation, mobilization, massage, traction), acupuncture, heat, transcutaneous electrical nerve stimulation, exercise, and/or ultrasound. **Stress self-management may include relaxation, balance and body awareness exercises, pain and stress self-management lectures and discussion. The multimodal care group received an average of 7 (range 4-8) sessions, compared to 11 (range 1-52) sessions for stress self-management group over 20 weeks.
For patients with persistent (over 3 months) neck pain grades I-II, we suggest manipulation in conjunction with soft tissue therapy.Remark: Evaluated after eight 20 min sessions (over a three week period). Does not include manipulation as a stand-alone treatment.
For patients with persistent (over 3 months) neck pain and associated disorders grade I-II, we suggest high-dose massage over wait listing based on patient preferences and resources available.Remark: Interventions were given 3 X 60 min a week over 4 weeks. Lower dosages and duration did not have therapeutic benefit, and we cannot suggest offering as an option.
For patients presenting with persistent (over 3 months) neck pain grades I-III, we suggest clinicians offer *multimodal care and/or **practitioner advice based on patient preference.Remark: * Multimodal care and exercises may consist of thrust/non-thrust joint manipulation; muscle energy, stretching and home exercises (cervical retraction, deep neck flexor strengthening, cervical rotation ROM). ** Multimodal minimal intervention may consist of postural advice; encouragement to maintain neck motion and daily activities; cervical rotation ROM exercise; instructions to continue prescribed medication; therapeutic pulsed (10%) ultrasound at 0.1 W/cm2 for 10 minutes applied to the neck and cervical ROM exercises.
For workers with persistent (over 3 months) neck and shoulder pain, we suggest mixed supervised and unsupervised high-intensity strength training or advice alone.Remark: For reduction in pain intensity, 3 sessions per week, each lasting 20 minutes over a 20-week period. Exercise includes strengthening. Extra resources are likely required for complete exercise intervention implementation.
Recommendations for Persistent Whiplash Associated Disorders (WAD)
For patients with persistent (over 3 months) grades I-II WAD, we suggest supervised exercises with advice or advice alone based on patient preference and resources available.Remark: Extra resources may be required for supervised exercises.
Recommendations proposed in this guideline are derived from the best available evidence for the treatment of Neck Pain Associated and Whiplash Associated Disorders. Clinicians should always aim to incorporate the best evidence available to inform clinical decision making.
About the quality and strength of the evidence for this guideline
Quality of the evidence [9]
The certainty in the evidence (also known as quality of evidence or confidence in the estimates) is assessed for each important outcome using these categories: high, moderate, low. Randomized trials begin as high quality evidence. Quality may be downgraded as a result of limitations in study design or implementation, imprecision of estimates (wide confidence intervals), variability in results, indirectness of evidence, or publication bias. The quality of the evidence of included randomized controlled trials ranged between low and moderate.
Strength of the evidence [9]
Based on available evidence, the quality of the recommendation indicates the extent to which one can be confident that adherence to the recommendation will do more good than harm. Strength of recommendation is determined by the balance between desirable and undesirable consequences of alternative management strategies, quality of evidence, variability in values and preferences, and resource use. Overall, the strength of the evidence of the recommendations for this guideline is weak. Weak recommendations mean that patients’ choices will vary according to their values and preferences, and clinicians must ensure that patients’ care is in keeping with their values and preferences.
Structured Patient Education
Recommendations for structured patient education are included in the exercise intervention section of this guideline. The panel decided not to repeat these findings in the current section and felt that the benefits of increasing the frequency and intensity of exercise regimes was not restricted to those working in an industrial environment, or to any specific population sub-group with the exception of older adults.
Work Disability Prevention Interventions
Evidence on Work Disability Prevention interventions was considered, but the panel decided not to provide practice recommendations because of the uncertainty surrounding judgments on the evidence.
Funding Sources and Conflicts of Interest
Funds provided by the Canadian Chiropractic Research Foundation. The views of the funding body have not influenced the content of the guideline. No conflicts of interest were reported for this study.
Guideline Disclaimer
The evidence-based practice guidelines published by the CCGI include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options. [21] Guidelines are intended to inform clinical decision making, are not prescriptive in nature, and do not replace professional chiropractic care or advice, which always should be sought for any specific condition. Furthermore, guidelines may not be complete or accurate because new studies that have been published too late in the process of guideline development or after publication are not incorporated into any particular guideline before it is disseminated. CCGI and its working group members, executive committee, and stakeholders (the “CCGI Parties”) disclaim all liability for the accuracy or completeness of a guideline, and disclaim all warranties, expressed or implied. Guideline users are urged to seek out newer information that might impact the diagnostic and/or treatment recommendations contained within a guideline. The CCGI Parties further disclaim all liability for any damages whatsoever (including, without limitation, direct, indirect, incidental, punitive, or consequential damages) arising out of the use, inability to use, or the results of use of a guideline, any references used in a guideline, or the materials, information, or procedures contained in a guideline, based on any legal theory whatsoever and whether or not there was advice of the possibility of such damages.
Through a comprehensive and systematic literature review, CCGI evidence-based CPGs incorporate data from the existing peer-reviewed literature. This literature meets the prespecified inclusion criteria for the clinical research question, which CCGI considers, at the time of publication, to be the best evidence available for general clinical information purposes. This evidence is of varying quality from original studies of varying methodological rigor. CCGI recommends that performance measures for quality improvement, performance-based reimbursement, and public reporting purposes should be based on rigorously developed guideline recommendations.
Contributorship Information
Concept development (provided idea for the research): A.B., J.O., G.S.
Design (planned the methods to generate the results): A.B., J.O., G.S.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): A.B., J.O., G.S.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): A.B., J.O., G.S., F.A.Z.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): J.O., A.B., M.D., M.S., C.H.
Literature search (performed the literature search): A.B., F.A.Z.
Writing (responsible for writing a substantive part of the manuscript): A.B., J.O., G.S., F.A.Z., J.S., J.W., M.D., M.S., I.P., S.P., C.H., J.H.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): A.B., J.O., G.S., F.A.Z., J.S., J.W., M.D., M.S., I.P., S.P., C.H., J.H.Acknowledgments
We thank the following people for their contributions to this paper: Dr. John Riva, DC, observer; Heather Owens, Research Coordinator, proofreading; Cameron McAlpine (Director of Communication & Marketing, Ontario Chiropractic Association), for assistance in producing the companion document intended for patients with NAD; members of the guideline panel who served on the Delphi consensus panel, who made this project possible by generously donating their expertise and clinical judgment.
References:
Ferrari, R. and Russell, A.
Regional musculoskeletal conditions: neck pain.
Best Pract Res Clin Rheumatol. 2003; 17: 57–70Hogg-Johnson, S., van der Velde, G., Carroll, L. J. et al (2008).
The Burden and Determinants of Neck Pain in the General Population:
Results of the Bone and Joint Decade 2000–2010 Task Force
on Neck Pain and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S39–51Holm LW, Carroll LJ, Cassidy JD, et al.
The Burden and Determinants of Neck Pain in Whiplash-associated
Disorders after Traffic Collisions: Results of the Bone and Joint
Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S52-59Cote P, van der Velde G, Cassidy JD, Carroll LJ, Hogg-Johnson S, Holm LW, et al.
The Burden and Determinants of Neck Pain in Workers: Results of the
Bone and Joint Decade 2000–2010 Task Force on Neck Pain
and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S60-74Vos, T., Flaxman, A., Naghavi, M. et al.
Years Lived with Disability (YLDs) for 1160 Sequelae of 289 Diseases
and Injuries 1990-2010: A Systematic Analysis for the
Global Burden of Disease Study 2010
Lancet. 2012 (Dec 15); 380 (9859): 2163–2196Cote P, Cassidy JD, Carroll L.
The Treatment of Neck and Low Back Pain: Seeks Care?
Who Goes Where?
Med Care. 2001 (Sep); 39 (9): 956–967Hoy, D.G., Protani, M., De, R., and Buchbinder, R.
The epidemiology of neck pain.
Best Pract Res Clin Rheumatol. 2010; 24: 783–792Murray CJL, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al.
The State of US Health, 1990-2010:
Burden of Diseases, Injuries, and Risk Factors
JAMA 2013 (Aug 14); 310 (6): 591–608Manchikanti, L., Singh, V., Datta, S., Cohen, S., and Hirsch, J.
Physicians. ASoIP. Comprehensive review of epidemiology, scope, and impact of spinal pain.
Pain Physician. 2009; 12: E35–E70Hincapié, C., Cassidy, J., Côté, P., Carroll, L., and Guzmán, J.
Whiplash injury is more than neck pain: a population-based study of pain
localization after traffic injury.
J Occup Environ Med. 2010; 52: 434–440Blincoe, L., Miller, T., Zaloshnja, E., and Lawrence, B.
The Economic and Societal Impact of Motor Vehicle Crashes, 2010. (Revised)
(Report No. DOT HS 812 013). National Highway Traffic Safety Administration,
Washington, DC; 2015Bannister, G., Amirfeyz, R., Kelley, S., and Gargan, M.
Whiplash injury.
J Bone Joint Surg. 2009; 91-B: 845–850Johansson, M., Boyle, E., Hartvigsen, J., Carroll, L., and Cassidy, J.
A Population-based, Incidence Cohort Study of Mid-back Pain
After Traffic Collisions: Factors Associated with Global Recovery
European Journal of Pain 2015 (Nov); 19 (10): 1486–1495Styrke, J., Stalnacke, B., Bylund, P., and Sojka, P.
A 10-year incidence of acute whiplash injuries after road traffic crashes
in a defined population in northern Sweden.
PM R. 2012; 4: 739–747Ontario MoFo.
Ontario Auto Insurance Anti-Fraud Task Force Interim Report.
(Available at:)
http://www.fin.gov.on.ca/en/autoinsurance/interim-report.pdfKarlsborg, M., Smed, A., Jespersen, H. et al.
A prospective study of 39 patients with whiplash injury.
Acta Neurol Scand. 1997; 95: 65–72Sterling, M., Jull, G., Vicenzino, B., Kenardy, J., and Darnell, R.
Development of motor system dysfunction following whiplash injury.
Pain. 2003; 103: 65–73Guzman, J., Hurwitz, E.L., Carroll, L.J. et al.
A New Conceptual Model Of Neck Pain: Linking Onset, Course, And Care:
The Bone and Joint Decade 2000-2010 Task Force
on Neck Pain and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S14–23Leaver, A., Maher, C., McAuley, J., Jull, G., and Refshauge, K.
Characteristics of a new episode of neck pain.
Man Ther. 2013; 18: 254–257Côté, P., Shearer, H., Ameis, A. et al.
Enabling recovery from common traffic injuries: a focus on the injured person.
UOIT-CMCC Centre for the Study of Disability Prevention and Rehabilitation. ; 2015Clar C, Tsertsvadze A, Court R, Hundt G, Clarke A, Sutcliffe P.
Clinical Effectiveness of Manual Therapy for the Management of
Musculoskeletal and Non-Musculoskeletal Conditions:
Systematic Review and Update of UK Evidence Report
Chiropractic & Manual Therapies 2014 (Mar 28); 22 (1): 12Bronfort, G., Evans, R., Anderson, A., Svendsen, K., Bracha, Y., and Grimm, R.
Spinal Manipulation, Medication, or Home Exercise With Advice
for Acute and Subacute Neck Pain: A Randomized Trial
Annals of Internal Medicine 2012 (Jan 3); 156 (1 Pt 1): 1–10Hurwitz, EL, Carragee, EJ, van der Velde, G et al.
Treatment of Neck Pain: Noninvasive Interventions: Results of the
Bone and Joint Decade 2000–2010 Task Force on Neck Pain
and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S123–152R. Bryans, P. Decina, M. Descarreaux, et al.,
Evidence-Based Guidelines for the Chiropractic Treatment
of Adults With Neck Pain
J Manipulative Physiol Ther 2014 (Jan); 37 (1): 42–63Shaw L, Descarreaux M, Bryans R, Duranleau M, Marcoux H, Potter B, Ruegg R, Watkin R, White E.
A Systematic Review of Chiropractic Management of Adults with
Whiplash-Associated Disorders: Recommendations for
Advancing Evidence-based Practice and Research
Work. 2010; 35 (3): 369–394in: G. Graham, M. Mancher, D. Miller Wolman, S. Greenfield, E. Steinberg (Eds.)
Clinical Practice Guidelines We Can Trust.
Institute of Medicine, Shaping the Future for Health.
National Academies Press, Washington, DC; 2011Côté, P., Wong, J.J., Sutton, D. et al.
Management of Neck Pain and Associated Disorders: A Clinical
Practice Guideline from the Ontario Protocol for Traffic
Injury Management (OPTIMa) Collaboration
European Spine Journal 2016 (Jul); 25 (7): 2000–2022Johnson, A.P., Sikich, N.J., Evans, G. et al.
Health technology assessment: a comprehensive framework for evidence-based
recommendations in ontario.
Int J Technol Assess Health Care. 2009; 25: 141–150Shukla, V., Bai, A., Milne, S., and Wells, G.
Systematic review of the evidence grading system for grading level of evidence.
German J Evid Qual Health Care. 2008; 102: 43Mustafa, R.A., Santesso, N., Brozek, J. et al.
The GRADE approach is reproducible in assessing the quality of evidence of
quantitative evidence syntheses.
J Clin Epidemiol. 2013; 66: 736–742.e5Woolf, S., Schunemann, H., Eccles, M., Grimshaw, J., and Shekelle, P.
Developing clinical practice guidelines: types of evidence and outcomes;
values and economics, synthesis, grading, and presentation and deriving recommendations.
Implementation Sci. 2012; 7: 61Tricco, A., Tetzlaff, J., and Moher, D.
The art and science of knowledge synthesis.
J Clin Epidemiol. 2011; 64: 11–20Guyatt, G., Eikelboom, J.W., Akl, E.A. et al.
A guide to GRADE guidelines for the readers of JTH..
J Thromb Haemost. 2013; 11: 1603–1608Adaptation. The ADAPTE Manual and Resource Toolkit V2.
G-I-N Adaptation Working Group. (Available at:)
http://www.g-i-n.net/working-groups/adaptationBrouwers, M., Kho, M., Browman, G. et al.
AGREE II: advancing guideline development, reporting and evaluation in health care.
J Clin Epidemiol. 2010; 63: 1308–1311Flottorp, S., Oxman, A.D., Cooper, J.G., Hjortdahl, P., Sandberg, S., and Vorland, L.H.
Retningslinjer for diagnostikk og behandling av sar hals.
Tidsskr Nor Laegeforen. 2000; 120: 1754–1760Grimshaw, J., Eccles, M., Lavis, J., Hill, S., and Squires, J.
Knowledge translation of research findings.
Implementation Sci. 2012; 7: 50Southerst D, Nordin M, Côté P, et al.
Is Exercise Effective for the Management of Neck Pain and
Associated Disorders or Whiplash-associated Disorders?
A Systematic Review by the Ontario Protocol for Traffic
Injury Management (OPTIMa) Collaboration
Spine J 2016 (Dec); 16 (12): 1503–1523Sutton, D., Cote, P., Wong, J. et al.
Is multimodal care effective for the management of patients with whiplash-associated
disorders or neck pain and associated disorders? A systematic review by the
Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration.
Spine J. 2014; ([S1529-9430(14):00650-0])Yu, H., Côté, P., Southerst, D., Wong, J. et al.
Does Structured Patient Education Improve the Recovery and Clinical Outcomes
of Patients with Neck Pain? A Systematic Review from the Ontario
Protocol for Traffic Injury Management (OPTIMa) Collaboration
Spine J. 2014; (pii: S1529-9430(14))Varatharajan, S., Côté, P., Shearer, H. et al.
Are work disability prevention interventions effective for the management of
neck pain or upper extremity disorders? A systematic review by the
Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration.
J Occup Rehabil. 2014; 24: 692–708Wong, J.J., Shearer, H.M., Mior, S. et al.
Are manual therapies, passive physical modalities, or acupuncture effective
for the management of patients with whiplash-associated disorders or neck pain
and associated disorders? An update of the Bone and Joint Decade Task Force
on Neck Pain and Its Associated Disorders by the Optima Collaboration.
Spine J. 2015; 20Shea, B., Grimshaw, J., Wells, G., Boers, M., Andersson, N., and Hamel, C.
Development of AMSTAR: a measurement tool to assess the methodological
quality of systematic reviews.
BMC Med Res Methodol. 2007; 7: 10Norman G, Streiner D.
Biostatistics: The Bare Essentials. 3rd ed.
Hamilton, ON: BC Decker; 2008.Ricci, S., Celani, M., and Righetti, E.
Development of clinical guidelines: methodological and practical issues.
Neurol Sci. 2006; 27: S228–S230van der Velde, G., van Tulder, M., Côté, P. et al.
The sensitivity of review results to methods used to appraise and
incorporate trial quality into data synthesis.
Spine. 2007; 32: 796–806Slavin, R.
Best evidence synthesis: an intelligent alternative to meta-analysis.
J Clin Epidemiol. 1995; 48: 9–18Network GI and GRADE Working Group. Resources. (Available at:)
http://www.g-i-n.net/working-groups/updating-guidelines/resourcesGuyatt GH, Oxman AD, Vist GE, et al.
GRADE: An Emerging Consensus on Rating Quality of Evidence
and Strength of Recommendations
British Medical Journal 2008 (Apr 26); 336 (7650): 924–926Guyatt, G., Oxman, A., Akl, E., Kunz, R., Vist, G., Brozek, J. et al.
GRADE guidelines 1. Introduction: GRADE evidence profiles and summary of findings tables.
J Clin Epidemiol. 2011; 64: 38–94Treweek, S., Oxman, A., Alderson, P. et al.
Developing and evaluating communication strategies to support informed
decisions and practice based on evidence (DECIDE): protocol and preliminary results.
Implementation Sci. 2013; 8: 6McCarthy, M., Grevitt, M., Silcocks, P., and Hobbs, G.
The Reliability of the Vernon and Mior Neck Disability Index,
and its Validity Compared With the Short Form-36
Health Survey Questionnaire
European Spine Journal 2007 (Dec); 16 (12): 2111–2117Stauffer, M., Taylor, S., Watson, D., Peloso, P., and Morrison, A.
Definition of nonresponse to analgesic treatment of arthritic pain:
an analytical literature review of the smallest detectable difference,
the minimal detectable change, and the minimal clinically important
difference on the pain visual analog scale.
Int J Inflam. 2011; 2011: 231926Hawker, G.A., Mian, S., Kendzerska, T., and French, M.
Measures of Adult Pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating
Scale for Oain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill
Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36
Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent
and Constant Osteoarthritis Pain (ICOAP)
Arthritis Care Res (Hoboken) 2011 (Nov); 63 Suppl 11: S240-252Blozik, E., Himmel, W., Kochen, M.M., Herrmann-Lingen, C., and Scherer, M.
Sensitivity to change of the Neck Pain and Disability Scale.
Euro Spine J. 2011; 20: 882–889Andrews, J., Guyatt, G., Oxman, A.D. et al.
GRADE guidelines: 14. Going from evidence to recommendations:
the significance and presentation of recommendations.
J Clin Epidemiol. 2013; 66: 719–725Andrews, J.C., Schünemann, H.J., Oxman, A.D. et al.
GRADE guidelines: Going from evidence to recommendation—determinants of a
recommendation's direction and strength.
J Clinl Epidemiol. 2013; 66: 726–735Black, N., Murphy, M., Lamping, D., McKee, M., Sanderson, C., and Askham, J.
Consensus development methods: a review of best practice in creating clinical guidelines.
J Health Serv Res Policy. 1999; 4: 236–248Seo, H.-J. and Kim, K.U.
Quality assessment of systematic reviews or meta-analyses of nursing interventions
conducted by Korean reviewers.
BMC Med Res Methodol. 2012; 12: 129Leaver, A., Maher, C., Herbert, R. et al.
A randomized controlled trial comparing manipulation with mobilization for
recent onset neck pain.
Arch Phys Med Rehabil. 2010; 91: 1313–1318Dunning, J., Cleland, J., Waldrop, M. et al.
Upper cervical and upper thoracic thrust manipulation versus nonthrust mobilization
in patients with mechanical neck pain: a multicenter randomized clinical trial.
J Orthop Sports Phys Ther. 2012; 42: 5–18Nagrale, A., Glynn, P., Joshi, A., and Ramteke, G.
The efficacy of an integrated neuromuscular inhibition technique on upper trapezius
trigger points in subjects with non-specific neck pain: a randomized controlled trial.
J Man Manip Ther. 2010; 18: 37–43McReynolds, T. and Sheridan, B.
Intramuscular ketorolac versus osteopathic manipulative treatment in the management
of acute neck pain in the emergency department: a randomized clinical trial.
JAOA. 2005; 105: 57–68Chou, R., Turner, J.A., Devine, E.B. et al.
The effectiveness and risks of long-term opioid therapy for chronic pain:
a systematic review for a National Institutes of Health Pathways to
Prevention Workshop Effectiveness and Risks of Long-Term Opioid Therapy for Chronic Pain.
Ann Inter Med. 2015; 162: 276–286Kuijper, B., Tans, J., Beelen, A., Nollet, F., and de Visser, M.
Cervical collar or physiotherapy versus wait and see policy for recent
onset cervical radiculopathy: randomised trial.
BMJ. 2009; 339: b3883Cassidy, J.
Mobilisation or immobilisation for cervical radiculopathy?
BMJ. 2009; 339: 3952Konstantinovic, L., Cutovic, M., Milovanovic, A. et al.
Low-level laser therapy for acute neck pain with radiculopathy:
a double-blind placebo-controlled randomized study.
Pain Med. 2010; 11: 1169–1178van den Heuvel, S., de Looze, M., Hildebrandt, V., and Thé, K.
Effects of software programs stimulating regular breaks and exercises on
work-related neck and upper-limb disorders.
Scand J Work Environ Health. 2003; 29: 106–116Lamb, S., Gates, S., Williams, M. et al.
Emergency department treatments and physiotherapy for acute whiplash:
a pragmatic, two-step, randomised controlled trial.
Lancet. 2013; 381: 546–556Ferrari, R., Rowe, B.H., Majumdar, S.R. et al.
Simple educational intervention to improve the recovery from acute whiplash:
results of a randomized, controlled trial.
Acad Emerg Med. 2005; 12: 699–706von Trott, P., Wiedemann, A., Lüdtke, R., Reißhauer, A., Willich, S., and Witt, C.
Qigong and exercise therapy for elderly patients with chronic neck pain (QIBANE):
a randomized controlled study.
J Pain. 2009; 10: 501–508Rendant, D., Pach, D., Ludtke, R. et al.
Qigong versus exercise versus no therapy for patients with chronic neck pain:
a randomized controlled trial.
Spine. 2011; 36: 419–427Michalsen, A., Traitteur, H., Lüdtke, R. et al.
Yoga for chronic neck pain: a pilot randomized controlled clinical trial.
J Pain. 2012; 13: 1122–1130Jeitler, M., Brunnhuber, S., Meier, L. et al.
Effectiveness of jyoti meditation for patients with chronic neck pain and
psychological distress-a randomized controlled clinical trial.
J Pain. 2015; 16: 77–86Hakkinen, A., Kautiainen, H., Hannonen, P., and Ylinen, J.
Strength training and stretching versus stretching only in the treatment of patients
with chronic neck pain: a randomized one-year follow-up study.
Clin Rehabil. 2008; 22: 593–600Salo, P., Ylonen-Kayra, N., Hakkinen, A., Kautiainen, H., Malkia, E., and Ylinen, J.
Effects of long-term home-based exercise on health-related quality of life in patients
with chronic neck pain: a randomized study with a 1-year follow-up.
Disabil Rehabil. 2012; 34: 1971–1977Evans, R., Bronfort, G., Schulz, G. et al.
Supervised Exercise With And Without Spinal Manipulation Performs Similarly
And Better Than Home Exercise For Chronic Neck Pain:
A Randomized Controlled Trial
Spine (Phila Pa 1976). 2012 (May 15); 37 (11): 903–914Maiers, M., Bronfort, G., Evans, R. et al.
Spinal Manipulative Therapy and Exercise For Seniors with Chronic Neck Pain
Spine J. 2014 (Sep 1); 14 (9): 1879–1889Griffiths, C., Dziedzic, K., Waterfield, J., and Sim, J.
Effectiveness of specific neck stabilization exercises or a general neck exercise
program for chronic neck disorders: a randomized controlled trial.
J Rheumatol. 2009; 36: 390–397Gustavsson, C., Denison, E., and von Koch, L.
Self-management of persistent neck pain: a randomized controlled trial of a multi-component
group intervention in primary health care.
Eur J Pain. 2010; 14: 630.e1-11Gustavsson, C., Denison, E., and von Koch, L.
Self-management of persistent neck pain: two-year follow-up of a randomized controlled
trial of a multicomponent group intervention in primary health care.
Spine. 2011; 36: 2105–2115Sherman, K., Cherkin, D., Hawkes, R., Miglioretti, D., and Deyo, R.
Randomized trial of therapeutic massage for chronic neck pain.
Clin J Pain. 2009; 25: 233–238Lin, J., Shen, T., Chung, R., and Chiu, T.
The effectiveness of Long's manipulation on patients with chronic mechanical neck pain:
a randomized controlled trial.
Manual Ther. 2013; 18: 308–315Lauche, R., Materdey, S., Cramer, H. et al.
Effectiveness of home-based cupping massage compared to progressive muscle relaxation
in patients with chronic neck pain—a randomized controlled trial.
PLoS One. 2013; 8: e65378Sherman, K., Cook, A., Wellman, R. et al.
Five-week outcomes from a dosing trial of therapeutic massage for chronic neck pain.
Ann Fam Med. 2014; 12: 112–120Walker, M.J., Boyles, R.E., Young, B.A. et al.
The effectiveness of manual physical therapy and exercise for mechanical neck pain:
a randomized clinical trial.
Spine (Phila Pa 1976). 2008; 33: 2371–2378Boyles, R., Walker, M., Young, B., Strunce, J., and Wainner, R.
The addition of cervical thrust manipulations to a manual physical therapy approach
in patients treated for mechanical neck pain: a secondary analysis.
J Orthop Sports Phys Ther. 2010; 40: 133–140Hoving, J.L., de Vet, H.C., Koes, B.W. et al.
Manual Therapy, Physical Therapy, or Continued Care by a General Practitioner
for Patients with Neck Pain. A Randomized, Controlled Trial
Clin J Pain. 2006; 22: 370–377Hoving JL, Koes BW, de Vet HC, van der Windt DA, Assendelft WJ, van Mameren H, et al.
Manual Therapy, Physical Therapy, or Continued Care by a
General Practitioner for Patients with Neck Pain.
A Randomized, Controlled Trial
Annals of Internal Medicine 2002 (May 21); 136 (10): 713–722Monticone, M., Baiardi, P., Vanti, C. et al.
Chronic neck pain and treatment of cognitive and behavioural factors:
results of a randomised controlled clinical trial.
Euro Spine J. 2012; 21: 1558–1566Zebis, M., Andersen, L., Pedersen, M. et al.
Implementation of neck/shoulder exercises for pain relief among industrial workers:
a randomized controlled trial.
BMC Musculoskelet Disord. 2011; 12: 205Zebis, M.K., Andersen, C.H., Sundstrup, E., Pedersen, M.T., Sjøgaard, G., and Andersen, L.L.
Time-wise change in neck pain in response to rehabilitation with specific
resistance training: implications for exercise prescription.
PLoS One. 2014; 9: e93867Andersen, C., Andersen, L., Gram, B. et al.
Influence of frequency and duration of strength training for effective management of
neck and shoulder pain: a randomised controlled trial.
Br J Sports Med. 2012; 46: 1004–1010Andersen, L., Jorgensen, M., Blangsted, A., Pedersen, M., Hansen, E., and Sjogaard, G.
A. randomized controlled intervention trial to relieve and prevent neck/shoulder pain.
Med Sci Sports Exerc. 2008; 40: 983–990Sjogren, T., Nissinen, K., Jarvenpaa, S., Ojanen, M., Vanharanta, H., and Malkia, E.
Effects of a workplace physical exercise intervention on the intensity of headache
and neck and shoulder symptoms and upper extremity muscular strength of
office workers: a cluster randomized controlled cross-over trial.
Pain. 2005; 116: 119–128Stewart, M., Maher, C., Refshauge, K., Herbert, R., Bogduk, N., and Nicholas, M.
Randomized controlled trial of exercise for chronic whiplash-associated disorders.
Pain. 2007; 128: 59–68Michaleff, Z. and Maher, C. Lin C-WC, et al.
Comprehensive physiotherapy exercise programme or advice for chronic whiplash
(PROMISE): a pragmatic randomised controlled trial.
Lancet. 2014; 384: 133–141Gram, B., Andersen, C., Zebis, M.K. et al.
Effect of training supervision on effectiveness of strength training for
reducing neck/shoulder pain and headache in office workers:
cluster randomized controlled trial.
BioMed Ress Int. 2014; 2014: 9Jull, G., Sterling, M., Kenardy, J., and Beller, E.
Does the presence of sensory hypersensitivity influence outcomes of
physical rehabilitation for chronic whiplash? A preliminary RCT..
Pain. 2007; 129: 28–34Jull, G., Kenardy, J., Hendrikz, J., Cohen, M., and Sterling, M.
Management of acute whiplash: a randomized controlled trial of
multidisciplinary stratified treatments.
Pain. 2013; 154: 1798–1806Wiangkham, T., Duda, J., Haque, S., Madi, M., and Rushton, A.
The effectiveness of conservative management for acute whiplash associated disorder
(WAD) II: a systematic review and meta-analysis of randomised controlled trials.
PLoS One. 2015; 10: e0133415Guyatt, G., Oxman, A.D., Sultan, S. et al.
GRADE guidelines: 11. Making an overall rating of confidence in effect estimates
for a single outcome and for all outcomes.
J Clin Epidemiol. 2013; 66: 151–157Walsh, D., Howe, T., Johnson, M., and Sluka, K.
Transcutaneous electrical nerve stimulation for acute pain.
Cochrane Database Syst Rev. 2009; : CD006142Nnoaham, K. and Kumbang, J.
Transcutaneous electrical nerve stimulation (TENS) for chronic pain.
Cochrane Database Syst Rev. 2008; : CD003222French, S., Cameron, M., Walker, B., Reggars, J., and Esterman, A.
Superficial heat or cold for low back pain.
Cochrane Database Syst Rev. 2006; : CD004750Malanga, G.A., Yan, N., and Stark, J.
Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury.
Postgrad Med. 2015; 127: 57–65Carnes, D., Mullinger, B., and Underwood, M.
Defining adverse events in manual therapies: a modified Delphi consensus study.
Manual Ther. 2010; 15: 2–6Haldeman, S., Carroll, L.J., and Cassidy, J.D.
The empowerment of people with neck pain: introduction:
the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders.
Spine. 2008; 33: S8–S13Maiers, M., Vihstadt, C., Hanson, L., and Evans, R.
Perceived Value of Spinal Manipulative Therapy and Exercise
Among Seniors With Chronic Neck Pain:
A Mixed Methods Study
J Rehabil Med. 2014 (Nov); 46 (10): 1022–1028Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al.
Noninvasive Treatments for Low Back Pain
Comparative Effectiveness Review no. 169
(Prepared by the Pacific Northwest Evidence-based Practice Center
under contract no. 290-2012-00014-I.)
AHRQ publication no. 16-EHC004-EF. Rockville:
Agency for Healthcare Research and Quality; February 2016Machado, G., Maher, C., Ferreira, P. et al.
Efficacy and safety of paracetamol for spinal pain and osteoarthritis:
systematic review and meta-analysis of randomised placebo controlled trials.
BMJ. 2015; 350: h1225Miller, M., Barber, C.W., Leatherman, S. et al.
Prescription opioid duration of action and the risk of unintentional overdose
among patients receiving opioid therapy.
JAMA Intern Med. 2015; 175: 608–615Volkow, N. and McLellan, A.
Opioid abuse in chronic pain—misconceptions and mitigation strategies.
N Engl J Med. 2016; 374: 1253–1263Foster, N., Hartvigsen, J., and Croft, P.
Taking responsibility for the early assessment and treatment of patients with
musculoskeletal pain: a review and critical analysis.
Arthritis Res Ther. 2012; 14: 205World Health Organization (WHO)
WHO Guidelines on Basic Training and Safety in Chiropractic
Geneva, Switzerland: (November 2005)Guzman J, Haldeman S, Carroll LJ, et al.
Clinical Practice Implications of the Bone and Joint Decade 2000-2010
Task Force on Neck Pain and Its Associated Disorders:
From Concepts and Findings to Recommendations
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S199–S212Dietl, M. and Korczak, D.
Over-, under- and misuse of pain treatment in Germany.
GMS Health Technol Assess. 2011; 7:Freburger, J., Carey, T., Holmes, G., Wallace, A., Castel, L., and Darter, J.
Exercise prescription for chronic back or neck pain: who prescribes it?
Who gets it? What is prescribed?
Arthritis Care Res. 2009; 61: 192–200Goode, A., Freburger, J., and Carey, T.
Prevalence, practice patterns, and evidence for chronic neck pain.
Arthritis Care Res. 2010; 62: 1594–1601Kamaleri, Y., Natvig, B., Ihlebaek, C.M., and Bruusgaard, D.
Localized or widespread musculoskeletal pain: does it matter?
Pain. 2008; 138: 41–46MacDermid, J., Miller, J., and Gross, A.
Knowledge translation tools are emerging to move neck pain research into practice.
Open Orthop J. 2013; 20: 582–593Medina-Mirapeix, F., Escolar-Reina, P., Gascon-Canovas, J., Montilla-Herrador, J., Jimeno-Serrano.
Predictive factors of adherence to frequency and duration components in
home exercise programs for neck and low back pain: an observational study.
BMC Musculoskelet Disord. 2009; 10: 155Kay, T., Gross, A., Goldsmith, C. et al.
Exercises for mechanical neck disorders. Cochrane
Database Syst Rev. 2012; 8: CD004250Bertozzi, L., Gardenghi, I., Turoni, F. et al.
Effect of therapeutic exercise on pain and disability in the management of
chronic nonspecific neck pain: systematic review and meta-analysis of randomized trials.
Phys Ther. 2013; 93: 1026–1036Hartvigsen, J., Natvig, B., and Ferreira, M.
Is it all about a pain in the back?
Best Pract Res Clin Rheumatol. 2013; 27: 613–623Ambrose, K. and Golightly, Y.
Physical exercise as non-pharmacological treatment of chronic pain: why and when.
Best Pract Res Clin Rheumatol. 2015; 29: 120–130Lee, I., Shiroma, E., Lobelo, F., Puska, P., Blair, S., and Katzmarzyk, P.
Effect of physical inactivity on major non-communicable diseases worldwide:
an analysis of burden of disease and life expectancy.
Lancet. 2012; 380: 219–229World Health Organization.
Global Recommendations on Physical Activity for Health
Geneva: WHO; 2010: ISBN: 978 92 4 159 997 9Carroll, L.J., Ferrari, R., Cassidy, J.D., and Cote, P.
Coping and recovery in whiplash-associated disorders: early use of passive coping
strategies is associated with slower recovery of neck pain and pain-related disability.
Clin J Pain. 2014; 30: 1–8Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D.
The Burden of Chronic Low Back Pain: Clinical Comorbidities,
Treatment Patterns, and Health Care Costs in
Usual Care Settings
Spine (Phila Pa 1976). 2012 (May 15); 37 (11): E668–677Bodenheimer T, MacGregor K, Charifi C.
Helping patients manage their chronic conditions.
Oakland, CA: California HealthCare Foundation; 2005.Ritzwoller, D., Crounse, L., Shetterly, S., and Rublee, D.
The association of comorbidities, utilization and costs for patients identified
with low back pain.
BMC Musculoskelet Disord. 2006; 7: 72Sallis, R., Franklin, B., Joy, L., Ross, R., Sabgir, D., and Stone, J.
Strategies for promoting physical activity in clinical practice.
Prog Cardiovasc Dis. 2015; 57: 375–386Von Korff, M., Crane, P., Lane, M. et al.
Chronic spinal pain and physical-mental comorbidity in the United States:
results from the national comorbidity survey replication.
Pain. 2005; 113: 331–339Bussières, A., Al Zoubi, F., Quon, J. et al.
Fast tracking the design of theory-based KT interventions through a consensus process.
Implementation Sci. 2015; 10: 18Gutnick, D., Reims, K., Davis, C., Gainforth, H., Jay, M., and Cole, S.
Brief action planning to facilitate behavior change and support patient self-management.
J Clin Outcomes Manag. 2014; 21: 17–29Dhopte, P., Ahmed, S., Mayo, N., French, S., Quon, J.A., and Bussières, A.
Testing the feasibility of a knowledge translation intervention designed to
improve chiropractic care for adults with neck pain disorders:
study protocol for a pilot cluster-randomized controlled trial.
Pilot and Feasibility Studies. 2016; 2: 1–11Turner, L., Shamseer, L., Altman, D. et al.
Consolidated standards of reporting trials (CONSORT) and the completeness
of reporting of randomised controlled trials (RCTs) published in medical journals.
Cochrane Database Syst Rev. 2012; 11: MR000030Quinlan, K., Annest, J., Myers, B., Ryan, G., and Hill, H.
Neck strains and sprains among motor vehicle occupants—United States, 2000.
Accid Anal Prev. 2004; 36: 21–27Titler, M.
The evidence for evidence-based practice implementation.
in: Patient Safety and Quality:
An Evidence-Based Handbook for Nurses. vol. 1.
AHRQ, Rockville, MD; 2008: 113–161The Canadian Agency for Drugs and Technologies in Health.
Rx for Change database. (Available at:)
https://www.cadth.ca/rx-changeGrimshaw, J., Thomas, R., MacLennan, G., Fraser, C., Ramsay, C., and Vale, L.
Effectiveness and efficiency of guideline dissemination and implementation strategies.
Health Technol Assess. 2004; 8: 1–72Bishop PB, Quon JA, Fisher CG, Dvorak MF.
The Chiropractic Hospital-based Interventions Research Outcomes
(CHIRO) Study: A Randomized Controlled Trial on the Effectiveness
of Clinical Practice Guidelines in the Medical and Chiropractic
Management of Patients with Acute Mechanical Low Back Pain
Spine J. 2010 (Dec); 10 (12): 1055-1064Grimshaw, J., Schunemann, H., Burgers, J., Cruz, A., Heffner, J., and Metersky, M.
Disseminating and implementing guidelines.
Article 13 in integrating and coordinating efforts in COPD guideline development.
Proc Am Thorac Soc. 2012; 9: 298–303Pronovost, P.
Enhancing physicians' use of clinical guidelines.
JAMA. 2013; 310: 2501–2502Schuster, MA, Elizabeth A, McGlynn R, Brook H.
How good is the quality of health care in the United States?
Milbank. 2005;83(4):843-895.Greenhalgh, T., Howick, J., and Maskrey, N.
Evidence based medicine: a movement in crisis?
BMJ. 2014; 348: g3725Canadian Institutes of Health Research.
Knowledge translation—definition. ; 2008. (Available at:)
http://www.cihr-irsc.gc.ca/e/29529.htmlGagliardi, A., Marshall, C., Huckson, S., James, R., and Moore, V.
Developing a checklist for guideline implementation planning:
review and synthesis of guideline development and implementation advice.
Implementation Sci. 2015; 10: 19Cochrane-Effective Practice and Organisation of Care (EPOC). (Available at:)
http://epoc.cochrane.org/our-reviewsGagliardi, A., Brouwers, M., and Bhattacharyya, O.
A framework of the desirable features of guideline implementation tools (GItools):
Delphi survey and assessment of GItools.
Implementation Sci. 2014; 9: 98Okelo, S., Butz, A., Sharma, R. et al.
Interventions to modify health care provider adherence to asthma guidelines:
a systematic review.
Pediatrics. 2013; 132: 517–534Murthy, L., Shepperd, S., Clarke, M. et al.
Interventions to improve the use of systematic reviews in decision-making
by health system managers, policy makers and clinicians.
Cochrane Database Syst Rev. 2012; 9: CD009401Garg, A., Adhikari, N., McDonald, H. et al.
Effects of computerized clinical decision support systems on practitioner
performance and patient outcomes: a systematic review.
JAMA. 2005; 293: 1223–1238Rebbeck, T., Macedo, L., and Maher, C.
Compliance with clinical guidelines for whiplash improved with a
targeted implementation strategy: a prospective cohort study.
BMC Health Serv Res. 2013; 13: 213Bussières, A., Côté, P., French, S. et al.
Creating a chiropractic practice-based research network (PBRN):
enhancing the management of musculoskeletal care.
J Can Chiropr Assoc. 2014; 58: 8–15Canadian Chiropractic Research Database (CCRD). National Report.
The Canadian Chiropractic Association:
A Comprehensive Inventory of Practical Information About Canada’s Licensed Chiropractors. ; 2011Becker, M., Neugebauer, E., and Eikermann, M.
Partial updating of clinical practice guidelines often makes more sense
than full updating: a systematic review on methods and the development
of an updating procedure.
J Clin Epidemiol. 2014; 67: 33–45Alonso-Coello, P., Martínez García, L., Carrasco, J.M., Solà, I., Qureshi, S., and Burgers, J.S.
The updating of clinical practice guidelines: insights from an international survey.
Implementation Sci. 2011; 6: 1–8Martínez García, L., Arévalo-Rodríguez, I., Solà, I., Haynes, R., Vandvik, P.
Strategies for monitoring and updating clinical practice guidelines:
a systematic review.
Implementation Sci. 2012; 7: 1–10Moher D, Tsertsvadze A, Tricco A, et al.
A systematic review identified few methods and strategies describing
when and how to update systematic reviews.
J Clin Epidemiol. 2007;60(11):1095.e1-11.Shekelle, P., Eccles, M., Grimshaw, J., and Woolf, S.
When should clinical guidelines be updated?
BMJ. 2001; 323: 155–157Vernooij, R., Sanabria, A., Sola, I., Alonso-Coello, P.
Guidance for updating clinical practice guidelines:
a systematic review of methodological handbooks.
Implement Sci. 2014; 9: 3Moher, D., Pham, B., Lawson, M., and Klassen, T.
The inclusion of reports of randomised trials published in languages
other than English in systematic reviews.
Health Technol Assess. 2003; 7: 1–90Morrison, A., Polisena, J., Husereau, D. et al.
The effect of English-language restriction on systematic review-based
meta-analyses: a systematic review of empirical studies.
Int J Technol Assess Health Care. 2012; 28: 138–144Harbour, R. and Miller, J.A.
new system for grading recommendations in evidence based guidelines.
BMJ. 2001; 323: 334–336Cleland, J., Mintken, P., Carpenter, K. et al.
Examination of a clinical prediction rule to identify patients with neck pain
likely to benefit from thoracic spine thrust manipulation and a general
cervical range of motion exercise: multi-center randomized clinical trial.
Phys Ther. 2010; 90: 1239–1250Escortell-Mayor, E., Riesgo-Fuertes, R., Garrido-Elustondo, S. et al.
Primary care randomized clinical trial: Manual therapy effectiveness in comparison
with TENS in patients with neck pain.
Man Ther. 2011; 16: 66–73Lamb, S., Williams, M., Williamson, E. et al.
Managing Injuries of the Neck Trial (MINT): a randomised controlled trial of
treatments for whiplash injuries.
Health Technol Assess. 2012; 16: 1–141Pool, J., Ostelo, R., Knol, D., Vlaeyen, J., Bouter, L., and de Vet, H.I.
a behavioral graded activity program more effective than manual therapy in
patients with subacute neck pain?: results of a randomized clinical trial.
Spine. 2010; 35: 1017–1024Skillgate, E., Bohman, T., Holm, L., Vingård, E., and Alfredsson, L.
The long-term effects of naprapathic manual therapy on back and neck pain.
Results from a pragmatic randomized controlled trial.
BMC Musculoskelet Disord. 2010; 11: 1–11Kongsted, A., Qerama, E., Kasch, H. et al.
Education of patients after whiplash injury: is oral advice any better than a pamphlet?
Spine. 2008; 33: E843–E848Andersen, L., Saervoll, C., Mortensen, O., Poulsen, O., Hannerz, H., and Zebis, M.
Effectiveness of small daily amounts of progressive resistance training for
frequent neck/shoulder pain: Randomised controlled trial.
Pain. 2011; 152: 440–446Cheng, A. and Hung, L.
Randomized controlled trial of workplace-based rehabilitation for work-related
rotator cuff disorder.
J Occup Rehab. 2007; 17: 487–503Feuerstein, M., Nicholas, R., Huang, G., Dimberg, L., Ali, D., and Rogers, H.
Job stress management and ergonomic intervention for work-related
upper extremity symptoms.
Appl Ergon. 2004; 35: 565–574van Eijsden-Besseling, M., Bart Staal, J., van Attekum, A., de Bie, R.A.
No difference between postural exercises and strength and fitness exercises for early,
non-specific, work-related upper limb disorders in visual display unit workers:
a randomised trial.
Aust J Physiother. 2008; 54: 95–101Cameron, I., Wang, E., and Sindhusake, D.A.
randomized trial comparing acupuncture and simulated acupuncture for subacute
and chronic whiplash.
Spine. 2011; 36: E1659–E1665Cleland, J.A., Glynn, P.E., Whitman, J.M. et al.
Short-term response of thoracic spine thrust versus non-thrust manipulation in
patients with mechanical neck pain: preliminary analysis of a randomized clinical trial.
J Manual Manipulat Ther. 2007; 14: 172Dundar, U., Evcik, D., Samli, F., Pusak, H., and Kavuncu, V.
The effect of gallium arsenide aluminum laser therapy in the management of
cervical myofascial pain syndrome: a double blind, placebo-controlled study.
Clin Rheumatol. 2007; 26: 930–934Fu, W., Zhu, X., Yu, P., and Zhang, J.
Analysis on the effect of acupuncture in treating 5 cervical spondylosis
with different syndrome types.
Chin J Integr Med. 2009; 15: 426–430Kanlayanaphotporn, R., Chiradejnant, A., and Vachalathiti, R.
The immediate effects of mobilization technique on pain and range of motion
in patients presenting with unilateral neck pain: a randomized controlled trial.
Arch Phys Med Rehabil. 2009; 90: 187–192Kanlayanaphotporn, R., Chiradejnant, A., and Vachalathiti, R.
Immediate effects of the central posteroanterior mobilization technique
on pain and range of motion in patients with mechanical neck pain.
Dis Rehab. 2010; 32: 622–628Klein, R., Bareis, A., Schneider, A., and Linde, K.
Strain-counterstrain to treat restrictions of the mobility of the cervical spine
in patients with neck pain: a sham-controlled randomized trial.
Complement Ther Med. 2013; 21: 1–7Liang, Z., Zhu, X., Yang, X., Fu, W., and Lu, A.
Assessment of a traditional acupuncture therapy for chronic neck pain:
a pilot randomised controlled study.
Complementary Ther Med. 2011; 19: S26–S32Masaracchio, M., Cleland, J.A., Hellman, M., and Hagins, M.
Short-term combined effects of thoracic spine thrust manipulation and cervical
spine nonthrust manipulation in individuals with mechanical neck pain:
a randomized clinical trial.
J Orthop Sports Phys. 2013; 43: 118–127Saavedra-Hernandez, M., Castro-Sanchez, A., Arroyo-Morales, M. et al.
Short term effects of kinesio taping versus cervical thrust manipulation
in patients with mechanical neck pain: a randomized clinical trial.
J Orthop Sports Phys Ther. 2012; 42: 724–730Sillevis, R., Hellman, M., and Beekhuizen, K.
Immediate effects of a thoracic spine thrust manipulation on the
autonomic nervous system: a randomized clinical trial.
J Manual Manipulat Ther. 2010; 18: 181–190White, P., Lewith, G., Prescott, P., and Conway, J.
Acupuncture versus placebo for the treatment of chronic mechanical neck pain:
a randomized, controlled trial.
Ann Inter Med. 2004; 141: 911–919Young, I., Cleland, J., Aguilera, A. et al.
Manual therapy, exercise, and traction for patients with cervical radiculopathy:
a randomized clinical trial.
Phys Ther. 2009; 89: 632–642
Return to WHIPLASH
Return to GUIDELINES Articles
Return to CHRONIC NECK PAIN
Return to NECK DISORDER GUIDELINES Page
Since 11–21–2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |